Abstract
Degranulating eosinophils have been described in most endometrial cancers. We hypothesized that endometriosis (ectopic, nonneoplastic endometrial tissue) would be an appropriate model system for determining whether eosinophil degranulation is part of a specific immune response to endometrial cancer or if it is related to the more general phenomenon of tissue remodeling (wound healing) that is common to both disorders. To test this hypothesis, we performed immunohistochemistry and Western blotting to evaluate the presence of eosinophil peroxidase (a marker of eosinophil degranulation) in normal endometrium (n = 20) and endometriosis samples (n = 24) and to define the coexpression of three eosinophil chemoattractants: interleukin-5 (IL-5), eotaxin, and regulated on activator-normal T cell expressed and secreted (RANTES). There was focally intense deposition of eosinophil peroxidase in the fibrotic connective tissue and blood vessels of 21 of 24 human endometriosis specimens; two samples showed weak staining, and only one tissue was negative for eosinophil degranulation. None of the 10 normal proliferative endometrial specimens had evidence of eosinophil degranulation, and four of 10 secretory tissues stained only weakly for eosinophil peroxidase. The presence of degranulating eosinophils was also associated with the presence of eotaxin and IL-5 in some samples and with RANTES in others. We conclude that the abundant presence of degranulating eosinophils in the fibrous regions of endometriosis supports the interpretation that eosinophils are involved in general tissue remodeling and wound healing rather than a tissue-directed immune response.
Endometriosis is characterized by the ectopic implantation of nonmalignant endometrial glands and stroma in regions remote from the uterine cavity. Fibrous adhesions frequently surround endometriotic lesions, and healed foci of endometriosis usually consist only of fibrous tissue and hemosiderin-laden macrophages. Some women with endometriosis remain asymptomatic , whereas others experience chronic pain. 1 Endometriosis is usually confined to the pelvis, but extrapelvic sites have been reported in nearly all organs of the abdominal cavity. In addition, the thorax, skin, muscles, peripheral nerves, brain, and spinal column are occasionally affected, as are surgical scars and the genital tract. Areas that are frequently involved include the abdominal wall, small intestines, appendix, urinary tract, and lymph nodes. 2
Eosinophil peroxidase (EPO) is an intracellular enzyme that is released from eosinophils as they degranulate. 3 The high arginine content of the enzyme results in an unusually high net positive charge, thereby allowing it to adhere strongly to the surface of negatively charged cells that are adjacent to degranulating eosinophils. 4 Degranulating eosinophils have been identified in many lymphomas, breast cancers, and in most cases of endometrial cancer that were studied. 5-7 In normal endometrium, eosinophils accumulate just before and during menstruation. 8 This process is regulated, at least in part, by estradiol, 9,10 which controls the production of an eosinophil chemoattractant factor in rat. 11,12
The role of eosinophils in endometrial and other cancers remains uncertain. One possible explanation is that the eosinophils play a role in the host immune response that is specifically directed against the tumor cells. An alternative, and more likely, explanation is that the eosinophils participate in a more general type of inflammatory response that is related to wound healing and tissue remodeling prompted by the growing tumor. There is a growing body of evidence, for example, that eosinophils regulate fibroblasts and stimulate collagen synthesis. 13-17
Human endometriosis provides a good model system for studying this issue because it consists of biologically benign endometrial tissue that behaves in a pseudomalignant fashion by growing in ectopic locations. Therefore, if eosinophils were part of an immune response specifically directed against endometrial cancer cells, we would not expect to find degranulating eosinophils in benign endometriosis tissues. On the other hand, if eosinophils are involved in generating a fibrous tissue response as part of the wound-healing mechanism, we would expect to see evidence of degranulating eosinophils in endometriosis. Here we describe EPO expression in association with fibrosis in human endometriosis specimens and the coexpression of eosinophil chemoattractants in those tissues.
Materials and Methods
Tissues
Fresh endometriosis tissues (n = 24) were supplied by the Eastern and Midwestern Divisions of the Cooperative Human Tissue Network (CHTN) of the National Disease Research Institute and by Dr. Alice Demoupolis (New York University, Department of Gynecologic Pathology). All lesions were black or red and came from ovarian/tubal sites. Normal proliferative (n = 10; one menses) and secretory (n = 10; two late secretory) endometrium was also supplied by Dr. Demoupolis. Fresh surgical specimens from CHTN were shipped overnight on ice in Dulbecco’s minimum essential medium. Cryostat sections of both types of tissue were immunohistochemically stained with anti-EPO or an isotype-matched irrelevant IgG (Ag8). Six to nine samples were also stained with antibodies to interleukin-5 (IL-5) or IL-5R or eosinophil-attracting chemokines (eg, RANTES, eotaxin) to understand the regulation of expression of EPO.
To determine the specificity of the eosinophil response to endometriosis, we also examined a variety of inflammatory intraperitoneal processes (chronic salpingitis, n = 2; chronic appendicitis, n = 2; and postoperative scarring, n = 1) and normal fallopian tubes (n = 2) for evidence of degranulating eosinophils. These tissues were supplied by Dr. Fritz Lin (Department of Pathology, University of California, Irvine, CA).
Antibodies
The following primary antibodies were used: anti-eosinophil peroxidase (EPO), polyclonal rabbit anti-huRANTES (500-P36; PeproTech, Rocky Hills, NJ), polyclonal rabbit anti-huEotaxin (500-P41; PeproTech), polyclonal rabbit anti-huIL-5 (SC-1765; (Santa Cruz Biotechnology, Santa Cruz, CA), and polyclonal rabbit anti-huIL-5Rα (SC-673; Santa Cruz Biotechnology). The IL-5R antibody does not cross-react with either IL-3 or granulocyte-macrophage colony-stimulating factor (GM-CSF). Goat anti-rabbit second-antibody (ALI3401) was purchased from Biosource (Camarillo, CA.).
Immunohistochemistry
Indirect immunoperoxidase staining was done by incubating tissue sections (5 μm) in 95% ethanol for 10 minutes and then washing slides rapidly with phosphate-buffered saline (PBS) (pH 7.4). Endogenous peroxidase activity was neutralized by flooding the slides with 0.3% H2O2 in methanol (1 ml of 3% H2O2 in 9 ml MeOH) and then washing for 5 minutes in PBS. Nonspecific binding was reduced with a 20-minute incubation with normal blocking solution. The blocking serum was adjusted to the species in which the secondary antibody was generated. Each section was then covered with 50 μl of primary antibody (10 μg/ml) for 30 minutes in a humid chamber. Anti-EPO antibody (Techniclone International, Tustin, CA) is a murine monoclonal antibody that is highly specific for EPO and does not bind to other cell types or to myeloperoxidase. 3 Its derivation, origin, and other properties are described in detail in previous publications. 3,5 Biotinylated horse anti-mouse second antibody (50 μl of a 1:200 dilution of stock) was added and incubated for 30 minutes under humid conditions. Horse serum (HS) from Vectastain (Vector Labs, Burlingame, CA) was the blocking agent. Excess second antibody was washed off with 5% HS-PBS. For rabbit primary antibodies, biotinylated goat anti-rabbit second antibody was the second antibody used, and goat serum was used for the blocking solution. Sections were washed again in PBS and processed using a Vectastain avidin-biotin complex (ABC) reagent for 30 minutes. Excess ABC was washed off and sections were finally covered with 100 μl DAB solution (a 1:1 solution of 1 mg/ml diaminobenzidine in 1 ml Tris buffer and 67 μl of 3% H2O2) for about 6 minutes. All incubations were done at room temperature. All primary antibodies were used at a concentration of 10 μg/ml. Slides were then washed three times with PBS and counterstained for 30 seconds with hematoxylin before coverslipping. The positive controls were cryostat sections of tissues containing Hodgkin’s disease, which typically contains numerous eosinophils and cytokine-positive inflammatory cells. An affinity-isolated rabbit IgG was used in place of the primary rabbit anti-chemokine antibodies as a negative control for the rabbit polyclonal stained sections in those studies.
Qualitative Immunohistochemical Analysis
Reactivity of antibody with endometriosis sections was assessed by determining the number of samples that were positive per number of samples tested. Reactivity was defined as follows: negative, faint staining (−/+), staining present with low to moderate intensity (1+), and strong and abundant staining intensity (2+). The microanatomical location of staining was also evaluated: eg, myometrium, glandular epithelium, luminal secretions, stroma/connective tissue, and perivascular. Slides were digitally imaged using a Spot Diagnostic digital camera (Sterling Heights, MI) and Image-Pro Plus imaging software (Media Cybernetics, Silver Spring, MD).
Electrophoresis and Immunoblotting
Tissue homogenates were prepared from stored (−70°C) surgical samples by mincing and homogenizing tissue at 4°C in homogenate buffer (10 mmol/L Tris, pH 7.6, 1% Triton X-100, 150 mmol/L NaCl, 5 mmol/L EDTA, 10 μg/ml leupeptin, 1 μg/ml aprotinin, 100 μmol/L phenylmethylsulfonyl fluoride) (Tissue-Tearer; Dremel, Racine, WI). After 5 minutes ’ incubation on ice, homogenates were centrifuged at 2000 × g for 5 minutes. Supernatants were transferred to a separate tube, and the protein content was determined by the method of Bradford (Sigma, St. Louis, MO). Both homogenates and pellets were stored at −70°C.
Specific proteins in homogenates were detected by immunoblotting. Proteins in the homogenate (aliquots of 10–20 μg) were fractionated by size with 10% or 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing (2% SDS, 100 mmol/L dithiothreitol) or nonreducing (for EPO) conditions. After transfer of protein to nitrocellulose filters (BA-S 85; Schleicher and Schuell, Keene, NH), the nonspecific binding sites were blocked with 0.2% casein/1% normal serum. Specific proteins were detected by sequential incubation in primary antibody (0.14 μg/ml anti-EPO antibody or 0.2 μg/ml anti-actin antibody). The horseradish peroxidase-conjugated goat anti-mouse second antibody was used at a dilution of 1:1000 (Vector Laboratories). EPO and actin bands were visualized with Luminol Reagent (Santa Cruz).
Results
EPO Expression in Endometriosis and Normal Endometria
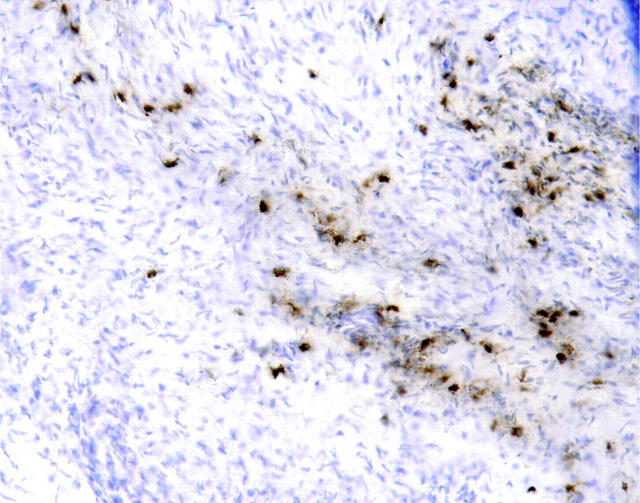
The results of this part of the study are presented in Table 1 ▶ . Specific EPO staining (+1 to +2 intensity) was observed on 21 of 24 samples of endometriosis tissue; two showed faint (±) staining, and only one sample was completely negative. The EPO was localized within intact eosinophils and as extracellular granular material, most commonly in the loose connective tissue (Figure 1A) ▶ and fibrous bands at the edges of the lesions (Figure 1B) ▶ . Frequently, the EPO was concentrated within apparently intact eosinophils in the lumen of small blood vessels (Figure 1C) ▶ . In one case, the EPO was also associated with extensive acute inflammation. Extracellular EPO was never observed in the myometrium or in the endometrial glands or lumen (Figure 1D) ▶ , but intact eosinophils were sometimes observed in the myometrium (Figure 1E) ▶ .
Table 1.
Eosinophil Peroxidase in Normal Endometrium and Endometriosis
| Tissue | Eosinophil peroxidase staining intensity | ||||
|---|---|---|---|---|---|
| − | +/− | +1 | +2 | Total | |
| Normal proliferative endometrium | 10 | 0 | 0 | 0 | 10 |
| Normal secretory endometrium | 6 | 4 | 0 | 0 | 10 |
| Endometriosis | 1 | 2 | 7 | 14 | 24 |
Figure 1.
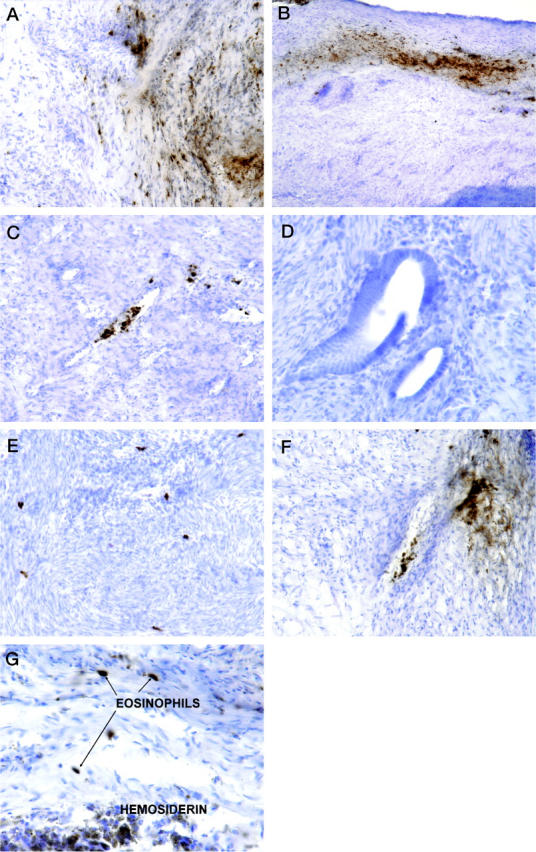
A: Endometriosis samples immunostained for EPO. There was positive staining in intact eosinophils (dark, red-brown deposits) and on free eosinophil granules in the loose connective tissue . B: Staining was especially intense in band-like deposits at the edges of lesions. C: Intact eosinophils with EPO activity were often detected within and near small blood vessels. Eosinophils and EPO were not detected in or near the glandular portions of endometriosis (D), but intact eosinophils were occasionally observed in the myometrium (E). A strikingly fibrillar distribution of EPO was also frequently observed (D, upper right corner). G: Hemosiderin-laden macrophages could be seen in proximity to eosinophils but had different tinctorial properties. Original magnifications: D and G, ×400; A, C, E, and F, ×200; B, ×100. Hematoxylin counterstain and DAB chromogen.
The distribution of EPO was generally quite patchy and sometimes had a strikingly fibrillar distribution (Figure 1F) ▶ , resembling the pattern previously described in Hodgkin’s disease and in breast cancer. 3,5,7 The intense, localized, red-brown staining of EPO and eosinophils was readily distinguishable from hemosiderin-laden macrophages, which had a granular, olive brown appearance (Figure 1G) ▶ that persisted even in the absence of primary antibody.
H&E-stained sections of these tissues showed a few intact eosinophils admixed with hemosiderin granules (Figure 2A) ▶ . Under high-power (×400) magnification, medusa cells, a connective tissue eosinophil that has assumed an ameboid or fibrillar shape, were readily identifiable in other areas (Figure 2 ▶ , B and C), along with free red granules suggestive of disintegrated eosinophils.
Figure 2.
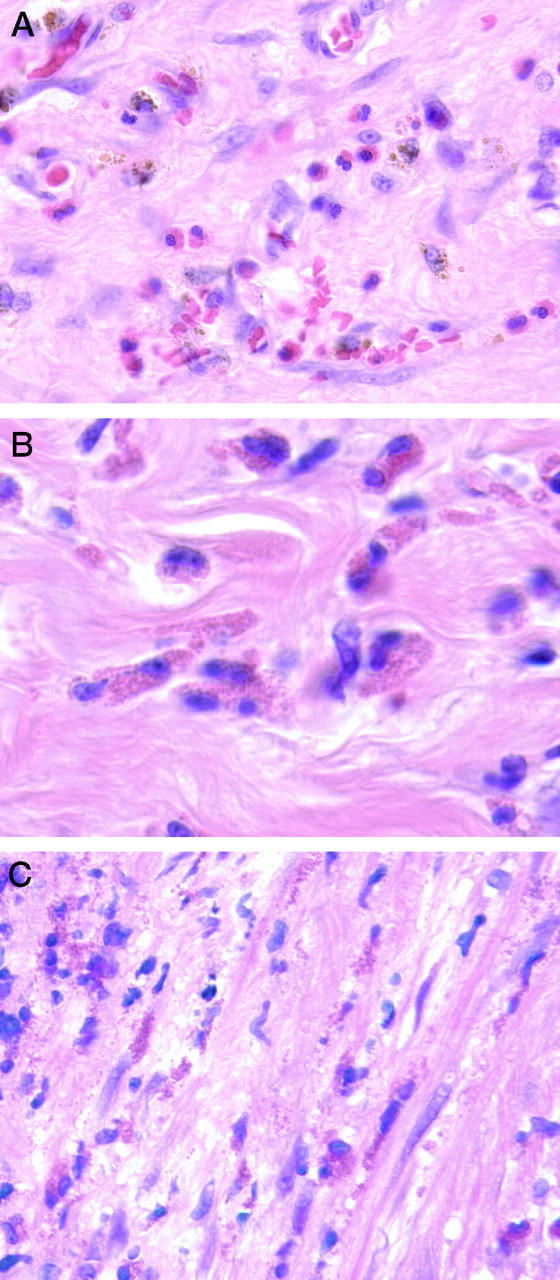
Hematoxylin and eosin-stained sections of endometriosis tissues. A: Intact eosinophils admixed with golden-brown hemosiderin granules could be readily identified. B and C: In other regions, medusa cells (eosinophils with an ameboid appearance embedded in fibrous stoma) were present (B and C), along with extracellular red granules that probably represented disintegrated eosinophils (C). Original magnification: all images were ×400. B and C were electronically enlarged.
No specific staining for EPO was observed in the 10 normal proliferative endometrial samples (Figure 3A) ▶ . Four of 10 normal secretory samples showed only faint (±) interstitial staining on a few intact eosinophils. The negative control slides from the endometriosis samples and normal endometrial biopsies had little or no specific staining (Figure 3B) ▶ . There was no evidence of degranulating eosinophils in the other inflammatory or normal intraperitoneal tissues that were examined, which may reflect the relatively late and mature stages of fibrosis in these tissues compared to the endometriosis samples.
Figure 3.
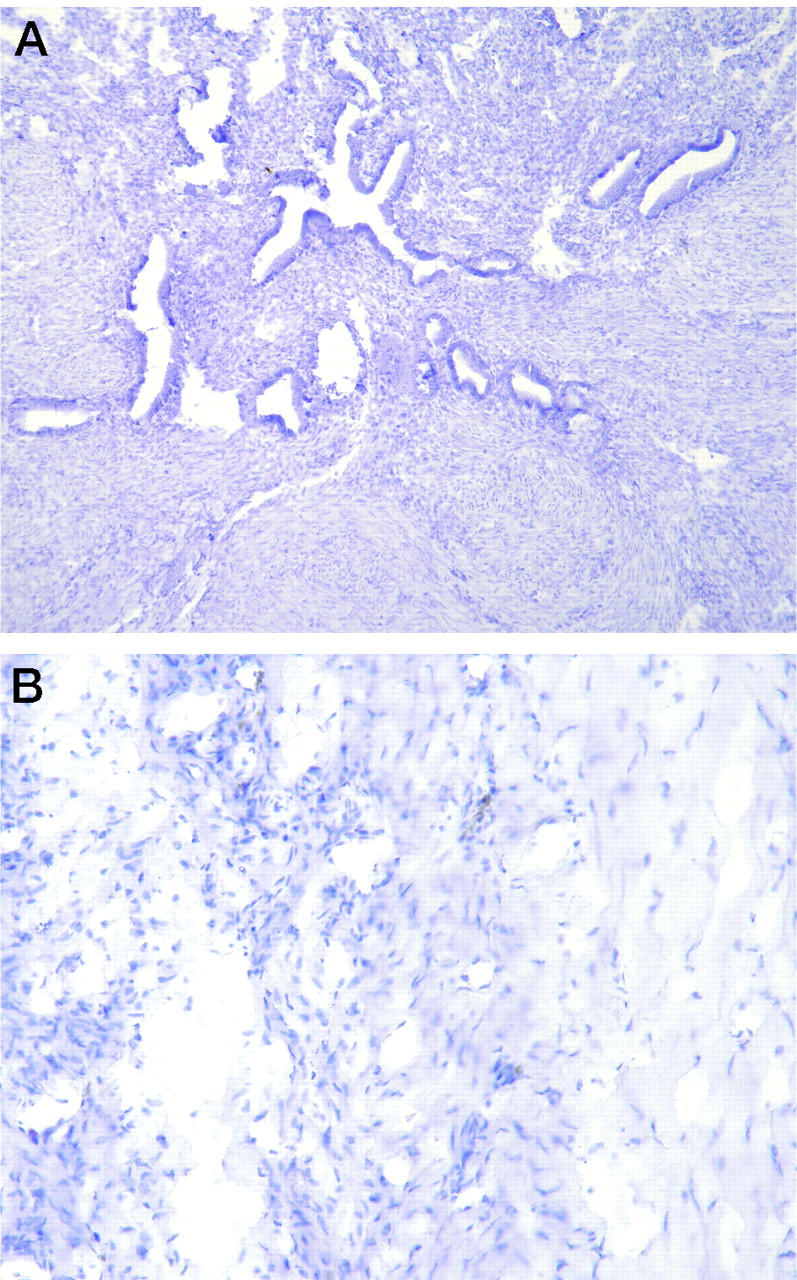
Control specimens. A: Normal proliferative endometrial samples had no evidence of EPO when immunostained with the monoclonal antibody directed against EPO. B: Substitution of an irrelevant monoclonal antibody (negative control) for the anti-EPO antibody produced no staining of any of the tissues, including the endometriosis sample shown here.
Cytokines in Endometriosis
In an effort to understand the regulation of expression of EPO in endometriosis, we then evaluated expression of three cytokines with chemotactic properties for eosinophils: IL-5, eotaxin, and RANTES. Because IL-5 is a chemoattractant for eosinophils, 18,19 we performed immunohistochemistry (IHC) to determine expression of IL-5 and its receptor in the same specimens (Figure 4) ▶ . IL-5 expression was increased in five of nine specimens studied (+1 intensity), and IL-5R was increased in 23 of 24 specimens (+1 to +2). There was no IL-5 staining in normal endometrium and only faint IL-5R staining in some secretory endometrium samples (results not shown), but not in proliferative endometrium. The staining for IL-5R in both endometriosis and normal endometrium generally paralleled the distribution and intensity of staining for EPO. This result suggested that IL-5R in the tissues probably represented receptor from disintegrated eosinophils within the soft tissue. The one sample of endometriosis that was negative for IL-5 and IL-5R was also negative for EPO, suggesting that the IL-5 system may at least mediate in part the infiltration by eosinophils. It is unknown at this time why this tissue sample did not stain for either IL-5 or for EPO.
Figure 4.
Endometriosis sample immunostained for IL-5 receptor. There was no detectable IL-5 in this tissue, but IL-5 receptor was present as fibrillar and amoeboid deposits resembling medusa cells, suggesting an origin from eosinophils. Original magnification, ×200.
In a follow-up study, both normal endometrium and endometriosis samples were stained with primary antibodies specific for IL-5, eotaxin, and RANTES (Table 2) ▶ . In four of six endometriosis samples, moderate to intense staining for eotaxin was observed in the glandular epithelium, perivascular region, stromal area, and inflammatory fibrotic area. In contrast, three of four normal endometrium (one proliferative and two secretory) samples only had faint ± staining concentrated exclusively on the luminal surface of the glandular epithelium. RANTES staining was negative in normal endometrium (0/4), but low to moderate staining was detected in three of six samples of endometriosis tissue, mostly where inflammation was present. Anti-IL-5 faintly (±) stained the glandular epithelium in one of the four normal samples and moderately (+1) stained three of six endometriosis samples. Endometriosis was often double positive for eotaxin and IL-5 (three of six). In no case was a sample positive for both RANTES and IL-5.
Table 2.
Coexpression of Cytokines in Endometrial Tissues
| Cytokine expression | Normal endometrium (n = 4) | Endometriosis (n = 6) |
|---|---|---|
| IL-5+/RANTES− | 1 (25%) | 4 (66%) |
| IL-5+/eotaxin− | 0 (0%) | 1 (16%) |
| IL-5+/RANTES+ | 0 (0%) | 0 (0%) |
| IL-5+/eotaxin+ | 1 (25%) | 3 (50%) |
| RANTES+/IL-5− | 0 (0%) | 2 (33%) |
| RANTES+/eotaxin− | 0 (0%) | 1 (16%) |
| RANTES+/eotaxin+ | 0 (0%) | 1 (16%) |
| Eotaxin+/IL-5− | 2 (50%) | 1 (16%) |
| Eotaxin+/RANTES− | 3 (75%) | 1 (16%) |
| IL-5+/RANTES+/eotaxin+ | 0 (0%) | 0 (0%) |
Western Blot Analysis of Endometriosis
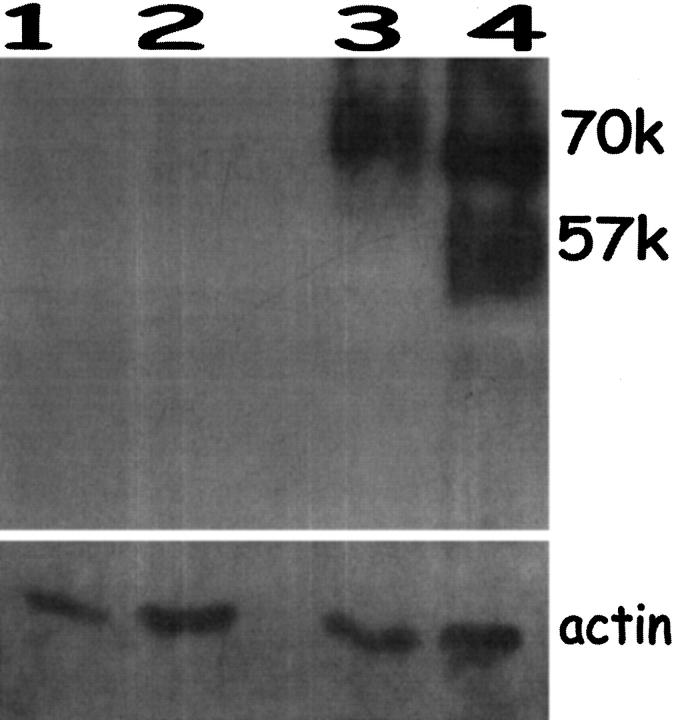
Immunoblots of total protein isolated from endometriosis specimens, as well as from endometrium from endometriosis patients (proliferative phase), were probed for EPO under nonreducing conditions (Figure 5) ▶ . EPO is a heterodimer composed of a 57 -kd subunit and an 11-kd subunit. 20 An EPO-specific band was seen in the two endometriosis samples (lanes 3 and 4) at ∼70 kd. One sample had a second band at 57 kd, suggesting some breakdown of the heterodimer despite the nonreducing conditions. The 11 -kd band was not seen, possibly because this smaller subunit did not express the epitope recognized by the anti-EPO antibody. The proliferative endometrium from endometriosis patients (lanes 1 and 2) was negative for EPO. These immunoblot results suggest that degranulating eosinophils are most likely localized in the endometriosis lesions and are not present in uterine endometrium with no specific pathological changes obtained from the same patients. Occasionally, endometrium from women without endometriosis demonstrated a weak signal at 57 kd (not shown). Pathology reports for these weakly positive samples documented the presence of disordered proliferative endometrium or polyps that may be associated with tissue remodeling.
Figure 5.
Immunoblotting for EPO in endometriosis lesions and endometrium from endometriosis patients. Lanes 1 and 2: normal endometrium from two endometriosis patients, proliferative phase. Lanes 3 and 4: endometriosis tissue from proliferative phase. Only the endometriosis samples contained abundant EPO. Size markers were included. Reprobing of these blots for actin indicated equal loading of protein in all lanes.
Discussion
We have presented three lines of evidence which indicate that degranulating eosinophils are present in the fibrotic connective tissue regions of endometriosis lesions: immunohistochemical detection of EPO, immunoblotting, and direct histological visualization of eosinophils. These results, therefore, support the interpretation that eosinophils are more likely to be involved in general tissue remodeling and wound healing rather than being a tumor-specific immune response directed against endometrial cancer. This is because endometriosis and endometrial cancer share the common property of tissue damage and wound healing.
Our findings that degranulating eosinophils are associated with the fibrotic portions of endometriosis are consistent with previous reports that eosinophils are linked to fibrosis. Bassett et al showed in 1977 that the collagenous fibers of healing, incised, dermal wounds in rats contained abundant eosinophils. 21 A subsequent study confirmed the presence of degranulating eosinophils in the collagen bands of nodular sclerosis Hodgkin’s disease. 3 Since then, a wide variety of other pathological disorders have also been shown to contain tissue eosinophilia and evidence of eosinophil degranulation in association with fibrosis. 22 Our current study, therefore, appears to add endometriosis to the list of conditions in which fibrosis is associated with eosinophil degranulation.
The mechanisms by which eosinophils promote fibrosis are becoming more understood. Birkland et al 13 demonstrated that human eosinophils stimulate DNA synthesis and matrix production in dermal fibroblasts. Using a model of pulmonary fibrosis, other investigators 14,15 then showed that tumor necrosis factor-α (TNF-α) might mediate pulmonary fibrosis via induction of IL-5-mediated eosinophil recruitment and fibrogenic cytokine production. More recently, IL-4-mediated tissue allograft infiltration by eosinophils has been shown to be associated with interstitial fibrosis. 16 Finally, Levi-Schaffer et al 17 have shown that transforming growth factor -β derived from human eosinophils directly regulates human lung- and skin-derived fibroblast proliferation and collagen synthesis.
Eosinophils in the uterus of estrogen-treated rats 10,23-25 and in normal human endometrium just before and during menstruation 8 may also be involved in tissue remodeling, but in a manner that is probably unrelated to fibrosis. In these situations, it has been proposed that matrix metalloproteinases liberated by eosinophils may account for tissue degradation during menstruation. 26 Thus it appears that eosinophils may play multiple, interrelated roles in the remodeling of uterine tissues: promotion of fibrosis during endometriosis, tissue breakdown during normal menstruation, and wound healing with fibrosis during progressive growth of endometrial cancer.
The nature of the eosinophil chemotactic factor in the uterus in these conditions is still incompletely understood. A variety of proteins that are chemotactic for eosinophils (eg, MCP-1, lysophosphatidylcholine) have recently been shown to be elevated in ectopic endometrium, 27,28 including a 17-kd estrogen-inducible protein in rat 29 and various factors ranging in molecular mass from 70 to 300 kd. 30 IL-5 (molecular mass = 45 kd) is capable of recruiting eosinophil infiltration, as well as inducing the degranulation of eosinophils 18,19 through the Jak 2-STAT-1 pathway. 31,32
Another very potent eosinophil chemotactic growth factor is eotaxin. 33,34 IL-5 and eotaxin (8 kd) have been shown to act synergistically to augment eosinophil accumulation. 35 Pelvic fluid concentrations of RANTES (8 kd), a cytokine with potent chemotactic activity for human monocytes and eosinophils, is elevated in the peritoneal fluid and in the stroma of women with endometriosis. The levels correlate with the severity of the disease. 36 However, RANTES is also produced by stroma of normal endometrium. 37 The levels of RANTES in endometriosis are significantly higher because stroma from endometriosis is under the direct influence of proinflammatory cytokines like IFN-γ and TNF-α, 38,39 which are capable of up-regulating RANTES expression. Our immunohistochemical results have demonstrated that eotaxin expression, possibly in combination with IL-5, may be a mechanism of eosinophilia associated with endometriosis. An alternative mechanism of eosinophil attraction might involve RANTES alone. The two mechanisms may operate at different times of the menstrual cycle under different hormonal influences. Other chemoattractants, such as MCP-1α, will also need to be considered. Additional ongoing studies are addressing this issue.
It remains to be determined whether or not EPO expression is expressed in all four stages of endometriosis and in all types of disease (nodular lesions, vesicular implants, papular implants, ovarian adhesions, and hemorrhagic cysts). In addition, the variability that we observed between specimens may be due to the stage of the menstrual cycle during which the specimen was obtained. This in turn may affect the expression of proinflammatory cytokines in the peritoneal fluid, chemokines, and/or their receptors within the endometriosis tissue or the process of degranulation of eosinophils once they infiltrate the tissue. The absence of degranulating eosinophils in the other chronic, inflammatory, intraperitoneal processes that we studied provides further evidence that eosinophil degranulation may be temporally related to a specific and active stage in the fibrotic process and may not be present in the final stage of fibrosis. In vitro studies now in progress are attempting to determine whether expression of EPO in endometriosis is influenced by estradiol (E2) and progesterone (P) and, if so, by what mechanism. Additional fresh clinical specimens from known phases of the menstrual cycle are also being evaluated for EPO expression, along with immunostaining of several relevant eosinophil chemoattractants.
Finally, our findings may have more immediate, practical implications with regard to developing a noninvasive imaging procedure for in situ localization of endometriosis. The high percentage of cases of endometriosis that were positive for eosinophil degranulation (∼88% at 1+ or higher in our study) gives us confidence that EPO will be a useful target for antibody-directed imaging and/or therapy of endometriosis using conjugated antibody to EPO. The monoclonal antibody directed against EPO that we used in this study has already been radiolabeled and administered to a variety of patients. 5 These studies have clearly shown that the antibody localizes only to sites of active eosinophil degranulation , such as those in certain tumors and allergic nasal polyps. Although eosinophil degranulation is not a specific property of endometriosis or cancer, it may well prove to be a useful marker for tissue remodeling that is associated with a variety of diseases. Further ongoing studies are attempting to develop novel direct and indirect chemistries to radiolabel the anti-EPO antibody for radioimmunodetection of endometriosis and other diseases.
Footnotes
Address reprint requests to Dr. Rosalyn D. Blumenthal, Garden State Cancer Center, 520 Belleville Avenue, Belleville, NJ 07109.
Supported in part by U.S. Public Health Service grant CA39841 from the National Institutes of Health (D. M. G.).
References
- 1.Mitchell G: Clinical Presentation and Diagnosis. Schenker R eds. Endometriosis: Contemporary Concepts and Clinical Management. 1989, :pp 307-328 Lippincott, Philadelphia [Google Scholar]
- 2.Robboy SJ, Guggan MA, Kurman RJ: The Female Reproductive System. Rubin E Farber JL eds. Pathology. 1994, :936-938 Lippincott, Philadelphia [Google Scholar]
- 3.Samoszuk M, Sholly S, Epstein A: Eosinophil peroxidase is detectable with a monoclonal antibody in collagen bands of nodular sclerosis Hodgkin’s disease. Lab Invest 1987, 56:394-400 [PubMed] [Google Scholar]
- 4.Samoszuk M, Peterson A, Gidanian F: Cytophilic and cytotoxic properties of human eosinophil peroxidase plus major basic protein. Am J Pathol 1988, 132:455-460 [PMC free article] [PubMed] [Google Scholar]
- 5.Samoszuk M, Andersen A, Ramzi E, Wang F, Braunstein P, Lutsky J, Majmundar H, Slater L: Radioimmunodetection of Hodgkin’s disease and non-Hodgkin’s lymphomas with monoclonal antibody to eosinophil peroxidase. J Nucl Med 1993, 34:1246-1253 [PubMed] [Google Scholar]
- 6.Samoszuk M, Lin F, Rim P, Strathearn G: New marker for blood vessels in human ovarian and endometrial cancers. Clin Cancer Res 1996, 2:1867-1871 [PubMed] [Google Scholar]
- 7.Samoszuk M: Eosinophils and human cancer. Histol Histopathol 1997, 12:807-812 [PubMed] [Google Scholar]
- 8.Jeziorska M, Salamonsen L, Woolley D: Mast cell and eosinophil distribution and activation in human endometrium throughout the menstrual cycle. Biol Reprod 1995, 53:312-320 [DOI] [PubMed] [Google Scholar]
- 9.Kelenyi G, Nemeth A: Tissue eosinophil leucocytes. II. Electron microscopy and histochemistry of the uterine eosinophil leucocytes in oestrogen-treated spayed rats. Acta Acad Sci Hung 1972, 23:253-267 [PubMed] [Google Scholar]
- 10.Brokelmann J, Fawcett D: The localization of endogenous peroxidase in the rate uterus and its induction by estradiol. Biol Reprod 1969, 1:59-71 [DOI] [PubMed] [Google Scholar]
- 11.Lee Y, Howe R, Sha S, Teuscher C, Sheehan D, Lyttle C: Estrogen regulation of an eosinophil chemotactic factor in the immature rat uterus. Endocrinology 1989, 125:3022-3028 [DOI] [PubMed] [Google Scholar]
- 12.Leiva M, Xu Q, Galman M, Lyttle C: Ontogeny of the production of an estrogen-regulated eosinophil chemotactic factor in the rat uterus. Biol Reprod 1991, 45:818-823 [DOI] [PubMed] [Google Scholar]
- 13.Birkland TP, Cheavens MD, Pincus SH: Human eosinophils stimulate DNA synthesis and matrix production in dermal fibroblasts. Arch Dermatol Res 1994, 286:312-318 [DOI] [PubMed] [Google Scholar]
- 14.Zhang K, Gharaee-Kermani M, McGarry B, Remick D, Phan SH: TNF-alpha-mediated lung cytokine networking and eosinophil recruitment in pulmonary fibrosis. J Immunol 1997, 158:954-959 [PubMed] [Google Scholar]
- 15.Gharaee-ermani M, Phan SH: The role of eosinophils in pulmonary fibrosis (review). Int J Mol Med 1998, 1:43-53 [PubMed] [Google Scholar]
- 16.Le Moine A, Flamand V, Demoo FX, Noel JC, Surquin M, Kiss R, Nahori MA, Pretolani M, Goldman M, Abramowicz D: Critical roles for IL-4, IL-5, and eosinophils in chronic skin allograft rejection. J Clin Invest 1999, 103:1659-1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levi-Schaffer F, Garbuzenko E, Rubin A, Reich R, Pickholz D, Gillery P, Emonard H, Nagler A, Maquart FA: Human eosinophils regulate human lung- and skin-derived fibroblast properties in vitro: a role for transforming growth factor beta (TGF-beta). Proc Natl Acad Sci USA 1999, 96:9660-9665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujisawa T, Terada A, Atsuta J, Iguchi K, Kamiya H, Sakurai M: IL-5 as a strong secretagogue for human eosinophils. Int Arch Allergy Immunol 1997, 114(Suppl 1):81-83 [DOI] [PubMed] [Google Scholar]
- 19.Shi H, Qin S, Huang G, Chen Y, Xiao C, Xu H, Liang G, Xie Z, Qin X, Wu J, Li G, Zhang C: Infiltration of eosinophils into the asthmatic airways caused by interleukin 5. Am J Respir Cell Mol Biol 1997, 16:220-224 [DOI] [PubMed] [Google Scholar]
- 20.Ten R, Pears L, McKean D, Bell M, Gleich G: Molecular cloning of the human eosinophil peroxidase. Evidence for the existence of a peroxidase multigene family. J Exp Med 1989, 169:1757-1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassett E, Baker J, de Souza P: A light microscopical study of healing incised dermal wounds in rats, with special reference to eosinophil leukocytes and to the collagenous fibres of the periwound areas. Br J Exp Pathol 1977, 58:581-605 [PMC free article] [PubMed] [Google Scholar]
- 22.Noguchi H, Kephart G, Colby T, Gleich G: Tissue eosinophilia and eosinophil degranulation in syndromes associated with fibrosis. Am J Pathol 1992, 140:521-528 [PMC free article] [PubMed] [Google Scholar]
- 23.Bassett E: Infiltration of eosinophils into the modified connective tissue of oestrous and pregnant animals. Nature 1962, 194:1259-1261 [Google Scholar]
- 24.Bergman F, Damber M, Linden U, Paul K: Mast cells and eosinophil granulocytes in the oestrogen-stimulated mouse uterus. Acta Endocrinol 1972, 69:77-86 [DOI] [PubMed] [Google Scholar]
- 25.King W, Alen T, DeSombre E: Localization of uterine peroxidase activity in estrogen-treated rats. Biol Reprod 1981, 25:859-870 [DOI] [PubMed] [Google Scholar]
- 26.Salamonsen LA, Woolley DE: Menstruation: induction by matrix metalloproteinases and inflammatory cells. J Reprod Immunol 1999, 44:1-27 [DOI] [PubMed] [Google Scholar]
- 27.Jolicoeur C, Boutouil M, Drouin R, Paradis I, Lemay A, Akoum A: Increased expression of monocyte chemotactic protein-1 in the endometrium of women with endometriosis. Am J Pathol 1998, 152:125-133 [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy A, Santanam M, Morales A, Parthasarathy S: Lysophosphatidyl choline, a chemotactic factor for monocytes/T-lymphocytes is elevated in endometriosis. J Clin Endocrinol Metab 1998, 83:2110-2113 [DOI] [PubMed] [Google Scholar]
- 29.Howe R, Lee Y, Fischkoff S, Teuscher C, Lyttle C: Glucocorticoid and progestin regulation of eosinophil chemotactic factor and complement C3 in the estrogen-treated rat uterus. Endocrinology 1990, 126:3193-3199 [DOI] [PubMed] [Google Scholar]
- 30.Hirashima M, Tashiro K, Sakata K, Muramoto K, Iyama K: Eosinophil chemotactic factors from granuloma of Kimura’s disease with special reference to T lymphocyte-derived factors. J Leukoc Biol 1986, 40:393-401 [DOI] [PubMed] [Google Scholar]
- 31.Pazdrak K, Stafford S, Alam R: The activation of the Jak-STAT 1 signaling pathway by IL-5 in eosinophils. J Immunol 1995, 155:397-402 [PubMed] [Google Scholar]
- 32.van der Bruggen T, Caldenhoven E, Kanters D, Coffer P, Raaijmakers J, Lammers J, Koenderman L: Interleukin-5 signaling in human eosinophils involves JAK2 tyrosine kinase and Stat1 alpha. Blood 1995, 85:1442-1448 [PubMed] [Google Scholar]
- 33.White J, Imburgia C, Dul E, Appelbaum E, O’Donnell E, O’Shannessy D, Brawner M, Fornwald J, Adamou J, Elshourbagy N, Kaiser K, Foley J, Schmidt DB, Johanson K, Macphee C, McNulty D, Scott G, Schleimer R, Sarau H: Cloning and functional characterization of a novel human CC chemokine that binds to the CCR3 receptor and activates human eosinophils. J Leukoc Biol 1997, 62:667-675 [DOI] [PubMed] [Google Scholar]
- 34.Forssman U, Uguccioni M, Loetscher P, Dahinden C, Langen H, Thelen M, Baggiolini L: Eotaxin-2, a novel CC chemokine that is selective for the chemokine receptor CCR3, and acts like eotaxin on human eosinophils and basophil leukocytes. J Exp Med 1997, 185:2171-2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins P, Marleau S, Griffiths-Johnson D, Jose P, Williams T: Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J Exp Med 1995, 182:1169-1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khorram O, Taylor R, Ryan I, Schall T, Landers D: Peritoneal fluid concentrations of the cytokine RANTES correlate with the severity of endometriosis. Am J Obstet Gynecol 1993, 169:1545-1549 [DOI] [PubMed] [Google Scholar]
- 37.Hornung D, Ryan IP, Chao VA, Vigne JL, Schriock ED, Taylor RN: Immunolocalization and regulation of the chemokine RANTES in human endothelial and endometriosis tissues and cells. J Clin Endocrinol Metab 1997, 82:1621-1628 [DOI] [PubMed] [Google Scholar]
- 38.Oral E, Olive D, Arici A: The peritoneal environment in endometriosis. Hum Reprod Update 1996, 2:385-398 [DOI] [PubMed] [Google Scholar]
- 39.Giudice L, Tazuke SI, Swiersz L: Status of current research on endometriosis. J Reprod Med 1998, 43(Suppl 3):252-262 [PubMed] [Google Scholar]