Abstract
When the monocyte infiltrates a tissue, adhesion to the extracellular matrix provides structural anchors, and the cell may be deformed through these attachments. To test the hypothesis that human monocytes/macrophages are mechanically responsive, we studied the effects of small cyclic mechanical deformations on cultured human monocytes/macrophages. When monocytes/macrophages were subjected to 4% strain at 1 Hz for 24 hours, neither matrix metalloproteinase (MMP)-1 nor MMP-3 was induced; however, in the presence of phorbol myristate acetate, strain augmented MMP-1 expression by 5.1 ± 0.7-fold (P < 0.05) and MMP-3 expression by 1.6 ± 0.1-fold (P < 0.05). In contrast, MMP-9 expression was not changed by mechanical strain in the presence or absence of phorbol myristate acetate. Deformation rapidly induced the immediate early response genes c-fos and c-jun. In addition, mechanical deformation induced the transcription factor PU.1, an ets family member that is essential in monocyte differentiation, as well as mRNA for the M-CSF receptor. These studies demonstrate that human monocytes/macrophages respond to mechanical deformation with selective augmentation of MMPs, induction of immediate early genes, and induction of the M-CSF receptor. In addition to enhancing the proteolytic activity of macrophages within repairing tissues, cellular deformation within tissues may play a role in monocyte differentiation.
Degradation and subsequent remodeling of the extracellular matrix is critical to maintaining a biomechanically functional structure; remodeling of the matrix is also important in embryological development, tumor invasion, ovulation, and many pathobiological processes such as arthritis and wound healing. 1 Monocytes/macrophages participate directly in extracellular matrix degradation. In 1980, Werb et al 2-4 demonstrated that macrophages degraded glycoprotein and elastin components of the matrix. Recent studies have demonstrated that macrophages modulate the turnover of extracellular matrix directly through secretion of matrix metalloproteinases (MMPs) and proteinase inhibitors and indirectly through secretion of cytokines. 5,6 The battery of MMPs secreted from monocytes/macrophages includes MMP-1, MMP-2, MMP-3, MMP-7, and MMP-9, as well as TIMP-1 and TIMP-2. In addition, the differentiation state of monocytes/macrophages strongly influences the MMP profile of these cells. 7
Many MMPs share similar promoter structures and are co-regulated in vitro and in vivo. 8-10 However, MMP expression may also be selective; for example, tumor necrosis factor (TNF) and interleukin (IL)-1 selectively induce MMP-9 in human macrophages. 11,12 In fibroblasts, transcription of MMP-1 is dependent on an upstream −72 AP-1 site; neighboring this is a PEA-3 site that is also implicated in control of MMP-1 gene expression. 13 Promoter deletion studies in monocytic cell lines indicate that regulation of MMP-1 requires the −72 AP-1 site but this site is not sufficient for MMP-1 expression. 14
Almost all cells are subjected to mechanical stresses in their environment. Most studies of mechanotransduction at the cellular level have focused on differentiated cells with clear mechanical roles, such as osteoblasts, vascular smooth muscle cells, and cardiac myocytes. These studies indicate that multiple transduction pathways may participate in converting mechanical signals into biochemical signals, including stretch-activated ion channels, paracrine growth factors, G proteins, mitogen-activated protein (MAP) kinases, integrins, tyrosine kinases, and phospholipid metabolism. 15-17 Rapid mechanotransduction signals such as tyrosine kinase activation are followed by other events over the ensuing minutes, including immediate-early gene induction. Transcription of c-fos, c-jun, and egr-1 is rapidly induced in many cell types. These events are followed by cell-specific responses that may include cell migration, changes in cell proliferation rate, and changes in extracellular matrix metabolism. 18-20
Once a monocyte infiltrates a tissue, it establishes extracellular matrix contacts and may be subjected to deformation through those contacts. Although the monocyte/macrophage participates in the wound response, remarkably little is known about how these cells respond to mechanical stimuli. Martin et al 21 hypothesized that changes in morphology are a common feature of macrophage activation and that stretch-activated ion channels may play a role. They identified an outwardly rectifying potassium channel that is inactive at rest but activated by adhesion of cells or stretch of the membrane. Thus, macrophages, like smooth muscle cells, 22 have stretch-activated channels which can transduce mechanical signals. Mastsumoto et al 23 studied morphology of the monocyte-like cell line U-937 and rat peritoneal macrophages and suggested that cyclic stretch inhibits the differentiation to vacuolized cells and facilitates the differentiation to spindle cells.
These studies support the hypothesis that monocytes/macrophages are indeed mechanoresponsive. However, little is known about specific gene expression changes in monocytes/macrophages subject to deformation, particularly genes relevant to extracellular matrix degradation. Therefore, we tested the hypothesis that human monocytes/macrophages respond to controlled deformation with relevant molecular responses by studying MMP regulation.
Materials and Methods
Materials
RPMI 1640 (Dulbecco’s modified Eagle’s medium) was obtained from BioWhittaker (Walkersville, MD). Human serum was from ICN Pharmaceuticals, Costa Mesa, CA. Dulbecco’s phosphate-buffered saline (PBS) solution, Hanks’ balanced salt solution, fibronectin, laminin, trypsin, phorbol 12-myristate 13-acetate (PMA), recombinant human IL-1α, TNF-α, and other materials required for tissue culture were purchased from Life Technologies, Inc. (Gaithersburg, MD). Lipopolysaccharide (LPS), n-acetyl-l-cysteine, Tris, glycine, sodium chloride, and sodium dodecyl sulfate were obtained from Sigma Chemical Co. (St. Louis, MO). Prestained low molecular weight markers and acrylamide gel buffer were purchased from Bio-Rad. [α -32P]dCTP (3000 Ci/mmol) was purchased from Dupont-NEN (Boston, MA).
Monocyte Isolation
Monocytes were isolated from human peripheral blood mononuclear cells by the Histopaque-Ficoll gradient method. 24 White cell packs from platelet donors were obtained from Brigham and Women’s Hospital Blood Donor bank. Aliquots of 15 ml of human peripheral blood mononuclear cells were gently layered on 15 ml of Histopaque solution (Sigma Co.). RPMI 1640 (10 ml) with 1% penicillin and streptomycin sulfate was supplemented with 10% human serum and added. After centrifugation for 20 minutes at 25°C, the cell layer was harvested and mixed with fresh RPMI 1640 medium and centrifuged at 400 g for 5 minutes. Precipitated pellets were resuspended with RPMI 1640 medium containing 10% human serum and plated in a T-150 flask for 2 hours. Lymphocytes were removed after extrinsic washing three times with Hanks’ Ca2+, Mg2+ solution and adhered monocytes were maintained with RPMI- 1640 medium supplemented with 10% human serum at 37°C, 5% CO2. The isolated monocytes have undergone adhesion during isolation and we refer to these cells as monocytes/macrophages.
Mechanical Strain
Mechanical deformation was applied to a thin and transparent membrane on which cells were cultured, an approach which produces controlled cellular strain as well as visualization of cells. This device provides a nearly homogeneous biaxial strain profile; that is, strains that are equal at all locations on the membrane and in all directions. 25 An advantage of this device over some commonly used systems is that it eliminates locations on the substrate which have very high strains (20 to 30%) in one direction. In our device, at the extreme perimeter of the dish, strains in the circumferential direction are reduced. 26 However, very few cells grow at the extreme periphery of the well, as commonly found in all culture dishes. We have previously measured membrane strains with a high-resolution video device; 26 the cams used for this study gave strains of 1.0 ± 0.1%, 4.2 ± 0.1%, and 9.5 ± 0.1% (n = 18 different locations for each). For the preparation of monocytes/macrophages to be subjected to mechanical strain, autoclaved membrane dishes were coated with 2 μg/ml of fibronectin in 13 ml of Hanks’ Ca2+, Mg2+solution for 6 to 12 hours at 4°C and then washed twice with 10 ml of PBS. Monocytes/macrophages were plated on the coated membrane at a density of 1,000,000 cells/dish in 13 ml of RPMI 1640 containing 10% human serum and incubated for 4 to 5 days. For culturing monocytes/macrophages on laminin, 1 μg/ml of laminin was used. Before mechanical strain or mitogen stimulation, media was replaced with 10 ml of fresh medium. Mechanical strain was then applied at 1 Hz and control dishes were treated identically but received no mechanical strain. In some experiments, LPS (1 μg/ml) or phorbol myristate acetate (162 μmol/L) was used.
Northern Analysis and Gel-Shift Assay
Total RNA was isolated by the guanidinium isothiocyanate and phenol chloroform method. 27 The full-length 1.47-kb MMP-1 (interstitial collagenase-1), full-length 1.5-kb MMP-3 (stromelysin), and 652 bp of TIMP-1 cDNAs were used as probes (gift of Merck Research Laboratories, West Point, PA). The full-length c-jun probe was obtained from Dr. Peter Libby and the cDNA for c-fms was obtained from Dr. D. Tenen (both of Harvard Medical School). For the preparation of the c-fos probe, human vascular smooth muscle cells were stimulated with PMA (162 μmol/L) for 1 hour after 48 hours serum deprivation. Purified RNA (2 μg) was used for the synthesis of cDNA by Moloney murine leukemia virus reverse transcriptase with a reverse transcriptase-polymerase chain reaction (PCR) system (Stratagene, La Jolla, CA). Synthesis of the c-fos cDNA was performed by PCR reaction with Taq polymerase (Perkin Elmer, Foster City, CA). The primer set for the synthesis of c-fos was 5′-CTA-CGA-GGC-GTC-ATC-CTC-CCG-3′-sense and 5′-TAC-GGC-GTT-GGC-CTC-CTC-CCT-CGA-3′-antisense oligonucleotides, yielding a 431-bp cDNA. We used a 438-bp 5′-coding region probe for human PU.1 (gift of Dr. Francoise Moreau-Gachelin, Institut Curie, Paris, France) which lacks the 3′ ets binding sequence to avoid hybridization to other ets families. 28 The cDNA probe for MMP-9 (92-kd gelatinase) was synthesized by PCR with the primer set: sense 5′-GGC-GCT-CAT-GTA-CCC-TAT-GT-3′ and antisense 5′-TCA-AAG-ACC-GAG-TCC-AGC-TT-3′ to generate a 468-bp PCR product. The probes were radiolabeled by the random priming method with [α-32P]dCTP and the Klenow fragment of DNA polymerase (Stratagene). For Northern blotting, 15 μg of RNA was loaded on a 1.0% agarose-formaldehyde gel (2.0 mol/L), transferred to a nylon membrane (Amersham Life Science, Arlington Heights, IL) and UV crosslinked with a UV Stratalinker (Stratagene). The probe was hybridized with ExpressHyb solution (Clontech, Palo Alto, CA) at 68°C for 1 hour. The membrane was washed with a 2× SSC, 0.05% sodium dodecyl sulfate (SDS) solution for 30 to 40 minutes, three times at the room temperature and 0.1× SSC, 0.1% SDS solution with continuous shaking at 50°C for 40 minutes. The membrane was exposed to X-ray film at −80°C. Quantitation of western analyses was performed by scanning densitometry using the Optimas 5.2 software package (Optimas Corp., Bothell, Washington). Electrophoretic mobility shift assays were performed as previously described by Pierce et al, 14 using the identical oligonucleotide with the MMP-1 AP-1 site.
Western Analysis
Conditioned media were concentrated by Centricon 10 miniconcentrators (Millipore, Bedford, MA). Samples were loaded on a 10% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane in 25 mmol/L Tris base (pH 8.5), 0.2 mol/L glycine, 20% methanol. The nitrocellulose membrane was blocked by 5% nonfat-dried milk in Tris-buffered saline (TBS) washing buffer containing 20 mmol/L Tris base (pH 7.6), 137 mmol/L NaCl, 0.1% Tween 20 for 2 hours. For the detection of MMP-1, the membrane was incubated with 1:2000 diluted rabbit anti-human MMP-1 polyclonal antibody (gift of Merck Research Laboratories) for 1 hour at 37°C and washed with TBS washing buffer for 30 minutes. The secondary antibody, goat anti-rabbit IgG coupled to peroxidase, was diluted 1:4000 and incubated with membrane for 30 minutes. After washing with TBS washing buffer for 30 minutes, the membrane was developed with the enhanced chemiluminescent method (Amersham Life Science).
Gelatin Zymography
Conditioned media were loaded on a 10% SDS-polyacrylamide gel containing 1 mg/ml of gelatin. Electrophoresis was performed in Tris-glycine buffer for 4 hours and the gel was then incubated in 2.5% Triton X-100 solution for 15 minutes twice to remove SDS. To detect gelatinase activity, the gel was incubated in reaction buffer containing 50 mmol/L Tris-HCl (pH 7.4), 10 mmol/L CaCl2, and 0.05% Brij 35 overnight at 37°C. The gelatinolytic activity was shown by staining with 0.1% (w/v) Coomassie brilliant blue R-250, 10% (v/v) glacial acetic acid, and 30% (v/v) methanol and destained with 10% (v/v) acetic acid and 30% (v/v) methanol.
All experiments presented were performed at least twice with representative data shown. Quantitative data are presented as the mean ± SD from at least three independent experiments, and comparisons were made by a two-sided Student’s t-test.
Results
Strain Regulates MMP-1 Expression by Human Monocytes/Macrophages
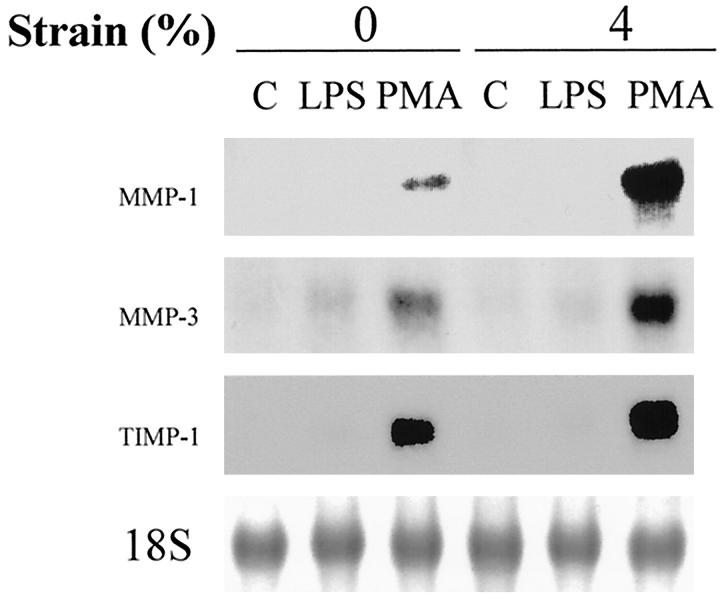
To study the effect of deformation on the regulation of MMP-1 expression, human monocytes/macrophages were subjected to 4% mechanical strain at 1 Hz for 24 hours. Total RNA was isolated and analyzed by Northern blotting (Figure 1) ▶ . Mechanical strain alone did not induce MMP-1 expression by human monocytes/macrophages. PMA induced MMP-1 expression, and strain augmented MMP-1 expression induced by PMA by (5.1 ± 0.7-fold, n = 4, P < 0.05). No apparent morphological changes were caused by strain at applied 4% amplitude at 1 Hz; we have observed evidence of monocyte cellular injury or detachment only at strains of at least 14% (data not shown). We then studied the effect of deformation on the regulation of MMP-3 expression. Co-expression of MMP-1 with MMP-3 level has been observed in vivo 10 and the promoters of MMP-1 and MMP-3 have similar structures. 8 Mechanical strain alone did not induce MMP-3 expression by human monocytes/macrophages, similar to the findings with MMP-1. PMA induced MMP-3 expression, and strain augmented MMP-3 expression induced by PMA by (1.6 ± 0.1-fold, n = 4, P < 0.05). TIMP-1 was induced by PMA, and this was minimally augmented by strain by (1.3 ± 0.1-fold, n = 4, P < 0.05).
Figure 1.
Strain regulates MMP-1 expression by human monocytes/macrophages. Monocytes/macrophages were cultured on fibronectin-coated membranes for 4 to 5 days, fresh medium was exchanged, and cells were subjected to 4% mechanical strain at 1 Hz for 24 hours. Total RNA was isolated and analyzed by Northern blotting for MMP-1, MMP-3, and TIMP-1.
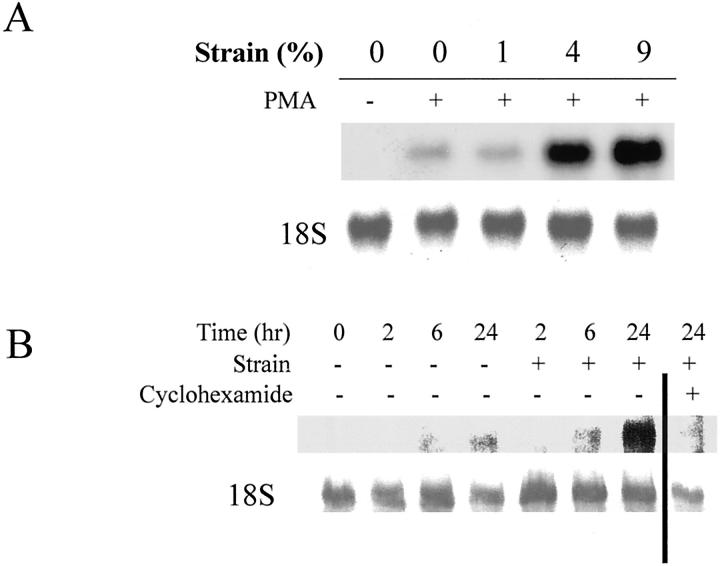
To study the amplitude dependence of this effect, cells were subjected to magnitudes of strain of 0%, 1%, 4%, or 9% at 1 Hz for 24 hours in the presence of PMA. Total mRNA was isolated and analyzed by Northern analysis (Figure 2A) ▶ . Strain of 4% or greater induced MMP-1 gene expression in monocytes/macrophages. Time course experiments showed that the enhanced MMP-1 gene expression occurred at 24 hours (and not at 6 hours or earlier), and cycloheximide (10 μmol/L) inhibited the enhanced expression of MMP-1 by strain (see Figure 4B ▶ ).
Figure 2.
A: Amplitude dependence of monocyte/macrophage augmentation of PMA-induced MMP-1. Human monocytes/macrophages cultured on fibronectin-coated membranes were subjected to 0%, 1%, 4%, and 9% strain for 24 hours. Deformation of 4% and above augmented MMP-1 expression. B: Time course of monocyte/macrophage augmentation of PMA-induced MMP-1 mRNA. All cells were treated with PMA in this experiment. Cyclohexamide blocked induction of MMP-1 mRNA.
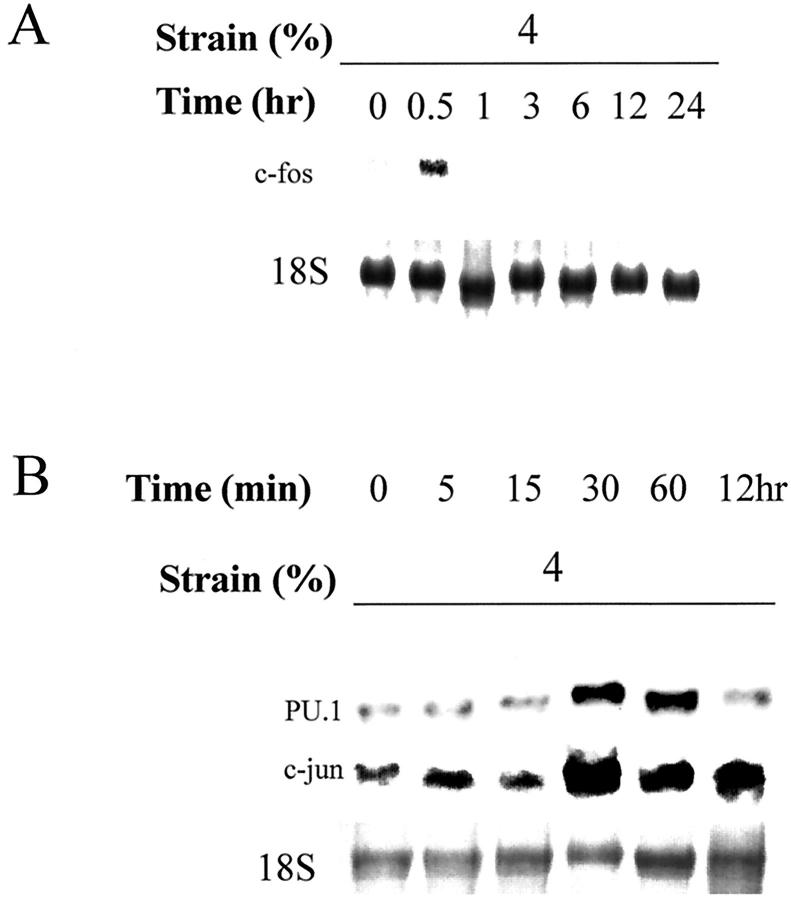
Figure 4.
Strain regulates immediate early gene expression. Human monocytes/macrophages were cultured on fibronectin (2 μg/ml)-coated membranes in RPMI-medium containing 10% human serum for 4 to 5 days. Deformation was applied with 4% strain at 1 Hz. Total RNA was isolated at each time point and analyzed with Northern blotting for c-fos (A) and PU.1 and c-jun (B).
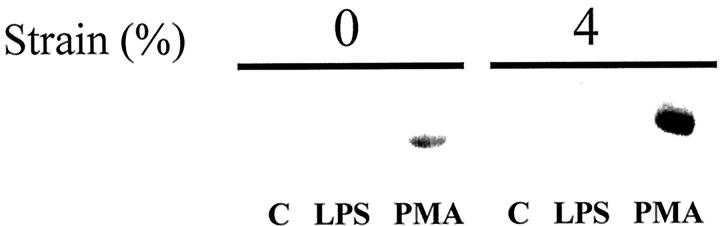
We then studied regulation of MMP-1 synthesis at the protein level by Western analysis (Figure 3) ▶ . As observed at the steady-state mRNA level, strain augmented MMP-1 synthesis in the media induced by PMA. No MMP-1 protein was detectable in lysates of the cell monolayer (data not shown). We have previously reported that strain (applied in an identical manner as used in this study) suppressed MMP-1 expression by human vascular smooth muscle cells. 29 Thus, deformation may have dramatic cell-specific effects on MMP-1 expression.
Figure 3.
Strain promotes MMP-1 expression. Human monocytes/macrophages were cultured on fibronectin-coated membranes. After culturing 4 to 5 days, medium was exchanged and cells were subjected to 4% mechanical strain at 1 Hz for 24 hours. Media were analyzed with anti-human MMP-1 polyclonal antibody. Strain augments MMP-1 secretion by monocytes/macrophages.
Effect of Strain on MMP-9
We then studied the effect of strain on MMP-9 (92-kd gelatinase) expression. Although many MMPs have similar promoter structures, expression of gelatinases may diverge from MMP-1 and MMP-3 and also can be tissue specific. 30,31 Northern analysis demonstrated that MMP-9 expression was constitutive and not influenced by PMA or LPS, even in the presence of strain (data not shown). We also analyzed MMP-9 and MMP-2 by gelatin zymography. MMP-9 (92-kd gelatinase) was the major gelatinolytic activity in monocytes/macrophages and was not changed by mechanical strain. MMP-2 (72-kd gelatinase) activity was trivial compared with MMP-9 activity and unchanged by strain (data not shown). These results demonstrated that the strain effect on MMPs in monocytes is specific for MMP-1 and MMP-3 expression.
Strain Regulates Immediate Early Gene Expression
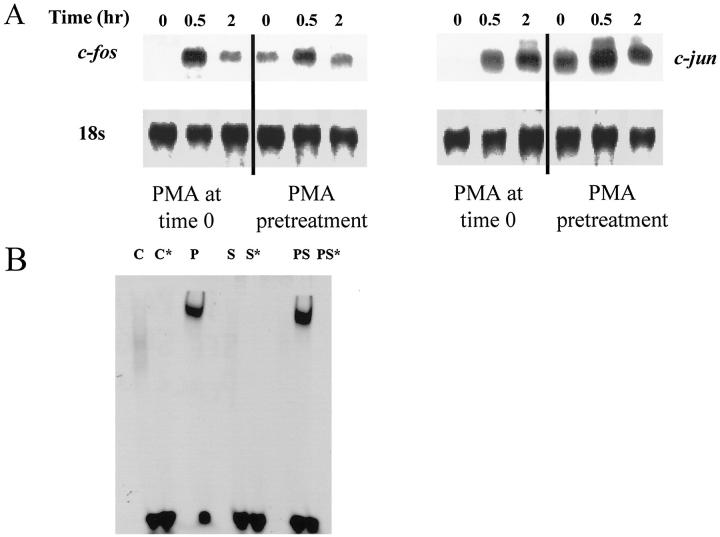
Transcriptional regulation of MMP-1 requires a −72 AP-1 site in the 5′-flanking region; this site is necessary, but not sufficient, for PMA induction of the MMP-1 gene. 13,14 It is likely that the induction of MMP-1 by strain is regulated by transcription factors that interact with these sites. We studied regulation of c-fos and ets-1 expression in monocytes/macrophages by mechanical deformation. Induction of c-fos was observed at 30 minutes after strain (Figure 4A) ▶ and was not observed in controls with simple media exchange. We have previously reported that mechanical deformation down-regulates ets-1, a transcription factor that interacts with PEA-3 sites, in vascular smooth muscle cells; we did not detect ets-1 expression in monocytes/macrophages in the presence or absence of mechanical deformation (data not shown).
We also studied regulation of PU.1, a transcriptional factor of the ets family, and c-jun by mechanical deformation, because a negative regulatory role of PU.1 interacting with c-jun on MMP-1 expression has been reported in fibroblasts. 32 In monocytes/macrophages, we observed PU.1 expression in unstrained cells, but both PU.1 and c-jun expression increased at 30 minutes after strain (Figure 4B) ▶ . Induction of these immediate early genes in monocytes by strain demonstrates mechanoresponsiveness of monocytes/macrophages; these transcriptional factors may participate in not only MMP-1 regulation but also possibly in differentiation of monocytes/macrophages.
Although mechanical induction of c-fos and c-jun was observed in the absence of PMA, MMP-1 induction was only observed in the presence of PMA. This observation suggests that induction of MMP-1 in the presence of PMA is not simply due to c-fos and c-jun increases. To explore this, monocytes/macrophages were cultured in the presence of PMA and subjected to strain (Figure 5A) ▶ . When PMA was added at the same time as strain, dramatic increases of c-fos and c-jun were observed. A similar induction of jun-B was observed, although induction of fos-B was not observed (data not shown). However, when PMA was added 2 hours before initiation of strain, c-fos and c-jun were noted to be increased in the absence of strain, such that further induction by strain was barely apparent. Because MMP-1 expression was measured over 24 hours, it is therefore possible that induction of c-fos and c-jun by strain is unrelated to induction of MMP-1 by strain in the presence of PMA, as PMA itself induces these factors. We also performed electrophoretic mobility shift assays using an oligonucleotide that contained the MMP-1 −72 AP-1 site. 14 Although PMA induced binding of nuclear extract to the AP-1 site, strain alone did not induce this activity. Strain only modestly induced further binding, suggesting that enhanced activation of the −72 AP-1 site alone does not explain the augmentation of MMP-1 synthesis by strain (Figure 5B) ▶ .
Figure 5.
A: Immediate early gene expression in the presence of PMA. Human monocytes/macrophages were cultured on fibronectin (2 μg/ml)-coated membranes in RPMI medium containing 10% human serum for 4 to 5 days. Deformation was applied with 4% strain at 1 Hz; PMA was added at the time strain was initiated or 2 hours before strain. Total RNA was isolated at each time point and analyzed with Northern blotting for c-fos (left) and c-jun (right). B: Electrophoretic mobility shift assay using oligonucleotides that contain the human MMP-1 AP-1 site; 14 conditions were C (control), P (PMA), S (strain), and PS (strain + PMA). A cold oligonucleotide excess of 5× was used to compete for labeled oligonucleotide in conditions designated by *, and supershift assays with antibodies to AP-1 confirmed specificity of the assay (not shown). Strain itself did not activate the AP-1 site, although it modestly augmented the effects of PMA.
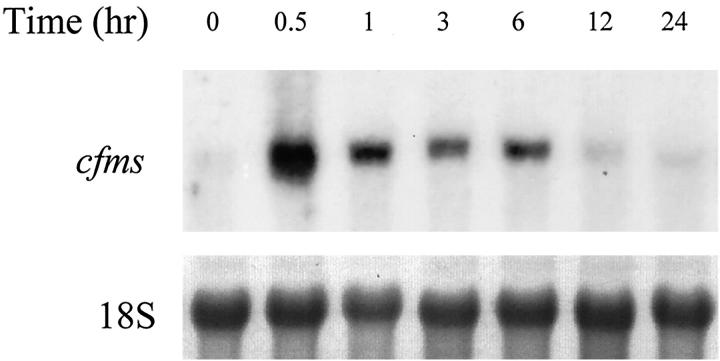
The increases in PU.1 in the presence of strain raised the hypothesis that the receptor for M-CSF (cfms) is induced by strain. In the absence of PMA, strain rapidly induced mRNA for cfms (>10-fold, n = 3, Figure 6 ▶ ). This induction was rapid (within 30 minutes), occurring at the same time as induction of PU.1.
Figure 6.
Strain regulates c-fms expression by human monocytes/macrophages. Monocytes/macrophages were cultured on fibronectin-coated membranes for 4 to 5 days, fresh medium was exchanged, and cells were subjected to 4% mechanical strain at 1 Hz. Total RNA was isolated and analyzed by Northern blotting for c-fms.
Cytokines and Antioxidant
To study potential factors mediating MMP-1 induction by strain, we measured the concentration of IL-1β released in conditioned medium by enzyme-linked immunosorbent assay assay. Strain at 4% did not induce IL-1β release; we have detected IL-1β release by deformed monocytes/macrophages, but only at strains of at least 14% (data not shown). We also studied MMP-1 regulation in direct response to exogenous recombinant human IL-1α and TNF-α (both 10 ng/ml added at the initiation of strain), because TNF-α is induced by PMA. 33 Northern analysis showed that neither IL-1α nor TNF-α at high concentrations (10 ng/ml) induced MMP-1 expression in the presence or absence of strain, whereas parallel experiments with the same cytokines demonstrated induction of MMP-1 in cultured human aortic smooth muscle cells (data not shown). Thus, these findings indicate that the effect of strain on the regulation of MMP-1 is not mediated by paracrine IL-1 or TNF-α release.
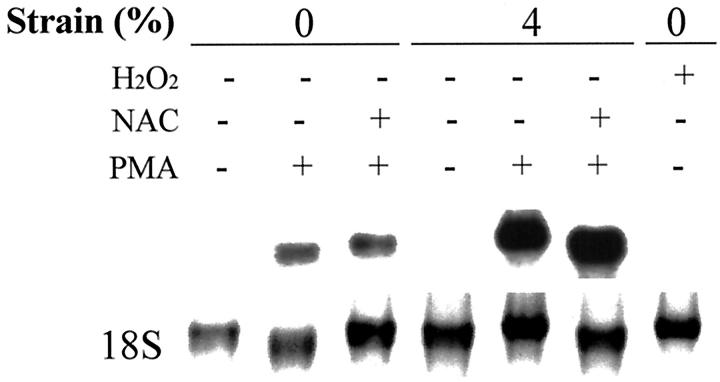
Several recent studies indicate that reactive oxygen species may participate in mechanotransduction. 34-36 In addition, Galis et al 37 showed that LPS and PMA induced MMP-9 expression via a reactive oxygen species mechanism. To test the hypothesis that MMP-1 induction by mechanical strain is mediated by reactive oxygen species, human monocytes/macrophages were pretreated with 10 mmol/L N-acetyl cysteine for 30 minutes. Northern analysis indicated that the effect of strain on the regulation of MMP-1 is not mediated by reactive oxygen species (Figure 7) ▶ .
Figure 7.
Effect of reactive oxygen species on MMP-1 regulation. Human monocytes/macrophages were cultured on fibronectin-coated membranes for 4 to 5 days. Before applying strain, cells were pretreated with 10 mmol/L of N-acetyl cysteine N-acetyl-l-cysteine for 30 minutes or 100 μmol of hydrogen peroxide (H2O2) and subjected to 4% mechanical strain at 1 Hz for 24 hours. Total RNA was isolated and analyzed with Northern blotting. N-acetyl-l-cysteine did not inhibit strain-augmentation of MMP-1.
Effect of Extracellular Matrix
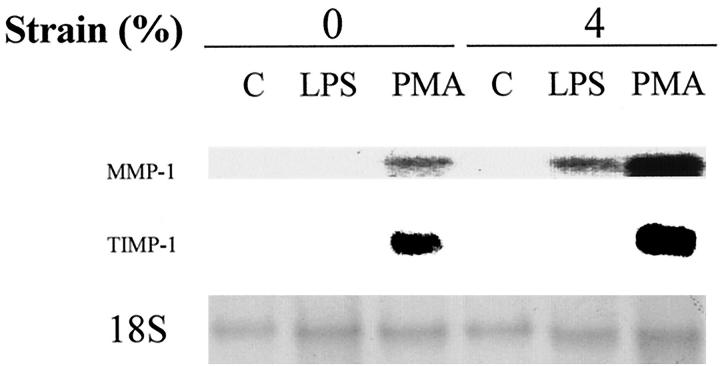
The extracellular matrix can strongly influence both mechanoresponsiveness and MMP-1 regulation. 38,39 We studied mechanoresponsiveness of MMP-1 regulation by monocytes/macrophages cultured on laminin (1 μg/ml) compared to fibronectin (we have been unable to attach monocytes/macrophages to the membrane by type I collagen). We observed the same efficiency of cellular adherence of human monocytes/macrophages to laminin compared to fibronectin, and morphological differences were not observed. After deformation, total mRNA was isolated and analyzed by Northern blotting (Figure 8) ▶ . Similar to monocytes/macrophages on fibronectin, monocytes/macrophages deformed on laminin augmented MMP-1 expression with negligible changes in TIMP-1 expression. However, on laminin, LPS alone did not induce MMP-1, but strain in the presence of LPS induced MMP-1 expression. This result shows that MMP-1 regulation by mechanical deformation is matrix dependent.
Figure 8.
Effect of strain on MMP-1 regulation cultured on laminin. Human monocytes/macrophages were cultured on laminin (1 μg/ml)-coated membranes. After 4 to 5 days, medium was exchanged with fresh medium and cells were subjected to 4% mechanical strain at 1 Hz for 24 hours. Total RNA was isolated and analyzed by Northern blotting with a cDNA probe for human MMP-1.
Discussion
Once a monocyte/macrophage has established residence in a tissue, it may be subjected to mechanical stimuli. A central goal of this study was to establish that the monocyte/macrophage is a mechanically sensitive cell. This study demonstrates that the monocyte/macrophage is highly sensitive to small biomechanical stimuli and rapidly induces immediate-early gene expression. Some—but not all—of the effects of strain were seen only in the presence of PMA; PMA may act through protein kinase C to promote macrophage differentiation. 40 Furthermore, the deformation in our experiments was imposed several days after adhesion of cells to a substrate. Therefore, this study suggests that deformation could provide a differentiation signal that is independent of other signals such as adhesion or protein kinase C activation, and that these signals may act in a stepwise manner or interactively to regulate phenotype.
Several lines of evidence indicate that mechanical events modulate MMP expression. Werb and colleagues 41,42 showed that expression of interstitial collagenase (MMP-1) and stromelysin (MMP-3) by rabbit synovial fibroblasts depended on alteration of cellular morphology through the cytoskeleton. In addition, MMP-1 can be induced in a monolayer culture of vascular smooth muscle cells by mechanical injury of the monolayer. 43 We recently reported that highly controlled deformation of vascular smooth muscle cells can, in fact, suppress MMP-1 expression. 29
We hypothesized that mechanical strain may regulate expression of MMPs by monocytes/macrophages. In this study, we found that mechanical strain induced MMP-1, MMP-3, and TIMP-1 expression in the presence of PMA, whereas MMP-9 expression was not changed. These data show that the effect of deformation on the regulation of MMPs is selective. Cytokines secreted by macrophages are known to play an important role in expression of MMPs. 44,45 In our study, exogenous TNF-α and IL-1α did not induce MMPs in the same manner that strain and PMA did, suggesting that these cytokines were not responsible. However, we cannot exclude the possibility that other cytokines are involved. In addition, PMA can cause changes in cell shape and spreading; in this study, after cells were cultured and spread for 4 to 5 days, we noted no changes in cell morphology after treatment with PMA.
Induction of c-fos and c-jun could potentially activate AP-1 sites and promote MMP-1 expression. In contrast, PU.1 may function as a negative regulator of MMP-1 expression. In this study, both PU.1 and MMP-1 were induced by deformation, suggesting that PU.1 was not functioning as a suppressor of MMP-1 expression. PU.1 is an essential gene for monocyte differentiation. 46-48 Interaction between PU.1 and c-jun in the activation of the promoter of macrosialin, a murine transmembrane protein associated with macrophage differentiation, has been reported. 49 We have also shown the induction of the gene for the MCSF receptor. This raises the intriguing possibility that mechanical deformation may promote monocyte to macrophage differentiation.
Contact with specific extracellular matrix components potently regulates mechanotransduction. 38 To test the hypothesis that MMP-1 regulation by mechanical deformation depends on matrix composition, we cultured monocytes/macrophages on laminin. We found that mechanical strain induced MMP-1 expression treated with PMA in the same manner as fibronectin. Interestingly, we also observed induction of MMP-1 by mechanical strain in cells treated with LPS on laminin, an effect not seen on fibronectin. It is possible that interactions with specific integrin subunits matrix may explain the differences between fibronectin and laminin in LPS-treated monocytes/macrophages. Because membranes coated with poly-l-lysine did not support adhesion of the cells, we were unable to determine whether adhesion without integrins changed the mechanostimulation effect.
It is important to note that all cell monolayer deformation devices will have shear forces caused by movement of fluid over the membrane, and we cannot exclude an effect of shear stress. In fact, in our device shear stresses are not uniform because the device has been designed to provide uniform strains rather than uniform shear stress. Preliminary results using computational fluid mechanic methods indicate that these shear stresses are extremely low and dependent on depth of media and frequency (Thomas Brown, personal communication). Furthermore, increases in expression of MMP-1 with strain were paralleled by increases in TIMP-1. In repeated experiments we found using a fluorescent MMP-1 substrate that the net activity of MMP-1 remained zero in the culture supernatants, possibly due to concomitant expression of TIMP-1, expression of other TIMPs, or inadequate activation of pro-MMP-1 (data not shown). Thus, our in vitro observations should not be extrapolated directly to the assumption that strain of a tissue with macrophages will increase collagen degradation.
Mechanical activation of monocytes/macrophages could have implications for wound repair. One particular circumstance where this effect could participate in human pathophysiology is the atherosclerotic lesion. In unstable atherosclerotic lesions, regions of high mechanical stress are frequently infiltrated with monocytes/macrophages overexpressing MMP-1. Degradation of collagen fibrils in this location may be an important factor in destabilizing the lesion. Our data suggest that mechanical factors may participate in promoting MMP synthesis in these locations.
Footnotes
Address reprint requests to Richard T. Lee, M.D., Cardiovascular Division, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115. E-mail: rtlee@bics.bwh.harvard.edu.
Supported in part by a Grant-in-Aid from the American Heart Association, and National Heart Lung and Blood Institute grants HL-54759 and HL-61794.
References
- 1.DiPietro LA: Wound healing: the role of the macrophage and other immune cells. Shock 1995, 4:233-240 [PubMed] [Google Scholar]
- 2.Werb Z, Banda MJ, Jones PA: Degradation of connective tissue matrices by macrophages. I. Proteolysis of elastin, glycoproteins, and collagen by proteinases isolated from macrophages. J Exp Med 1980, 152:1340-1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones PA, Werb Z: Degradation of connective tissue matrices by macrophages. II. Influence of matrix composition on proteolysis of glycoproteins, elastin, and collagen by macrophages in culture. J Exp Med 1980, 152:1527-1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werb Z, Bainton DF, Jones PA: Degradation of connective tissue matrices by macrophages. III. Morphological and biochemical studies on extracellular, pericellular, and intracellular events in matrix proteolysis by macrophages in culture. J Exp Med 1980, 152:1537-1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell EJ, Cury JD, Lazarus CJ, Welgus HG: Monocyte procollagenase and tissue inhibitor of metalloproteinases. Identification, characterization, and regulation of secretion. J Biol Chem 1987, 262:15862-15868 [PubMed] [Google Scholar]
- 6.Lee E, Grodzinsky AJ, Libby P, Clinton SK, Lark MW, Lee RT: Human vascular smooth muscle cell-monocyte interactions and metalloproteinase secretion in culture. Arterioscler Thromb Vasc Biol 1995, 15:2284-2289 [DOI] [PubMed] [Google Scholar]
- 7.Campbell EJ, Cury JD, Shapiro SD, Goldberg GI: Neutral proteinases of human mononuclear phagocytes. Cellular differentiation markedly alters cell phenotype for serine proteinases, metalloproteinases, and tissue inhibitor of metalloproteinases. J Immunol 1991, 146:1286-1293 [PubMed] [Google Scholar]
- 8.Crawford H, Matrisian LM: Mechanisms controlling the transcription of matrix metalloproteinase genes in normal and neoplastic cells. Enzyme Protein 1996, 49:20-37 [DOI] [PubMed] [Google Scholar]
- 9.Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH: Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol 1989, 109:877-889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galis ZS, Sukhova GK, Kranzhöfer R, Clark S, Libby P: Macrophage foam cells from experimental atheroma constitutively produce matrix-degrading proteinases. Proc Natl Acad Sci USA 1995, 92:402-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarén P, Welgus HG, Kovanen PT: TNF-alpha and IL-1beta selectively induce expression of 92-kDa gelatinase by human macrophages. J Immunol 1996, 157:4159-4165 [PubMed] [Google Scholar]
- 12.Saarialho-Kere UK, Welgus HG, Parks WC: Distinct mechanisms regulate interstitial collagenase and 92-kDa gelatinase expression in human monocytic-like cells exposed to bacterial endotoxin. J Biol Chem 1993, 268:17354-17361 [PubMed] [Google Scholar]
- 13.Tremble P, Damsky CH, Werb Z: Components of the nuclear signaling cascade that regulate collagenase gene expression in response to integrin-derived signals. J Cell Biol 1995, 129:1707-1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierce RA, Sandefur S, Doyle GAR, Welgus HG: Monocytic cell type-specific transcriptional induction of collagenase. J Clin Invest 1996, 97:1890-1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banes AJ, Tauzaki M, Yamamoto J, Fischer T, Brigman B, Brown T, Miller L: Mechanoreception at the cellular level: the detection, interpretation, and diversity of responses to mechanical signals. Biochem Cell Biol 1995, 73:349-365 [DOI] [PubMed] [Google Scholar]
- 16.Davies PF, Barbee KA, Volin MV, Robotewskyj A, Chen J, Joseph L, Griem ML, Wernick MN, Jacobs E, Polacek DC, DePaola N, Barakat AI: Spatial relationships in early signaling events of flow-mediated endothelial mechanotransduction. Annu Rev Physiol 1997, 59:527-749 [DOI] [PubMed] [Google Scholar]
- 17.Takahashi M, Ishida I, Traub O, Corson MA, Berk BC: Mechanotransduction in endothelial cells: temporal signaling events in response to shear stress. J Vasc Res 1997, 34:212-219 [DOI] [PubMed] [Google Scholar]
- 18.Sadoshima J, Izumo S: The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol 1997, 59:551-571 [DOI] [PubMed] [Google Scholar]
- 19.Sumpio BE, Banes AJ, Link GW, Johnson G: Enhanced collagen production by smooth muscle cells during repetitive mechanical stretching. Arch Surg 1988, 123:1213-1266 [DOI] [PubMed] [Google Scholar]
- 20.Vadiakas G, Banes AJ: Verapamil decreases cyclic load-induced calcium incorporation in ROS 17/2.8 osteosarcoma cell cultures. Matrix 1992, 12:439-447 [DOI] [PubMed] [Google Scholar]
- 21.Martin DK, Bootcov MR, Campbell TJ, French PW, Breit SN: Human macrophages contain a stretch-sensitive potassium channel that is activated by adherence and cytokines. J Membrane Biol 1995, 147:305-315 [DOI] [PubMed] [Google Scholar]
- 22.Dopico AM, Kirber MT, Singer JJ, Walsh JV, Amer J: Membrane stretch directly activates large conductance Ca(2+)-activated K+ channels in mesenteric artery smooth muscle cells. Hypertension 1994, 7:82-89 [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto T, Delafontaine P, Schnetzer KJ, Tong BC, Nerem RM: Effect of uniaxial, cyclic stretch on the morphology of monocytes/macrophages in culture. J Biomech Engin 1996, 118:420-422 [DOI] [PubMed] [Google Scholar]
- 24.Boyum A: Isolation of mononuclear cells and granulocytes from human blood: isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scan J Clin Lab Invest 1968, 21:77-89 [PubMed] [Google Scholar]
- 25.Schaffer JL, Rizen M, Italien GJ, Benbrahim A, Megerman J, Gerstenfeld LC, Gray ML: Device for the application of a dynamic biaxially uniform and isotropic strain to a flexible cell culture membrane. J Orthop Res 1994, 12:709-719 [DOI] [PubMed] [Google Scholar]
- 26.Cheng GC, Briggs WH, Gerson DS, Libby P, Grodzinsky AJ, Gray ML, Lee RT: Mechanical strain tightly controls fibroblast growth factor-2 release from cultured human vascular smooth muscle cells. Circ Res 1997, 80:28-36 [DOI] [PubMed] [Google Scholar]
- 27.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 28.Ray D, Culine S, Tavitain A, Moreau-Gachelin F: The human homologue of the putative proto-oncogene Spi-1: characterization and expression in tumors. Oncogene 1990, 5:663-668 [PubMed] [Google Scholar]
- 29.Yang J-H, Briggs WH, Libby P, Lee RT: Small mechanical strains selectively suppress matrix metalloproteinase-1 expression by human vascular smooth muscle cells. J Biol Chem 1998, 273:6550-6555 [DOI] [PubMed] [Google Scholar]
- 30.Lijnen HR, Silence J, Van Hoef B, Collen D: Stromelysin-1 (MMP-3)-independent gelatinase expression and activation in mice. Blood 1998, 91:2045-2053 [PubMed] [Google Scholar]
- 31.Matrisian LM: The matrix-degrading metalloproteinases. Bioessays 1992, 14:455-463 [DOI] [PubMed] [Google Scholar]
- 32.Westermarck J, Seth A, Kähäri VM: Differential regulation of interstitial collagenase (MMP-1) gene expression by ETS transcription factors. Oncogene 1997, 14:2651-2660 [DOI] [PubMed] [Google Scholar]
- 33.Xie B, Laouar A, Huberman F: Autocrine regulation of macrophage differentiation and 92-kDa gelatinase production by tumor necrosis factor-alpha via alpha5 beta1 integrin in HL-60 cells. J Biol Chem 1998, 272:11583-11588 [DOI] [PubMed] [Google Scholar]
- 34.Cheng JJ, Chao YJ, Wung BS, Wang DL: Cyclic strain-induced plasminogen activator inhibitor-1 (PAI-1) release from endothelial cells involves reactive oxygen species. Biochem Biophys Res Commun 1996, 225:100-105 [DOI] [PubMed] [Google Scholar]
- 35.Wung BS, Cheng JJ, Hsieh HJ, Shyy YJ, Wang DL: Cyclic strain-induced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein 1. Circ Res 1997, 81:1-7 [DOI] [PubMed] [Google Scholar]
- 36.Chin JJ, Wung BS, Jhon YJ, Shyy YJ, Hsieh HJ, Wang DL: Reactive oxygen species are involved in shear stress-induced intercellular adhesion molecule-1 expression in endothelial cells. Arterioscler Thromb Vasc Biol 1997, 17:3570-3577 [DOI] [PubMed] [Google Scholar]
- 37.Galis ZS, Asanuma C, Godin D, Meng X: N-acetyl-cysteine decreases the matrix-degrading capacity of macrophage-derived foam cells: new target for antioxidant therapy? Circulation 1998, 97:2445-2453 [DOI] [PubMed] [Google Scholar]
- 38.Wilson E, Sudhir K, Ives HE: Mechanical strain of rat vascular smooth muscle cells is sensed by specific extracellular matrix/integrin interactions. J Clin Invest 1995, 96:2364-2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tremble P, Chiquet-Ehrisman R, Werb Z: The extracellular matrix ligands fibronectin and tenascin collaborate in regulating collagenase gene expression in fibroblasts. Mol Biol Cell 1994, 5:439-453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monick MM, Carter AB, Gudmundsson G, Geist LJ, Hunnighake GW: Changes in PKC isoforms in human alveolar macrophages compared with blood monocytes. Am J Physiol 1998, 275:L389-L397 [DOI] [PubMed] [Google Scholar]
- 41.Werb Z, Hembry RM, Murphy G, Aggeler J: Commitment to expression of the metalloendopeptidases, collagenase and stromelysin: relationship of inducing events to changes in cytoskeletal architecture. J Cell Biol 1986, 102:697-702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kheradmand F, Werner E, Tremble P, Symons M, Werb Z: Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science 1998, 280:898-902 [DOI] [PubMed] [Google Scholar]
- 43.James TW, Wagner R, White LA, Zwolak RM, Brickerhoff C: Induction of collagenase and stromelysin gene expression by mechanical injury in a vascular smooth muscle-derived cell line. J Cell Physiol 1993, 157:427-437 [DOI] [PubMed] [Google Scholar]
- 44.La Fleur M, Underwood JL, Rappolee DA, Werb Z: Basement membrane and repair of injury to peripheral nerve: defining a potential role for macrophages, matrix metalloproteinases, and tissue inhibitor of metalloproteinases-1. J Exp Med 1996, 184:2311-2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Girolamo N, Lioyd A, McCluskey P, Filipic M, Wakefield D: Increased expression of matrix metalloproteinases in vivo in scleritis tissue and in vitro in cultured human scleral fibroblasts. Am J Pathol 1997, 150:653-666 [PMC free article] [PubMed] [Google Scholar]
- 46.Chen HM, Zhang P, Voso MT, Hohaus S, Gonzalez DA, Glass CK, Zhang DE, Tenen DG: Neutrophils and monocytes express high levels of PU.1 (Spi-1) but not Spi-B. Blood 1995, 85:2918-2928 [PubMed] [Google Scholar]
- 47.Henkel GW, Mckerchen SR, Yamamoto H, Anderson KL, Oshima RG, Maki RA: PU.1 but not ets-2 is essential for macrophage development from embryonic stem cells. Blood 1996, 88:2917-2926 [PubMed] [Google Scholar]
- 48.Olweus J, Thompson PA, Lund-Johansen F: Granulocytic and monocytic differentiation of CD34hi cells is associated with distinct changes in the expression of the PU.1-regulated molecules, CD64 and macrophage colony-stimulating factor receptor. Blood 1996, 88:3741-3754 [PubMed] [Google Scholar]
- 49.Li AC, Guidez FBR, Collier JG, Glass CK: The macrosialin promoter directs high levels of transcriptional activity in macrophages dependent on combinatorial interactions between PU.1 and c-Jun. J Biol Chem 1998, 273:5389-5399 [DOI] [PubMed] [Google Scholar]