Abstract
Objective:
Appendicitis has been declining in frequency for several decades. During the past 10 years, its preoperative diagnosis has been made more reliable by improved computed tomography (CT) imaging. Thresholds for surgical exploration have been lowered by the increased availability of laparoscopic exploration. These innovations should influence the number of appendectomies performed in the United States. We analyzed nationwide hospital discharge data to study the secular trends in appendicitis and appendectomy rates.
Methods:
All appendicitis and appendiceal operations reported to the National Hospital Discharge Survey (NHDS) 1970–2004 were classified as perforated, nonperforated, negative, and incidental appendectomies and analyzed over time and by various demographic measures. Secular trends in the population-based incidence rates of nonperforated and perforated appendicitis and negative and incidental appendectomy were examined.
Results:
Nonperforated appendicitis rates decreased between 1970 and 1995 but increased thereafter. The 25-year decreasing trend was accounted for almost entirely by a decreasing incidence in the 10–19 year age group. The rise after 1995 occurred in all age groups above 5 years and paralleled increasing rates of CT imaging and laparoscopic surgery on the appendix. Since 1995 the negative appendectomy rate has been falling, especially in women, and incidental appendectomies, frequent in prior decades, have been rarely performed. Despite these large changes, the rate of perforated appendicitis has increased steadily over the same period. Although perforated and nonperforated appendicitis rates were correlated in men, they were not significantly correlated in women nor were there significant negative correlations between perforated and negative appendectomy rates.
Conclusion:
The 25-year decline in nonperforated appendicitis and the recent increase in appendectomies coincident with more frequent use of CT imaging and laparoscopic appendectomies did not result in expected decreases in perforation rates. Similarly, time series analysis did not find a significant negative relationship between negative appendectomy and perforation rates. This disconnection of trends suggests that perforated and nonperforated appendicitis may have different pathophysiologies and that nonoperative management with antibiotic therapy may be appropriate for some initially nonperforated cases. Further efforts should be directed at identifying preoperative characteristics associated with nonperforating appendicitis that may eventually allow surgeons to defer operation for those cases of nonperforating appendicitis that have a low perforation risk.
Appendicitis has been thought to be decreasing in incidence. We have found the declining trend reversed itself in 1995 and has been steadily increasing since. Perforating and nonperforating appendicitis follow different patterns suggesting that in some cases these diseases are independent of each other.
The fear of appendicitis complications results in more emergency general surgical operations than any other disease. Approximately 280,000 appendectomies are performed each year in the United States.1 Most are performed as emergencies to avoid the complications of perforated appendicitis; an entity believed to result from delay in surgical removal of the appendix after the appendix has become inflamed. Because of the devastating impact of perforated appendicitis, a certain rate of negative explorations for suspected appendicitis is accepted as good surgical practice. This is especially true for women in whom establishing the diagnosis may be difficult and the possibility of infertility resulting from perforated appendicitis is very real. Negative exploration rates as high as 30% are considered acceptable for women presenting with lower abdominal pain.2
Emergency appendectomy was originally advocated because of the very high mortality of perforated appendicitis and the assumption that acute appendicitis evolved to perforated disease, a pathophysiologic hypothesis that has never been proven. This notion was first proposed by Reginald Fitz, the originator of the term appendicitis, in 1886.3 Fitz was the first to identify inflammation of the appendix as a cause for right lower quadrant infections, previously known as thyphilitis. In making the argument that the appendix causes this entity, however, Fitz incidentally noted that one-third of patients undergoing autopsy in the preappendectomy era had evidence of prior appendiceal inflammation, suggesting that appendicitis often resolved spontaneously without surgery. Later evidence from submariners who developed appendicitis while at sea and received delayed surgical therapy has shown that in most cases the disease can resolve with nonoperative antibiotic and supportive therapy.4–6
Given that appendicitis does not necessarily progress to perforating disease and that it may resolve spontaneously, in prior eras of more limited diagnostic technology many patients with appendicitis but ambiguous clinical signs would not have had surgery. The morbidity of negative explorations necessitated reasonably firm diagnostic certainty to initiate surgery.7–10 For the past decade, however, computed tomography (CT) imaging has been able to establish the diagnosis of appendicitis with 100% sensitivity and 95% specificity,11,12 and laparoscopic appendectomy providing low morbidity for negative explorations has lowered the threshold for operating laparoscopically on patients with abdominal pain of uncertain etiology.13–15
How these changes have impacted appendectomy rates remains unknown. Secular trend analyses published to date16–22 have not included the years in which changes in CT imaging and laparoscopic appendectomy practice patterns would have affected appendectomy rates. We therefore undertook an epidemiologic analysis of a national database to extend analyses of longitudinal trends into the recent era of CT diagnosis and laparoscopic surgery of the appendix and to determine whether improved diagnostic tools and lower risk operative management have reduced the perforation rate.
METHODS
The yearly NHDS databases for the years 1970–2004 were acquired from the Centers for Disease Control (CDC) (http://www.cdc.gov/nchs/about/major/hdasd/nhds.htm) and the Inter-University Consortium for Political and Social Research web sites (http://www.icpsr.umich.edu/index-medium.html). The databases for 1971 and 1972 could not be obtained. The NHDS is the principal database used by the U.S. Government for monitoring hospital utilization. Each year approximately 300,000 hospital discharges are selected for the NHDS from the 35,000,000 total discharges nationally. The NHDS uses a complex, multistage design to ensure that the database is representative of the U.S. hospitalized population. Using U.S. Census information, the CDC provides statistical weighting factors for each patient entry in the NHDS database so that incidence and prevalence estimates of hospitalized disease can be made for the entire U.S. population. These weighting factors were used to determine the national prevalence of appendicitis. The estimated U.S. population for each year of the study was obtained from the U.S. Census Bureau as accessed through the CDC web site (http://wonder.cdc.gov/population.html).
From the main NHDS database, all records including a code for appendectomy or appendicitis (ICD-9 diagnosis codes ranging from 54.00 to 54.39 or procedure codes ranging from 470.00 to 479.99) were extracted into a secondary database for analysis. Appendicitis was defined as a patient having any of the following seven NHDS discharge diagnostic codes: 540.9 (acute appendicitis), 541.0 (appendicitis-unqualified), 542.0 (other appendicitis), 543.0 (other diseases of the appendix), 543.0 (unspecified disease of the appendix). Perforated appendicitis (540.0) or appendiceal abscess (540.1) were aggregated into a single category called “perforated appendicitis”. Nonperforated appendicitis was defined as having any appendicitis diagnostic code except for 540.0 or 540.1. Procedures codes used were: appendectomy (47.0), laparoscopic appendectomy (47.01), other appendectomy (47.09), incidental appendectomy (47.1), laparoscopic incidental appendectomy (47.11), other incidental appendectomy (47.19), drainage of an appendiceal abscess (47.2), “other” appendiceal operation (47.9), appendicostomy (47.91), closure of an appendiceal fistula (47.92), and other appendiceal operation (47.99). Incidental appendectomies were considered to have been performed if there were any of the following 3 procedure codes: 47.1, 47.11, or 47.19. Nonincidental appendectomies were defined as having any appendectomy procedure codes excluding those for incidental procedures. A negative appendectomy was defined as having any procedure code: 47.0, 47.01, or 47.09 without any diagnostic coding for appendicitis. Computed tomography (CT) scans were assumed to be performed if procedure codes 88.01 or 88.02 were present. The first diagnosis listed among the 7 NHDS discharge codes for those patients classified as undergoing a negative appendectomy was considered the patient’s primary diagnosis.
Between 1970 and 1978 ICD-8 codes were used for the NHDS. For these years perforated appendicitis was encoded as 540.1, nonperforated appendicitis (540.9), unspecified appendicitis (541.0), other appendicitis (542.0), and other disease of the appendix (543.0). Surgical codes were drainage of appendiceal abscess (41.0), appendectomy (41.1), appendicostomy (41.2), closure of appendiceal fistula (41.3), and other operations on the appendix (41.9). Of note, there were no ICD-8 codes for incidental appendectomy precluding analysis of these procedures prior to 1979.
To compare the reasons for negative appendectomies between different eras, we aggregated all records for the years 1979–1982 to represent the early era and 2001–2004 to represent the most recent era. The year 1979 was chosen as the start of the early era because it was the first year after the conversion from the ICD-8 to the ICD-9 hospital discharge coding systems in the NHDS database. Percentages of patients with each diagnostic category in the 2 eras were compared by χ2 or Fisher exact test as appropriate.
Time series analysis was performed by estimating the residuals of an autoregressive model for the data. Relationships between 2 time series were examined using cross-correlation analysis of the corresponding residual series.23
RESULTS
In our analyses of appendicitis rates, the nonperforated appendicitis rate for both men and women fell from the early 1970s to a nadir in 1995, after which it began rising (Fig. 1). During its 25-year progressive fall, the nonperforated appendicitis rate had a highly oscillatory behavior with unusually large peaks (possible outbreaks), mostly in the male population, occurring in 1977, 1981, 1984, 1987, 1994, and 1998. The U- or J-shaped (hockey stick) secular trend was similar for both men and women.
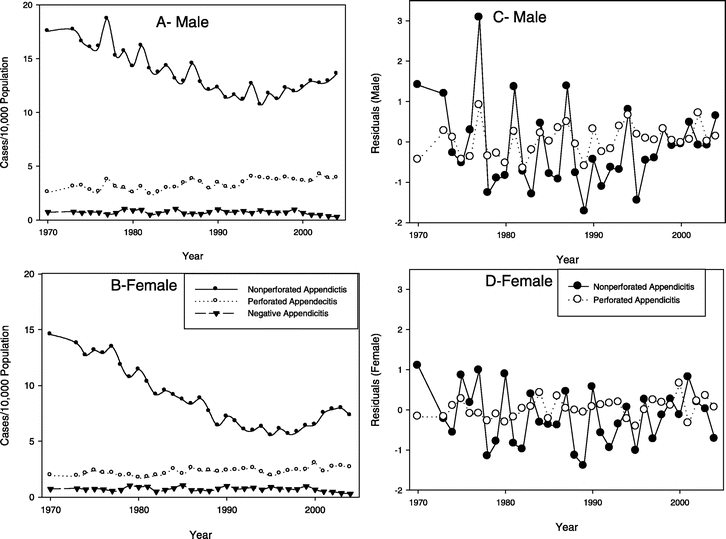
FIGURE 1. Population adjusted secular trends for perforated and nonperforated appendicitis and negative appendectomy rates in males (A) and females (B). Nonperforated appendicitis was determined from diagnostic codes for the disease entity appendicitis. Time series analysis found a significant year-to-year correlation between residuals of nonperforated and perforated appendicitis rates in males (r = 0.55, P = 0.001) (C) but not in females (r = 0.21, P = 0.25) (D). The year-to-year changes in the residuals of the nonperforated appendicitis rates of males and females were correlated (r = 0.49, P = 0.004), as were those of negative appendectomy rates in males and females (r = 0.45, P = 0.009; data not shown).
In contrast, in both men and women the rates of perforation and/or abscess formation (hereafter referred to as the perforation rate) appear to increase with time over the period of the study, apparently unassociated with the rate of nonperforated appendicitis cases (Fig. 1). The rate of negative appendectomy (number of appendectomies not associated with a concomitant diagnostic code for appendicitis) in both men and women remained low and relatively constant throughout the study period, with a slight decline possibly beginning after 2000 (Fig. 1).
Cross-correlation analysis shows that there is a significant positive cross-correlation at lag zero between perforated and nonperforated appendicitis in men (P = 0.001) but not in women. Although there was a negative correlation between negative appendectomy and perforation rates, this did not achieve statistical significance.
In our analyses of surgical trends for appendicitis (Fig. 2), the appendectomy rate (based on coding for operations) followed the same U- or J-shaped longitudinal pattern as the appendicitis rate (based on diagnostic ICD-9-CM codes). When open and laparoscopic appendectomies were distinguished, however, we found that the rate of open appendectomies continued to decline after 1995, but the rise in the appendectomy rate after 1995 was entirely accounted for by a progressive increase in the rate of laparoscopic appendectomies. The separate tracking of laparoscopic appendectomies begins in 1997 because that is the year that specific codes for this procedure were added to the ICD-9-CM system.
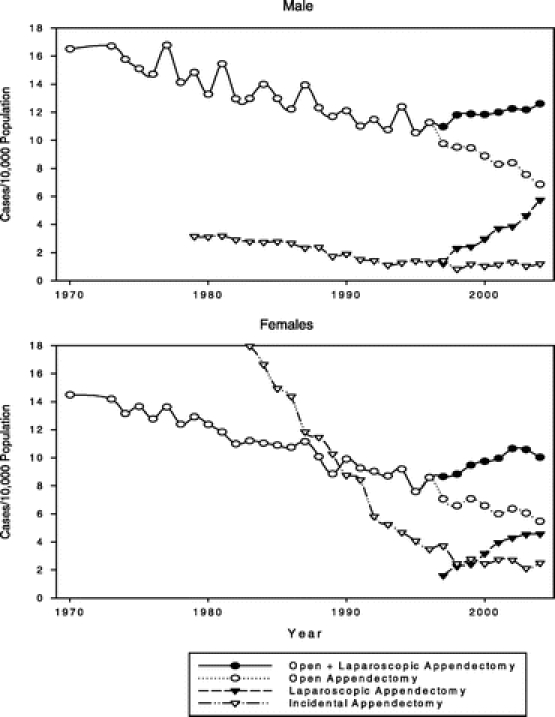
FIGURE 2. Surgical trends for appendicitis 1979–2003. ICD-9-CM coding for laparoscopic appendectomy began in 1997. Before 1997 the total appendectomy rate was also the same as the open rate.
The rate of incidental appendectomies in men, always relatively uncommon, gradually declined from 1979 (the year they were identified with a separate ICD-8 code) until 1998 after which it leveled off. In the early 1980s incidental appendectomies were far more common in women—more then 5 times the rate in men—but their rate fell precipitously through the 1980s and 1990s until 1998 after which they leveled off at a rate only twice that in men (Fig. 2).
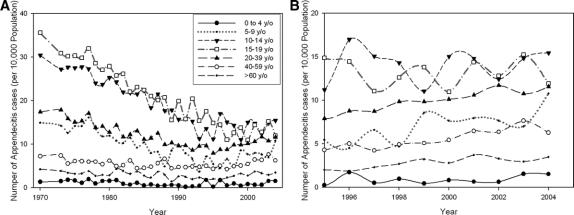
Stratification by age revealed that the progressive reduction in the appendicitis rate before 1995 occurred in all age groups but was substantially more pronounced in the 10–19 year age group, followed by those in the 5–9 year and 20–39 year age groups (Fig. 3A). In the years 1995–2004 after the end of the 25-year progressive decline, the appendicitis rates in ages 5–19 years continued to show an oscillating pattern; whereas, those in ages 0–4 years and ages older than 20 years and older showed slightly increasing trends with no more oscillations (Fig. 3B).
FIGURE 3. Rates of nonperforated appendicitis stratified by age (A) for 1970–2004 and detailed view (B) of 1995–2004.
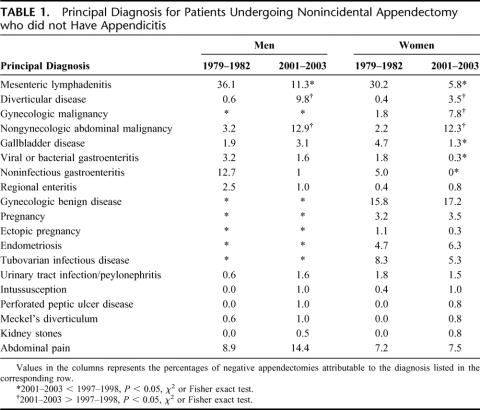
Although the rate of negative appendectomies remained relatively unchanged over the 34 years of the study, the underlying cause that precipitated the negative appendectomies changed substantially (Table 1). In 1979–1981, nearly one-third of both men and women who underwent appendectomy but were found to have no appendicitis were diagnosed with mesenteric adenitis, a diagnosis that is relatively uncommon in 2001–2004. Over the same interval the rate of malignancies and diverticular disease associated with negative appendectomy increased substantially in both men and women. The proportion of patients with abdominal pain not explained by any other operative finding did not change significantly.
TABLE 1. Principal Diagnosis for Patients Undergoing Nonincidental Appendectomy who did not Have Appendicitis
The rate of CT scan use during hospitalization of patients with appendicitis began increasing in the late 1980s and further accelerated in the late 1990s (Fig. 4). These trends did not differ significantly between men and women.
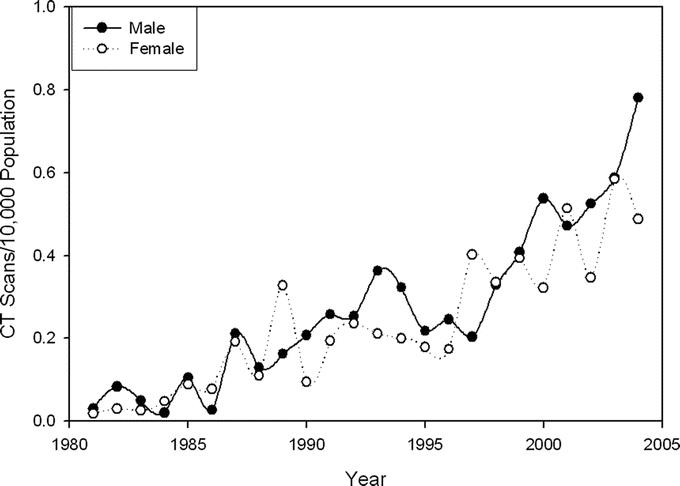
FIGURE 4. Rate of computed tomography (CT) utilization for hospitalized patients with appendicitis (number of CT scans per 10,000 population).
DISCUSSION
Perforated and nonperforated appendicitis have followed radically different epidemiologic trends over the past 2 decades. While perforated appendicitis slowly but steadily increased in incidence, nonperforated appendicitis followed a U- or J-shaped curve, its incidence progressively declining until 1995 when the trend reversed and began to increase at a rate similar to that at which it had been previously declining. If perforated appendicitis was simply the result of appendicitis that was not surgically treated early enough, the trends should have been more nearly parallel throughout all the time periods we studied. Time series analysis showed that on a year-to-year basis, there was a significant positive correlation between perforated and nonperforated appendicitis for men but not for women. These unassociated epidemiologic trends suggest that the pathophysiology of these diseases is different. If true, it might follow that many patients presenting with nonperforated appendicitis do not require surgical intervention and might experience spontaneous resolution without perforation. There is historical, clinical, and immunologic evidence to support this hypothesis.
It is generally assumed that untreated appendicitis will eventually perforate. This assumption dates back to the first description of appendicitis by Fitz in 1886. Correlating the clinical and pathologic features of what had previously been called thyphlitis, Fitz deduced that most cases of right lower quadrant inflammation were actually due to disease of the appendix and not primarily of the cecum.3 Reasoning from the fact that patients with perforations generally had a history of abdominal pain of longer duration than those without perforation, Fitz deduced that appendicitis had the potential to progress from nonperforated to perforated disease. He did acknowledge, however, that there was substantial heterogeneity in the course of appendicitis and that in autopsy series one-third of the preappendectomy era population had pathologic evidence of spontaneously resolved appendicitis.3 Although appendicitis could resolve spontaneously, perforated appendicitis was associated with a very high mortality in the preantibiotic era. Consequently, early appendectomy for suspected appendicitis was advocated at the turn of the century and, indeed, was responsible for a substantial reduction in the appendicitis-related death rate.
Do all cases of appendicitis that are not treated by emergency surgery perforate? Evidence from submarine personnel that develop appendicitis suggests not.4 Sailors with appendicitis while stationed on submarines do not have access to prompt surgical care. They are treated by corpsmen with antibiotics and fluids days to weeks following the initial attack until the ship can surface and they can be transferred to a hospital. Review of patients undergoing appendectomy following prolonged delay of surgical treatment demonstrates that perforation is unusual with the patients having pathologic evidence of resolving appendicitis.24 Our findings show that the rates of appendectomy and the diagnosis of appendicitis have risen in the recent era of increasing utilization of CT imaging and laparoscopic appendectomy. One possible explanation is that before 1995 patients with abdominal pain of unclear origin may have had appendicitis that resolved during a period of observation. Similar patients today would have the diagnosis of appendicitis established by CT imaging11,12,25–32 resulting in a higher likelihood of undergoing exploratory surgery because of the availability of low-morbidity laparoscopic procedures.
Further evidence that perforated and nonperforated appendicitis may be fundamentally different diseases is derived from study of the immune response to these disorders. Appendiceal inflammation results in a local pro-inflammatory cytokine response that is associated with a systemic anti-inflammatory cytokine profile.33 There is an IL-6 promoter single nucleotide polymorphism that is much more prevalent in patients with noncomplicated appendicitis and results in higher systemic and peritoneal IL-6 levels in patients with complicated appendicitis. This could cause increased local tissue thrombosis and greater inflammation because of IL-6’s ability to promote neutrophilic degranulation and inhibit apoptosis.34 Conceivably an exuberant, uninhibited immune response to appendicitis might result in immune-mediated tissue destruction causing perforation. This hypothesis is supported by our observation that perforation rates have been relatively flat when compared with the large swings in incidence of nonperforated disease. If perforation were caused by an inherent immune response to a similar pathogen that causes nonperforated appendicitis for most individuals, perforation rates would reflect the proportion of the population with the exuberant host response. The slow but steady increase in perforation rates that we observed might be due to a constant genetic predisposition coupled with a slowly increasing prevalence of one or more common pathogens that cause perforating appendicitis.
We also found that the perforation rate has been slowly increasing with time, while the rate of negative explorations has not changed substantially. Time series analysis of these disease entities showed no statistically significant negative correlation, ie, in times when negative appendectomy rates were high, there was no corresponding decrease in perforation rates. It is commonly thought that a negative relationship exists between negative explorations and perforation—the hypothesis being that less aggressive operative intervention, reflected by fewer negative explorations, results in delayed surgeries with higher perforation rates. Although we did not find a definite change in the negative exploration rate, the underlying causes of negative explorations has markedly changed. In the early years of the study period one-third of all negative explorations were attributable to mesenteric adenitis. This diagnosis was reasonably common in the era where aggressive exploration was advocated and this disease entity was commonly found when the appendix was normal. With better diagnostic technology, this diagnosis has become rare and the majority of negative appendectomies are now found in patients that undergo surgical exploration for abdominal pain thought due to appendicitis but at operation are unexpectedly found to have malignancies or diverticulitis. The radical shift in diagnoses associated with negative exploration for appendicitis most likely resulted from the advances in imaging technology with better preoperative diagnoses of surgical disease entities.
The 25-year declining trend in appendicitis and appendectomy rates has been reported from several previous epidemiologic studies.16–22 These studies have consistently found similar rates of decline, more rapid decline in adolescents and young adults where rates are highest, and the observation that perforation rates have remained relatively flat in the face of declining rates of nonperforated appendicitis. Our study extends prior findings into the recent period where CT imaging and laparoscopic surgery have been more widely applied in patients with abdominal pain.35 By documenting the sudden reversal of the long term decreasing trend in the rate of nonperforating appendicitis due to these changes in diagnostic and operative technology and still no consistent impact on the rate of perforating appendicitis more firmly establishes the lack of connection between nonperforated and perforated appendicitis.
Aside from demonstrating the changing epidemiological trends for appendicitis and appendectomy, our findings suggest that appendicitis is a more complex and heterogeneous disease than previously thought. Despite more than 100 years of study, our understanding of the underlying cause for the most common disease requiring emergency abdominal surgery remains incomplete. Given that secular trends for nonperforating and perforating appendicitis radically differ, it is unlikely that perforated appendicitis is simply the progression of appendicitis resulting from delayed treatment. Because there is biologic evidence to support the hypothesis that severe appendicitis might result because of an exuberant immune response, we suspect that most patients develop perforated disease before seeking health care for abdominal pain as a consequence of their genetic makeup possibly in conjunction with infection with one or more pathogens of slowing increasing population prevalence. In contrast, nonperforating appendicitis may result from infection with one or more different, more common pathogens that occurs in cyclic patterns, punctuated by sudden widespread epidemics.
Nonperforated and perforated appendicitis rates were correlated for men but not for women. Additionally, the rates for nonperforated appendicitis are much higher in men than women. Clearly there is a gender-specific difference in the susceptibility to the disease and the propensity to develop perforations. Many diseases can be attributable to selective expression genes residing on the X-chromosome.36 There are sex-specific syndromes such as the X-linked susceptibility to mycobacteria that result from mutations of X-chromosome genes.37 More research is needed to better understand the gender differences in appendicitis and the molecular basis for these. Better understanding of the etiology of appendicitis and the immunologic response to the disease may alter its surgical management. Many patients presenting with nonperforated appendicitis may be undergoing surgery unnecessarily and might recover with nonoperative management, including antibiotic therapy and supportive care, in much the same way patients with diverticulitis do. Further clinical investigation seems warranted to understand better this common disease and explore alternative management approaches, including biomarkers that might predict which cases of appendicitis will not perforate.
ACKNOWLEDGMENTS
The authors thank Robert Munford, MD, for his review of the manuscript.
Footnotes
Supported in part by the Hudson-Penn Endowment.
Reprints: Edward H. Livingston, MD, FACS, Divisions of Gastrointestinal and Endocrine Surgery, UT Southwestern Medical Center, 5323 Harry Hines Blvd Room E7-126, Dallas, Texas 75390-9156. E-mail: edward.livingston@utsouthwestern.edu.
REFERENCES
- 1.National Center for Health Statistics. Ambulatory and Inpatient Procedures in the United States, 1996. National Center for Health Statistics Series 13[No. 139]. 2004. [PubMed]
- 2.Larsson PG, Henriksson G, Olsson M, et al. Laparoscopy reduces unnecessary appendicectomies and improves diagnosis in fertile women. A randomized study. Surg Endosc. 2001;15:200–202. [DOI] [PubMed] [Google Scholar]
- 3.Fitz RH. Perforating inflammation of the vermiform appendix with special reference to its early diagnosis and treatment. Trans Assoc Am Physicians. 1886;1:107–144. [Google Scholar]
- 4.Campbell MR, Johnston SL III, Marshburn T, et al. Nonoperative treatment of suspected appendicitis in remote medical care environments: implications for future spaceflight medical care. J Am Coll Surg. 2004;198:822–830. [DOI] [PubMed] [Google Scholar]
- 5.Coldrey E. Five years of conservative treatment of acute appendicitis. J Int Coll Surg. 1959;32:255–261. [Google Scholar]
- 6.Rice BH. Conservative, non-surgical management of appendicitis. Mil Med. 1964;129:903–920. [PubMed] [Google Scholar]
- 7.Andersson RE. Small bowel obstruction after appendicectomy. Br J Surg. 2001;88:1387–1391. [DOI] [PubMed] [Google Scholar]
- 8.Bijnen CL, Van Den Broek WT, Bijnen AB, et al. Implications of removing a normal appendix. Dig Surg. 2003;20:215–219. [DOI] [PubMed] [Google Scholar]
- 9.Flum DR, Koepsell T. The clinical and economic correlates of misdiagnosed appendicitis: nationwide analysis. [see comment]. Arch Surg. 2002;137:799–804. [DOI] [PubMed] [Google Scholar]
- 10.Lau WY, Fan ST, Yiu TF, et al. Negative findings at appendectomy. Am J Surg. 1984;148:375–378. [DOI] [PubMed] [Google Scholar]
- 11.Rao PM, Rhea JT, Novelline RA. Helical CT of appendicitis and diverticulitis. Radiol Clin North Am. 1999;37:895–910. [DOI] [PubMed] [Google Scholar]
- 12.Rao PM, Rhea JT, Novelline RA, et al. Helical CT technique for the diagnosis of appendicitis: prospective evaluation of a focused appendix CT examination. Radiology. 1997;202:139–144. [DOI] [PubMed] [Google Scholar]
- 13.McGreevy JM, Finlayson SR, Alvarado R, et al. Laparoscopy may be lowering the threshold to operate on patients with suspected appendicitis. Surg Endosc. 2002;16:1046–1049. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen AG, Petersen OB, Wara P, et al. Randomized clinical trial of laparoscopic versus open appendicectomy. [see comment]. Br J Surg. 2001;88:200–205. [DOI] [PubMed] [Google Scholar]
- 15.Sauerland S, Lefering R, Neugebauer EA. Laparoscopic versus open surgery for suspected appendicitis [review]. Cochrane Database Syst Rev. 2002;CD001546. [124 refs]. [DOI] [PubMed]
- 16.Addiss DG, Shaffer N, Fowler BS, et al. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132:910–925. [DOI] [PubMed] [Google Scholar]
- 17.Al Omran M, Mamdani M, McLeod RS. Epidemiologic features of acute appendicitis in Ontario, Canada. Can J Surg. 2003;46:263–268. [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson R, Hugander A, Thulin A, et al. Indications for operation in suspected appendicitis and incidence of perforation. Br Med J. 1994;308:107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bisset AF. Appendicectomy in Scotland: a 20-year epidemiological comparison. J Public Health Med. 1997;19:213–218. [DOI] [PubMed] [Google Scholar]
- 20.Blomqvist P, Ljung H, Nyren O, et al. Appendectomy in Sweden 1989–1993 assessed by the inpatient registry. J Clin Epidemiol. 1998;51:859–865. [DOI] [PubMed] [Google Scholar]
- 21.Kang JY, Hoare J, Majeed A, et al. Decline in admission rates for acute appendicitis in England. [see comment]. Br J Surg. 2003;90:1586–1592. [DOI] [PubMed] [Google Scholar]
- 22.Williams NM, Jackson D, Everson NW, et al. Is the incidence of acute appendicitis really falling? Ann R Coll Surg Engl. 1998;80:122–124. [PMC free article] [PubMed] [Google Scholar]
- 23.Haugh LD. Checking the independence of two covariance-stationary time series: a univariate residual cross-correlation approach. J Am Stat Assoc. 1976;71:378–385. [Google Scholar]
- 24.Bowers WF, Hughes CW, Bonilla KB. The treatment of acute appendicitis under suboptimal conditions. US Armed Forces Med J. 1958;9:1545–1557. [PubMed] [Google Scholar]
- 25.Funaki B, Grosskreutz SR, Funaki CN. Using unenhanced helical CT with enteric contrast material for suspected appendicitis in patients treated at a community hospital. AJR Am J Roentgenol. 1998;171:997–1001. [DOI] [PubMed] [Google Scholar]
- 26.Garcia Pena BM, Mandl KD, Kraus SJ, et al. Ultrasonography and limited computed tomography in the diagnosis and management of appendicitis in children. JAMA. 1999;282:1041–1046. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs JE, Birnbaum BA, Macari M, et al. Acute appendicitis: comparison of helical CT diagnosis focused technique with oral contrast material versus nonfocused technique with oral and intravenous contrast material. Radiology. 2001;220:683–690. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser S, Frenckner B, Jorulf HK. Suspected appendicitis in children: US and CT–a prospective randomized study. Radiology. 2002;223:633–638. [DOI] [PubMed] [Google Scholar]
- 29.Lane MJ, Katz DS, Ross BA, et al. Unenhanced helical CT for suspected acute appendicitis. AJR Am J Roentgenol. 1997;168:405–409. [DOI] [PubMed] [Google Scholar]
- 30.Rao PM, Rhea JT, Novelline RA, et al. Helical CT combined with contrast material administered only through the colon for imaging of suspected appendicitis. AJR Am J Roentgenol. 1997;169:1275–1280. [DOI] [PubMed] [Google Scholar]
- 31.Schuler JG, Shortsleeve MJ, Goldenson RS, et al. Is there a role for abdominal computed tomographic scans in appendicitis? Arch Surg. 1998;133:373–376. [DOI] [PubMed] [Google Scholar]
- 32.Weltman DI, Yu J, Krumenacker J Jr, et al. Diagnosis of acute appendicitis: comparison of 5- and 10-mm CT sections in the same patient. Radiology. 2000;216:172–177. [DOI] [PubMed] [Google Scholar]
- 33.Rivera-Chavez FA, Wheeler H, Lindberg G, et al. Regional and systemic cytokine responses to acute inflammation of the vermiform appendix. Ann Surg. 2003;237:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivera-Chavez FA, Peters-Hybki DL, Barber RC, et al. Innate immunity genes influence the severity of acute appendicitis. Ann Surg. 2004;240:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flum DR, Morris A, Koepsell T, et al. Has misdiagnosis of appendicitis decreased over time? A population-based analysis. [see comment]. JAMA. 2001;286:1748–1753. [DOI] [PubMed] [Google Scholar]
- 36.Migeon BR. The role of X inactivation and cellular mosaicism in women’s health and sex-specific diseases. JAMA. 2006;295:1428–1433. [DOI] [PubMed] [Google Scholar]
- 37.Filipe-Santos O, Bustamante J, Haverkamp MH, et al. X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. J Exp Med. 2006;203:1745–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]