Abstract
Objective:
We conducted a randomized controlled trial of adjuvant interferon therapy in patients with predominantly hepatitis B-related hepatocellular carcinoma (HCC) to investigate whether the prognosis after hepatic resection could be improved.
Summary Background Data:
Recurrence is common after hepatic resection for HCC. Interferon possesses antiviral, immunomodulatory, antiproliferative, and antiangiogenic effects and may be an effective form of adjuvant therapy.
Patients and Methods:
Since February 1999, patients with no residual disease after hepatic resection for HCC were randomly assigned with stratification by pTNM stage to receive no treatment (control group), interferon alpha-2b 10 MIU/m2 (IFN-I group) or 30 MIU/m2 (IFN-II group) thrice weekly for 16 weeks. Enrollment to the IFN-II group was terminated from January 2000 because adverse effects resulted in treatment discontinuation in the first 6 patients. By June 2002, 40 patients each had been enrolled into the control group and IFN-I group. The baseline clinical, laboratory, and tumor characteristics of both groups were comparable.
Results:
The 1- and 5-year survival rates were 85% and 61%, respectively, for the control group and 97% and 79%, respectively, for the IFN-I group (P = 0.137). After adjusting for the confounding prognostic factors in a Cox model, the relative risk of death for interferon treatment was 0.42 (95% CI, 0.17–1.05; P = 0.063). Exploratory subset analysis showed that adjuvant interferon had no survival benefit for pTNM stage I/II tumor (5-year survival 90% in both groups; P = 0.917) but prevented early recurrence and improved the 5-year survival of patients with stage III/IVA tumor from 24% to 68% (P = 0.038).
Conclusion:
In a group of patients with predominantly hepatitis B-related HCC, adjuvant interferon therapy showed a trend for survival benefit, primarily in those with pTNM stage III/IVA tumors. Further larger randomized trials stratified for stage are needed.
A randomized controlled trial in patients with predominantly hepatitis B-related hepatocellular carcinoma suggested that adjuvant interferon therapy after hepatic resection may prevent early recurrence and improve survival in patients with high-risk tumors.
Hepatocellular carcinoma (HCC) is common worldwide, particularly in Asia where chronic hepatitis B virus (HBV) infection is the most common etiology.1 Potentially curative treatments for HCC include liver resection, liver transplantation, and local ablative therapy.2 Among these, liver resection is regarded as a standard treatment that offers a chance of cure for patients with anatomically localized tumors and preserved liver function. With advances in surgical techniques and perioperative management, the short-term outcome of liver resection has dramatically improved over the last decade.3 The long-term prognosis, however, remains guarded because of frequent development of locoregional tumor recurrence, which, together with concomitant hepatic decompensation, is the main cause of death. Recurrence in the liver remnant may originate from metastasis from the primary tumor or multicentric new primaries in a cirrhotic liver.4 DNA fingerprinting and HBV integration suggested that both mechanisms can contribute to the development of recurrence.5–7 Hence, the ideal agent for adjuvant treatment should have both tumoricidal and chemopreventive effects.
Interferons are cytokines possessing a variety of biologic properties, including antiviral, immunomodulatory, antiproliferative, and antiangiogenic effects.8–10 Interferon is effective in suppressing the replication of HBV and is the first agent approved for the treatment of chronic HBV infection. Response to interferon treatment is associated with improved clinical outcome and less cirrhosis-related complications,11 and long-term studies suggested that it may decrease the rate of HCC in patients with HBV-related cirrhosis.12,13 In addition, interferon has tumoricidal effect against a number of tumors including HCC. Two randomized trials from Hong Kong14,15 showed that high doses of interferon induced more tumor regression and better survival as compared with conservative management or adriamycin in Chinese patients with inoperable HCC that was predominantly HBV-related. Hence, we hypothesized that adjuvant therapy with interferon might be of benefit to patients who underwent resection of HCC owing to a combination of its modulating effect on hepatic function, tumoricidal effect on residual tumor cells, or chemopreventive effect on multicentric hepatocarcinogenesis. The beneficial effect of adjuvant interferon therapy was suggested by some retrospective as well as small-scale prospective studies.16,17 Accordingly, we conducted a randomized controlled trial to evaluate the safety and efficacy of adjuvant interferon therapy after hepatic resection in a group of patients with predominantly HBV-related HCC.
PATIENTS AND METHODS
Patients
Consecutive patients aged between 18 and 75 years who underwent elective curative hepatic resection for primary HCC from February 1999 were considered for entry into this single-center, open-label, randomized trial of postoperative adjuvant interferon therapy. Our selection criteria and technique of hepatic resection have been described previously.3 At the time of surgery, intraoperative ultrasonography was routinely performed to verify whether all macroscopic disease had been extirpated. At 1 month after surgery, a 3-phase contrast-enhanced computed tomography (CT) scan was performed. Hepatic resection was considered curative only when the histologic resection margin was clear and the postoperative CT scan showed no residual tumors at 1 month after surgery.
Eligible patients were excluded if they refused to participate or had one or more of the following criteria: history of previous treatment by regional or systemic chemotherapy, hormonal therapy or immunotherapy, history of psychiatric illness, poor hepatic function (presence of hepatic encephalopathy, ascites not controlled by diuretics, history of variceal bleeding within the last 3 months, a serum total bilirubin level over 50 μmol/L, a serum albumin level below 30 g/L or a prothrombin time of more than 4 seconds over the control), serum creatinine level over 180 μmol/L, absolute neutrophil count <1.5 × 109/L or platelet count <75 × 109/L, or poor performance status (Eastern Cooperative Oncology Group performance status rating18 grade 3 or 4).
Study Design
The study was initially designed to test the efficacy of 2 different dosages of adjuvant interferon therapy. At 1 month after hepatic resection, eligible patients were randomly assigned in equal numbers to receive no treatment (control group), interferon alpha-2b (Intron-A, Schering-Plough, Kenilworth, NJ) 10 MIU/m2 subcutaneously 3 times weekly for 16 weeks (IFN-I group), or interferon alpha-2b 30 MIU/m2 subcutaneously 3 times weekly for 16 weeks (IFN-II group). Because of adverse effects that resulted in discontinuation of interferon treatment in all the first 6 patients assigned to the IFN-II group, the Institutional Review Board agreed in January 2000 that further enrollment of patients to this treatment arm would be unethical and should be stopped. A revised protocol was then approved to continue the trial with 2 treatment groups (control and IFN-I groups) only, and the sample size in each remained unchanged.
Patients assigned to the interferon therapy group received treatment in hospital immediately after randomization within 60 days after surgery. Interferon was administered subcutaneously 3 times weekly starting with 2 doses each of one third and then two thirds of full dose escalating to full dose by the fifth dose. Oral acetaminophen 500 mg was given before each of the first few doses of interferon and then every 6 hours if necessary. Patients tolerating the interferon therapy and in stable condition were discharged from hospital and were treated by self-injection at home or at outpatient clinic. The dosage was reduced by one half or more for patients with significant neutropenia <1 × 109/L, thrombocytopenia <50 × 109/L, or other grade 3 adverse effects. Interferon treatment would be discontinued if severe adverse effects continued or when recurrence was detected.
Randomization
Randomization was performed with stratification according to International Union Against Cancer pathologic tumor-node-metastasis (pTNM) stage19 (5th edition, 1997) on histology (pTNM stages I/II and III/IVA) by drawing consecutively numbered sealed envelopes and implementation was ensured by a research assistant. The use of placebo to maintain double blinding was considered impractical because of the almost universal side effect of flu-like syndrome during initial interferon treatment. The study was conducted according to guidelines as stated in the Declaration of Helsinki and the protocol was approved by the Institutional Review Board. Patient's decision to participate in the study was voluntary and informed consent was obtained from each patient.
Assessment of Outcome
Because interferon possesses antiviral, immunomodulatory, antiproliferative, and antiangiogenic effects, it might potentially alter the outcome of the study patients not only by preventing recurrence but by modulating the behavior of recurrence and liver function. Hence, both recurrence and survival rate calculated from the date of operation were the primary outcome measures. Secondary outcome measures included patient tolerance, virologic response, and liver function. Prescription cards for interferon treatment were issued and patients were asked to record each dose administered. All patients were followed at the outpatient clinic weekly for 16 weeks, monthly for the first year and then with increasing intervals. At each visit to the clinic, the adverse effects were documented and blood tests were taken for complete blood counts, coagulation profile, and renal and liver function. Serum alpha-fetoprotein, hepatitis B virus serology, including hepatitis B surface antigen (HBsAg), hepatitis B antigen (HBeAg), and HBV DNA (Cobas Amplicor, Roche Diagnostics, Branchburg, NJ), chest radiography, and contrast-enhanced 3-phase CT scan were performed every 3 months. Patients with suspected recurrent disease would be investigated by further imaging studies, and histologic or cytologic confirmation would be obtained if possible. When recurrence was confirmed, adjuvant treatment would be stopped and the patient treated with various therapeutic modalities as deemed appropriate. In general, reresection by partial hepatectomy and local ablation by intralesional alcohol injection, cryotherapy or more recently, radiofrequency ablation were the preferred approaches. Patients with unresectable and nonablatable recurrence who fulfilled special criteria were considered for liver transplantation. Transarterial lipiodol chemoembolization was performed for other patients who had adequate hepatic reserve. Follow-up was continued through January 1, 2005 when all surviving patients had a minimum follow-up of 30 months.
Statistical Analysis
At the time of planning, it was decided that a sample size of 40 patients per treatment group would provide the study with a statistical power of 80% at the 0.05 level of significance to detect a reduction in recurrence rate from 60% to 30%.20 The sample size per treatment group remained unchanged when the protocol was revised in the year 2000. Comparison between groups was made on an intention-to-treat basis. Continuous variables were expressed as median (range) and compared using the Mann-Whitney U test. Categorical variables were compared using the χ2 test or Fisher exact test. Survival and recurrence-free survival were measured from the date of operation to the time of death and to the time when recurrent tumor was first diagnosed, respectively. Since transplantation as a salvage therapy could prevent death due to recurrent tumor or hepatic decompensation, it was regarded as a terminal event. The event was uncommon and the date of transplant was taken as the date of death. All-cause mortalities were included in the calculation of survival and disease-free survival. Survival rate was estimated by the life-table method and compared with the use of log-rank test. Univariate analysis was performed to identify the prognostic indicators for survival in the study patients. The confounding effect of these prognostic factors was assessed by including them with interferon treatment in a Cox proportional hazard model to assess the adjusted risk ratio for survival. Statistical analysis was performed with the SPSS (SPSS Inc., Chicago, IL) computer software program.
The study was initiated, designed, and conducted by the investigators. Data were collected by a dedicated research assistant funded by the investigators’ institution and analyzed by the investigators. The manuscript was also written jointly by the investigators. Schering-Plough provided the study medication free of charge but did not offer any other financial support. Staffs from Schering-Plough had no access to the data and no role in data analysis or manuscript preparation.
RESULTS
Patients
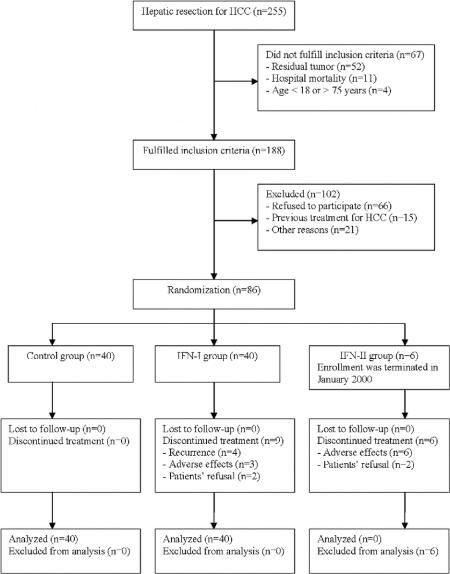
From February 1999 to June 2002, 255 patients underwent primary hepatic resection for HCC at the Department of Surgery, the University of Hong Kong at Queen Mary Hospital (Fig. 1). Sixty-seven patients were not considered for this study because of evidence of incomplete resection or residual tumor as shown on CT scan at 1 month after surgery (52 patients), hospital mortality (11 patients), or age <18 or >75 years (4 patients). A total of 102 patients who fulfilled the inclusion criteria were excluded because they refused to participate (66 patients), had previous medical treatment of HCC (15 patients), or were not suitable as a result of poor hepatic function (7 patients), low white cell or platelet counts (6 patients), poor performance status (6 patients), or poor renal function (2 patients). Of the remaining 86 patients, 15 patients who underwent operation between February 1999 and December 1999 were randomly allocated to the control group (4 patients), IFN-I group (5 patients), and IFN-II group (6 patients). Following the termination of enrollment to the IFN-II group and revision of the design of the trial in January 2000, 71 additional patients were randomly allocated to the control group (36 patients) and IFN-I group (35 patients). For the purpose of the current report, because there were only 6 patients assigned to the IFN-II group, comparison will only be made between the control group and the IFN-I group. The outcome of patients in the IFN-II group would be described separately.
FIGURE 1. Flow diagram of the progress of the trial.
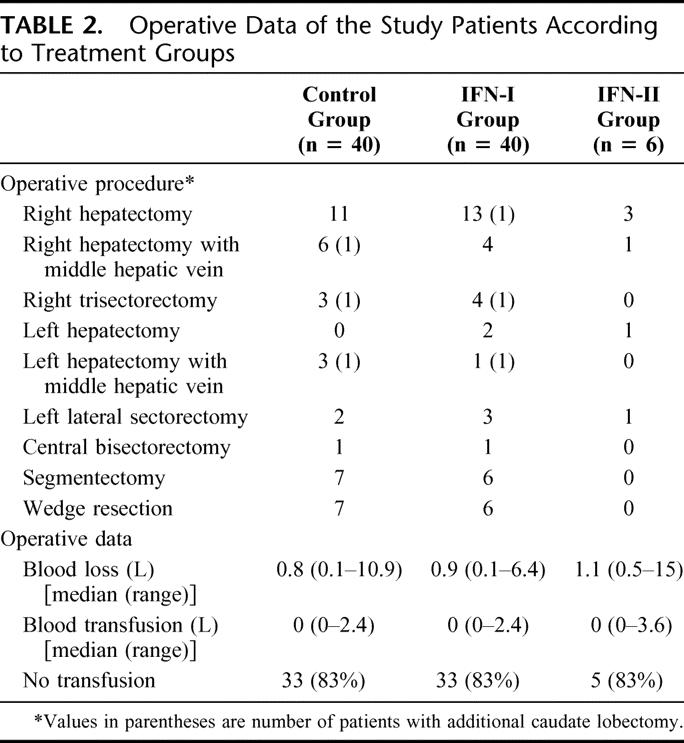
The baseline characteristics and operative data of the study patients are presented in Tables 1 and 2, respectively. There was no significant difference in any of the parameters between the control group and the IFN-I group. Thirty-five patients (88%) of the control group and 37 (93%) of the IFN-I group were seropositive for HBsAg. All except 1 patient (98%) of the control group and 2 patients (95%) of the IFN-I group were positive for at least one of the serum markers for HBV. Only 2 patients (5%) of the control group and 1 (3%) of the IFN-I group were seropositive for antibody against hepatitis C virus. Approximately half of all the study patients were of pTNM stage III or IVA and randomization with stratification ensured an identical proportion of patients with pTNM stage I/II or stage III/IVA tumors in each group. Nineteen patients (48%) of each group had histologic evidence of cirrhosis in the nontumorous liver.
TABLE 1. Baseline Characteristics of the Study Patients According to Treatment Groups
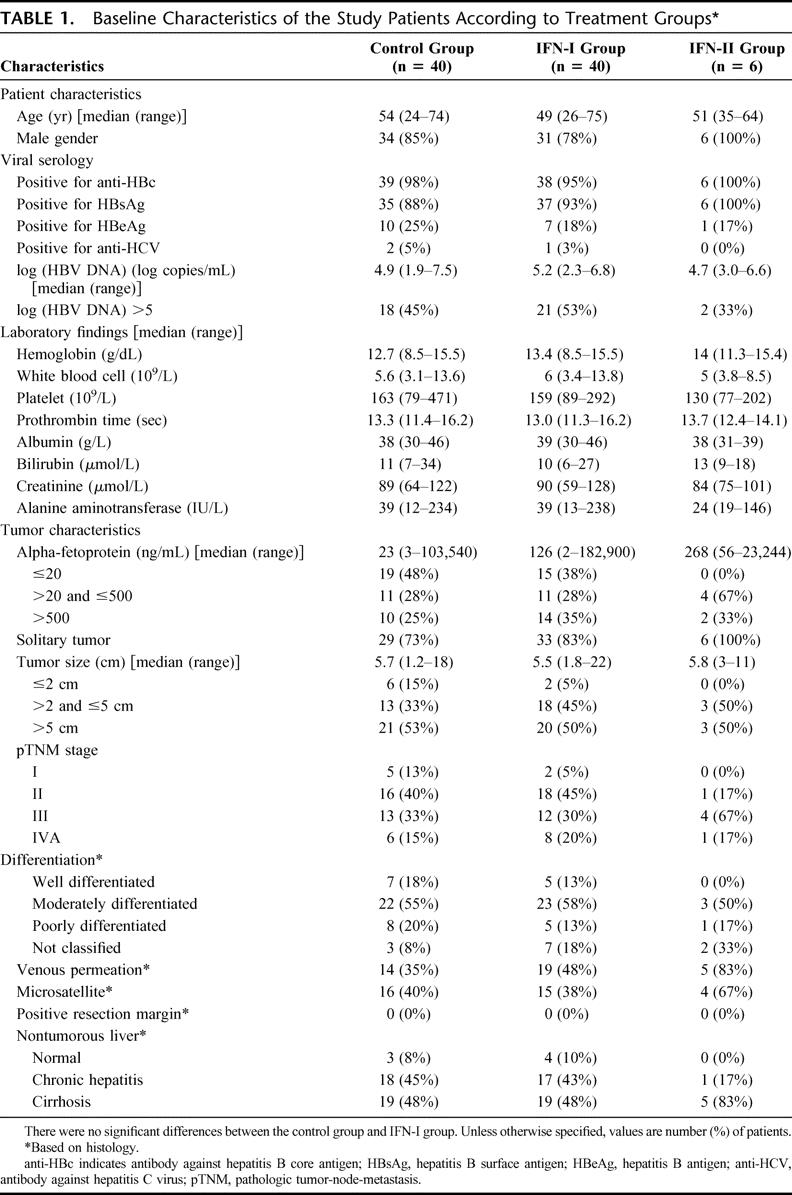
TABLE 2. Operative Data of the Study Patients According to Treatment Groups
Treatment Tolerance and Side Effects
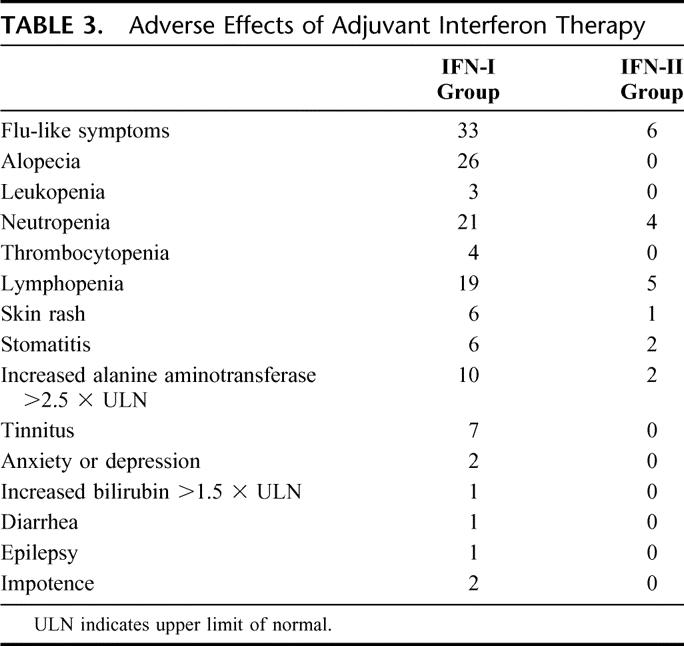
Two patients allocated to the IFN-I group refused to continue treatment after 2 and 3 doses of interferon and the remaining 38 patients showed adherence to the treatment. Thirty-one patients completed the 16-week course of adjuvant treatment, but dose reduction was required at some stage in 22 patients. The median interferon dosage was 16.7 MIU (range, 14–19.4 MIU) per dose. In the remaining 7 patients, interferon therapy was stopped prematurely because of recurrence in 4 patients and serious adverse effects in 3 patients (1 patient each due to grade 4 lymphopenia, convulsion, and hepatotoxicity). Flu-like symptoms were almost universally present and the other adverse effects are listed in Table 3.
TABLE 3. Adverse Effects of Adjuvant Interferon Therapy
Recurrence and Treatment of Recurrence
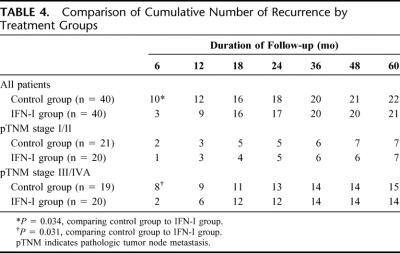
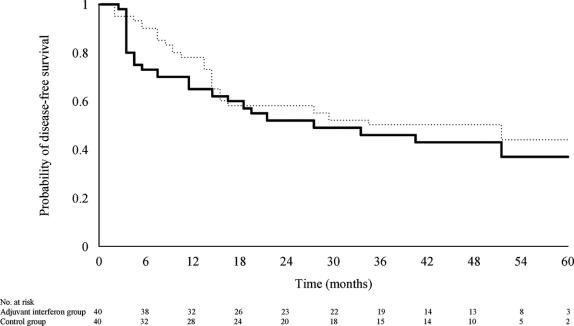
No patient was lost to follow-up. The cumulative number of patients with recurrence is presented in Table 4. There were significantly fewer recurrences at 6 months after surgery in the IFN-I group (10 of 40 patients vs. 3 of 40 patients; P = 0.034), and this difference in early recurrence rate was seen only in patients with pTNM stage III/IVA (8 of 19 patients vs. 2 of 20 patients; P = 0.031) but not in those with stage I/II tumor (2 of 21 patients vs. 1 of 20 patients; P = 1.0). With longer follow-up, however, this initial difference in recurrence rate was lost, and there was no significant difference in the overall disease-free survival (Fig. 2).
TABLE 4. Comparison of Cumulative Number of Recurrence by Treatment Groups
FIGURE 2. Probability of disease-free survival in patients treated with adjuvant interferon therapy (dotted line) and in patients of the control group (solid line) (log-rank test, P = 0.311).
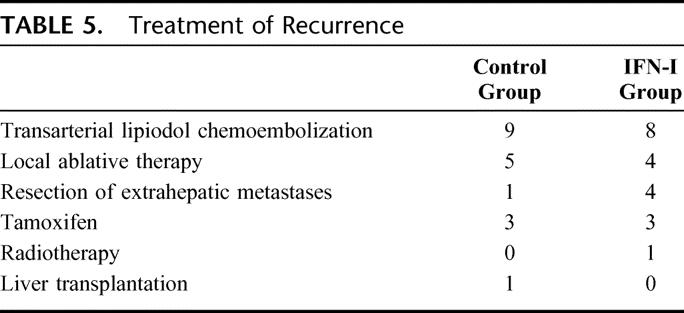
The sites of recurrence included the liver remnant in 17 patients, extrahepatic organs in 4, and both sites in 1 patient of the control group, and the liver remnant in 12 patients, extrahepatic organs in 5, and both sites in 4 patients of the IFN-I group. Recurrences were treated by various modalities according to the location of recurrence and liver function (Table 5).
TABLE 5. Treatment of Recurrence
Survival
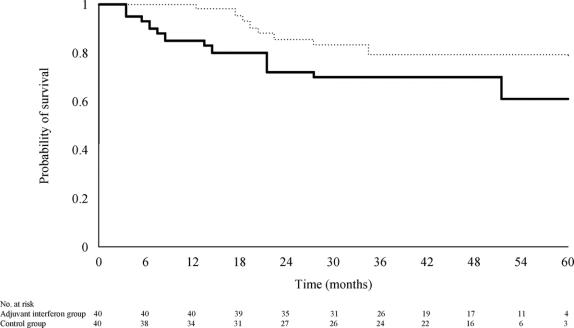
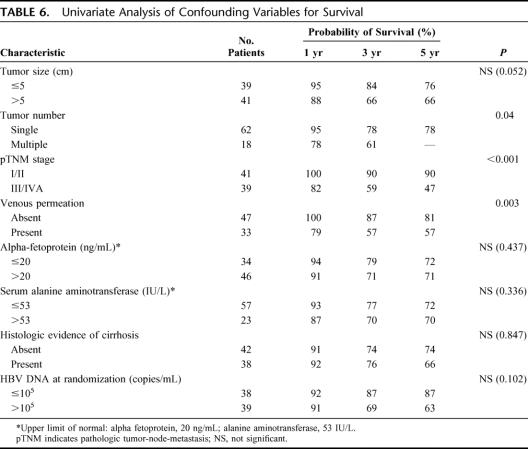
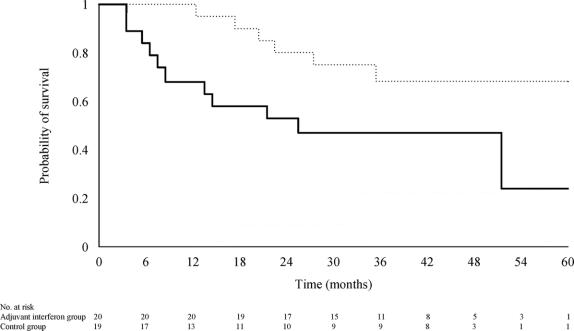
Thirteen patients (33%) of the control group and 8 (20%) of the IFN-I group had died or underwent transplantation. The 1-, 3-, and 5-year survival rates were 85%, 70%, and 61%, respectively, for the control group, and 97%, 79%, and 79%, respectively for the IFN-I group (relative risk of death in the IFN-I group = 0.53; 95% CI, 0.22–1.275; P = 0.137; Fig. 3). Univariate analysis identified tumor number, pTNM stage, and venous permeation on histology as the most significant prognostic factors for survival (Table 6). In the Cox model for survival that included these 3 confounding factors with interferon treatment, only pTNM stage significantly influenced the time to death (relative risk of death for pTNM stage III/IVA as compared with stage I/II = 1.94; 95% CI, 1.01–3.75; P = 0.049). The adjusted relative risk of death for patients treated with interferon was 0.42 (95% CI, 0.17–1.05; P = 0.063) as compared with that of the control group.
FIGURE 3. Probability of survival in patients treated with adjuvant interferon therapy (dotted line) and in patients of the control group (solid line) (log-rank test, P = 0.137).
TABLE 6. Univariate Analysis of Confounding Variables for Survival
Exploratory analysis for the survival benefit of adjuvant interferon treatment stratified by pTNM stage was performed. Adjuvant interferon therapy had no significant effect on the survival of patients with pTNM stage I/II tumors (1-, 3-, and 5-year survival of 100%, 90%, and 90%, respectively in both groups; P = 0.917). In patients with stage III/IVA disease, however, interferon therapy was associated with a significant improvement in survival (1-, 3-, and 5-year survival of 68%, 47%, and 24%, respectively, in the control group and 95%, 68%, and 68%, respectively, in the IFN-I group; relative risk of death = 0.38; P = 0.038; Fig. 4). Eleven of 19 patients (58%) with stage III/IVA tumor in the control group had died of progressive tumor recurrence (n = 10) or underwent liver transplantation for intrahepatic recurrence with concomitant hepatic decompensation (n = 1). Six of 20 patients (30%) with stage III/IVA tumor in the IFN-I group had died of recurrent tumor.
FIGURE 4. Probability of survival in patients with pTNM stage III/IVA tumors treated with adjuvant interferon therapy (dotted line) and in patients of the control group (solid line) (log-rank test, P = 0.038).
Virologic and Biochemical Changes
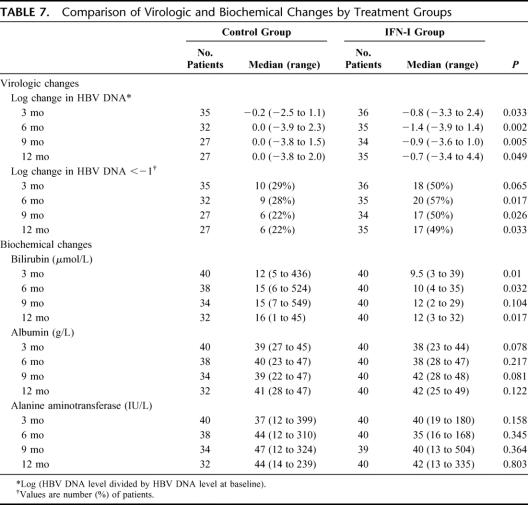
As only a small proportion of study patients had baseline serum alanine aminotransferase level >2 times that of upper limit of normal (7 of 80 patients), positive serology for HBeAg (17 of 80), or HBV DNA >105 copies/mL (39 of 80 patients), we did not assess the usual biochemical or virologic response rate of interferon therapy (Table 7). Nonetheless, interferon therapy induced a significantly greater reduction in HBV DNA level relative to the baseline and was more likely to be associated with a >1 log reduction in HBV DNA level at various time points within the first year of the study period. There was no significant difference in the serum alanine aminotransferase level or albumin level, but patients who received interferon therapy had a lower serum bilirubin level than those of the control group at 3, 6, and 12 months after operation
TABLE 7. Comparison of Virologic and Biochemical Changes by Treatment Groups
Outcome of 6 Patients Allocated to the IFN-II Group
None of the 6 patients of the IFN-II group completed the 16-week course of interferon therapy. Two patients refused treatment after 1 and 3 doses of interferon. Interferon was stopped in the other 4 patients because of grade 3 toxicity (neutrophil count <1 × 109/L) despite dose reduction. On subsequent follow-up, 3 patients developed recurrence at 6, 9, and 16 months after surgery. One died of tumor progression at 34 months and the other 2 were alive with tumors at 61 and 67 months. Of the remaining 3 patients, 1 patient underwent liver transplantation for liver failure in the absence of recurrence at 24 months and another 1 died of primary lung cancer proven on histology at 14 months. The last patient was alive and free of recurrence at 69 months. The survival and disease-free survival rates at 5 years were 50% and 17%, respectively.
DISCUSSION
Attempts to improve the long-term outcome with adjuvant therapy after potentially curative treatment of HCC have produced limited success.21 Systemic and regional chemotherapy or chemoembolization has largely been shown to have no survival benefit in randomized trials.20,22,23 Other adjuvant modalities, including oral acyclic retinoid acid,24 adoptive immunotherapy,25 and intraarterial radioiodine therapy,26 have shown promising benefit in individual trials, usually in small-scale and preliminary settings. Since the initiation of the present study in 1999, there have been at least 3 reported randomized trials27–29 that have shown positive benefit of adjuvant interferon therapy after resection or ablation of HCC. All 3 studies, however, involved small samples with only 10 to 15 patients each in the control or treatment arm, and there was little consideration of statistical power in the design. In the 2 studies from Japan27,28 that involved patients with hepatitis C-related HCC, the recurrence rates of 80% to 100% at 2 to 3 years in the control group were unexpectedly high, despite the inclusion of early tumors <5 cm in diameter only. In the study reported by Lin et al from Taiwan29 in which 30 patients who were treated by percutaneous ethanol injection with or without transarterial chemoembolization were randomized into 3 groups to receive no treatment, continuous, or intermittent adjuvant interferon therapy, the authors concluded that interferon therapy was effective in reducing recurrence in HBV-related HCC but not in hepatitis C-related HCC. Nonetheless, such a conclusion was only based on 16 patients with HBV-related HCC: 12 receiving adjuvant interferon therapy and 4 in the controlledarm.
Our study was designed to involve a larger sample size and was the first randomized controlled trial that tested the efficacy of adjuvant interferon therapy in patients with predominantly HBV-related HCC. Over 95% of the study patients had evidence of previous HBV infection and 90% were seropositive for HBsAg. To ensure that the interferon therapy was truly adjuvant, we have adopted the definition of a curative resection, as described by Nagasue et al,30 which included the criteria of a complete resection with clear microscopic margin and no residual tumor detected on imaging 1 month after surgery. Unlike previous trials,27,29 which had included patients treated by medical ablation, all patients in our study had surgical resection and had detailed histologic examination of the complete tumor and the surrounding nontumorous liver. Patients could thus be stratified by pTNM stage for randomization. Since tumor histologic factors and staging as well as underlying cirrhosis are the most important predictors of recurrence and survival after hepatic resection,4,31 the availability of these information allowed us to detect any other bias in randomization and to estimate the adjusted treatment benefit by including these confounding factors in a multivariate model.
The present study did not show a significant effect of adjuvant interferon therapy on the overall recurrence rate for the total patient population, but there was a significant reduction in the incidence of early recurrence and a trend toward a reduction in the adjusted hazard of death by over 50% in patients who had received adjuvant interferon. Exploratory subset analyses after stratification by pTNM stage showed that patients with pTNM stage III and IVA tumors benefited from adjuvant interferon treatment with prevention of early recurrence and statistically significant improvement in survival. As these were unplanned subset analyses, the results must be interpreted with caution. Nonetheless, there were 39 patients in the subset of stage III and IVA tumors in our trial, which was more than the total number of study patients in any of the 3 previously reported randomized trials.27–29 The significant increase in survival in this subset of patients who are known to have very poor prognosis is highly clinically relevant. The survival rate of the controlled group of patients with pTNM stage III and IVA tumors in our study (5-year survival rate of 24%) was comparable to what we have previously reported in patients with HBV-related HCC (5-year survival rate of 32% for pTNM stage III and 5% for stage IVA).31 Adjuvant interferon therapy after surgery for these relatively advanced tumors resulted in a remarkable improvement in 5-year survival rate to 68%. This survival benefit of interferon therapy could be attributed to its efficacy in preventing early recurrence. A previous study has shown that early recurrence carried a much worse prognosis, and patients with early recurrence had a median survival that was only half of that of patients with late recurrence.4 Our data also suggested the efficacy of adjuvant interferon in suppressing viral replication and in modulating liver function. The significance of this mode of action in contributing to the survival benefit is uncertain. Theoretically, modulation in liver function may not only affect survival directly but indirectly by influencing the patient's tolerance of various treatments for recurrence.
Recurrence due to metastatic disease presents early and that of multicentric primaries presents late. In a randomized trial on adjuvant interferon therapy in patients who underwent resection of solitary hepatitis C-related HCC less than 5 cm in diameter, Kubo et al28 showed that treatment prevented late recurrence that developed 2 years after operation but had no effect on early recurrence, suggesting that the benefit of adjuvant interferon in these patients was largely on suppression of multicentric recurrence. In contrast, our study included mostly HBV-related HCC and about 50% of the tumors were larger than 5 cm in diameter or of pTNM stage III/IVA. All the prognostic factors for survival identified on univariate analysis were related to tumor characteristics but not to the viral activity or presence of liver cirrhosis. On multivariate analysis, the pTNM stage was the only significant prognostic factor for survival, indicating that in this group of patients with HBV-related HCC, recurrence due to metastasis from a more advanced primary tumor was a more important issue than multicentric primaries in a cirrhotic liver remnant. The ability of adjuvant interferon treatment to prevent early recurrence and to improve survival only in patients with more advanced pTNM stage III/IVA tumors suggested that its action was likely to be related to its antiproliferative or antiangiogenic effect in suppressing the growth of micrometastases. The mechanism of interferon alpha on inhibition of metastasis and recurrence after resection of human HCC xenografts in nude mice was shown to be mediated by antiangiogenesis through down-regulating expression of vascular endovascular growth factor.10 Although it would be premature to conclude that the beneficial effects of adjuvant interferon therapy in HBV-related HCC are limited to patients with pTNM stage III or IVA tumors and not stage I or II tumors, the relatively low incidence of recurrence (33%) and a 5-year survival of 90% in the control arm of our patients with pTNM stage I/II tumors would make it necessary to have a much larger sample size to enable any adjuvant therapy to confer a statistically significant clinical benefit.
The mode of recurrence and the primary target of adjuvant interferon therapy may also be related to the dosage and duration of treatment. Previous randomized trials mostly in patients with HCV-related HCC had used a relatively low dosage of interferon (9–18 MIU per week) for prolonged period (24–36 months).27–29 In nude mice bearing human HCC xenografts, systemic interferon therapy has been shown to have a dose-dependent inhibitory effect on tumor growth, recurrence, and metastasis.32 Among previous studies on interferon treatment of inoperable HCC, only 2 reports14,15 using very high doses of interferon (60–150 MIU/m2 per week) had demonstrated significant tumor regression and a survival benefit in patients with HBV-related HCC. Hence, in our initial design of the present trial, we decided to use a higher dosage of interferon, including a very high dosage group of 30 MIU/m2 thrice weekly. This very high dosage of interferon proved to be poorly tolerated by the postoperative patients, resulting in premature discontinuation of treatment in all patients and termination of this treatment arm. The lower dosage of 10 MIU/m2 thrice weekly was better tolerated, but dose reduction was still required in many patients. Considering patient compliance and the relative importance of metastatic recurrence, we avoided prolonged course of treatment in the present trial and had administered interferon for 16 weeks, which was the usual duration of treatment of chronic hepatitis B.11,33 Whether the beneficial effect of adjuvant interferon can be extended by a more prolonged course of treatment remains to be defined. The efficacy of pegylated form of interferon, which has better pharmacokinetics,34 more convenient once-weekly dosing, and superior clinical results in patients with chronic hepatitis B,35 deserves further investigation.
CONCLUSION
This trial of adjuvant interferon therapy in a group of patients who underwent hepatic resection for predominantly HBV-related HCC suggests that the subgroup of patients with high-risk tumors (pTNM stage III or IVA) are likely to benefit from a 16-week course of treatment. Further randomized trials stratified for stage are warranted to verify these findings and to investigate the optimal dosage and duration of the adjuvant treatment.
ACKNOWLEDGMENTS
The authors thank Schering-Plough Company for providing the study medication.
Footnotes
Supported by Sun C.Y. Research Foundation for Hepatobiliary and Pancreatic Surgery of the University of Hong Kong.
Reprints: Chung Mau Lo, FRACS, FRCS (Edin), FACS, Department of Surgery, University of Hong Kong, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong, China. E-mail: chungmlo@hkucc.hku.hk.
REFERENCES
- 1.Xavier Bosch F. Global epidemiology of hepatocellular carcinoma. In: Okuda K, Tabor E, eds. Liver Cancer. New York: Churchill Livingstone, 1997:13–28. [Google Scholar]
- 2.Bruix J. Treatment of hepatocellular carcinoma. Hepatology. 1997;25:259–262. [DOI] [PubMed] [Google Scholar]
- 3.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poon RT, Fan ST, Ng IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–507. [PubMed] [Google Scholar]
- 5.Chen PJ, Chen DS, Lai MY, et al. Clonal origin of recurrent hepatocellular carcinomas. Gastroenterology. 1989;96:527–529. [DOI] [PubMed] [Google Scholar]
- 6.Esumi M, Aritaka T, Arii M, et al. Clonal origin of human hepatoma determined by integration of hepatitis B virus DNA. Cancer Res. 1986;46:5767–5771. [PubMed] [Google Scholar]
- 7.Sheu JC, Huang GT, Chou HC, et al. Multiple hepatocellular carcinomas at the early stage have different clonality. Gastroenterology. 1993;105:1471–1476. [DOI] [PubMed] [Google Scholar]
- 8.von Marschall Z, Scholz A, Cramer T, et al. Effects of interferon alpha on vascular endothelial growth factor gene transcription an tumor angiogenesis. J Natl Cancer Inst. 2003;95:437–448. [DOI] [PubMed] [Google Scholar]
- 9.Dinney CP, Bielenberg DR, Perrotte P, et al. Inhibition of basic fibroblast growth factor expression, angiogenesis and growth of human bladder carcinoma in mice by systemic interferon-alpha administration. Cancer Res. 1998;58:808–814. [PubMed] [Google Scholar]
- 10.Wang L, Wu WZ, Sun HC, et al. Mechanism of interferon alpha on inhibition of metastasis and angiogenesis of hepatocellular carcinoma after curative resection in nude mice. J Gastrointest Surg. 2003;7:587–594. [DOI] [PubMed] [Google Scholar]
- 11.Niederau C, Heintges T, Lange S, et al. Long-term follow-up of HBeAg positive patients treated with interferon alfa for chronic hepatitis B. N Engl J Med. 1996;334:1422–1427. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda K, Saitoh S, Suzuki Y, et al. Interferon decreases hepatocellular carcinogenesis in patients with cirrhosis caused by the hepatitis B virus: a pilot study. Cancer. 1998;82:827–835. [DOI] [PubMed] [Google Scholar]
- 13.Lin SM, Sheen IS, Chien RN, et al. Long-term beneficial effect of interferon therapy in patients with chronic hepatitis B virus infection. Hepatology. 1999;29:971–975. [DOI] [PubMed] [Google Scholar]
- 14.Lai CL, Wu PC, Lok AS, et al. Recombinant alpha 2 interferon is superior to doxorubicin for inoperable hepatocellular carcinoma: a prospective randomised trial. Br J Cancer. 1989;60:928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai CL, Lau JY, Wu PC, et al. Recombinant interferon-alpha in inoperable hepatocellular carcinoma: a randomized controlled trial. Hepatology. 1993;17:389–394. [PubMed] [Google Scholar]
- 16.Oon CJ. Long-term survival following treatment of hepatocellular carcinoma in Singapore: evaluation of Wellferon in the prophylaxis of high-risk pre-cancerous conditions. Cancer Chemother Pharmacol. 1992;31(suppl):137–142. [DOI] [PubMed] [Google Scholar]
- 17.Lygidakis NJ, Pothoulakis J, Konstantinidou AE, et al. Hepatocellular carcinoma: surgical resection versus surgical resection combined with pre- and post-operative locoregional immunotherapy-chemotherapy: a prospective randomized study. Anticancer Res. 1995;15:543–550. [PubMed] [Google Scholar]
- 18.Falkson G, MacIntyre JM, Moertel CG, et al. Primary liver cancer: an Eastern Cooperative Oncology Group trial. Cancer. 1984;54:970–977. [DOI] [PubMed] [Google Scholar]
- 19.Sobin LH, Whitekind C. TNM Classification of Malignant Tumors, 5th ed. New York: John Wiley, 1997. [Google Scholar]
- 20.Lai EC, Lo CM, Fan ST, et al. Postoperative adjuvant chemotherapy after curative resection of hepatocellular carcinoma: a randomized controlled trial. Arch Surg. 1998;133:183–188. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz JD, Schwartz M, Mandeli J, et al. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: review of the randomised clinical trials. Lancet Oncol. 2002;3:593–603. [DOI] [PubMed] [Google Scholar]
- 22.Ono T, Nagasue N, Kohno H, et al. Adjuvant chemotherapy with epirubicin and carmofur after radical resection of hepatocellular carcinoma: a prospective randomized study. Semin Oncol. 1997;24(suppl):618–625. [PubMed] [Google Scholar]
- 23.Ono T, Yamanoi A, Nazmy EL, et al. Adjuvant chemotherapy after resection of hepatocellular carcinoma causes deterioration of long-term prognosis in cirrhotic patients: metaanalysis of three randomized controlled trials. Cancer. 2001;91:2378–2385. [PubMed] [Google Scholar]
- 24.Muto Y, Moriwaki H, Ninomiya M, et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma: Hepatoma Prevention Study Group. N Engl J Med. 1996;334:1561–1567. [DOI] [PubMed] [Google Scholar]
- 25.Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802–807. [DOI] [PubMed] [Google Scholar]
- 26.Lau WY, Leung TW, Ho SK, et al. Adjuvant intra-arterial lipiodol iodine-131-labelled lipiodol for resectable hepatocellular carcinoma: a prospective randomised trial. Lancet. 1999;353:797–801. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda K, Arase Y, Saitoh S, et al. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor: a prospective randomized study of hepatitis C virus-related liver cancer. Hepatology. 2000;32:228–232. [DOI] [PubMed] [Google Scholar]
- 28.Kubo S, Nishiguchi S, Hirohashi K, et al. Effects of long-term postoperative interferon-alpha therapy on intrahepatic recurrence after resection of hepatitis C virus-related hepatocellular carcinoma: a randomized, controlled trial. Ann Intern Med. 2001;134:963–967. [DOI] [PubMed] [Google Scholar]
- 29.Lin SM, Lin CJ, Hsu CW, et al. Prospective randomized controlled study of interferon-alpha in preventing hepatocellular carcinoma recurrence after medical ablation therapy for primary tumors. Cancer. 2004;100:376–382. [DOI] [PubMed] [Google Scholar]
- 30.Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology. 1993;105:488–494. [DOI] [PubMed] [Google Scholar]
- 31.Poon RT, Fan ST, Lo CM, et al. Long-term prognosis after resection of hepatocellular carcinoma associated with hepatitis B-related cirrhosis. J Clin Oncol. 18:1094–1101. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Tang ZY, Qin LX, et al. High-dose and long-term therapy with interferon-alfa inhibits tumor growth and recurrence in nude mice bearing human hepatocellular carcinoma xenografts with high metastatic potential. Hepatology. 2000;32:43–48. [DOI] [PubMed] [Google Scholar]
- 33.Lok AS, Wu PC, Lai CL, et al. A controlled trial of interferon with or without prednisone priming for chronic hepatitis B. Gastroenterology. 1992;102:2091–2097. [DOI] [PubMed] [Google Scholar]
- 34.Perry CM, Jarvis B. Peginterferon-alpha-2a (40 kD): a review of its use in the management of chronic hepatitis C. Drugs. 2001;61:2263–2288. [DOI] [PubMed] [Google Scholar]
- 35.Cooksley WG, Piratvisuth T, Lee SD, et al. Peginterferon alpha-2a(40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepatol. 2003;10:298–305. [DOI] [PubMed] [Google Scholar]