Abstract
Objective:
To examine modifications of acid-base balance of cirrhotic patients undergoing hepatectomy for hepatocellular carcinoma (HCC).
Summary Background Data:
Acid-base disorders are frequently observed in cirrhotics; however, modifications during hepatectomy and their impact on prognosis have never been investigated.
Methods:
Two hundred and two hepatectomies for HCC on cirrhosis were reviewed. Arterial blood samples were collected immediately before and at the end of resection. Preresection and postresection acid-base parameters were compared and related to patient characteristics and postoperative course. The accuracy of acid-base parameters in predicting postoperative liver failure, defined as an impairment of liver function after surgery that led to patient death or required transplantation, was assessed using receiver operating characteristic analysis (ROC).
Results:
All patients showed a significant reduction in pH, bicarbonate, and base excess at the end of hepatectomy (P < 0.001 in all cases), worsened by intraoperative blood loss (P < 0.010) and preoperative Model for end-stage liver disease score ≥11 (P < 0.010). ROC curve analysis identifies patients with postresection bicarbonate <19.4 mmol/L at high risk for liver failure (50.0%) whereas levels >22.1 mmol/L did not lead to the event (0%; P < 0.001). Postoperative prolongation of prothrombin time and increases in bilirubin, creatinine, and morbidity were also more frequent in patients with lower postresection bicarbonate, resulting in a longer in-hospital stay.
Conclusion:
In cirrhotic patients, a trend toward a relative acidosis can be expected during surgery and is worsened by the severity of the underlying liver disease and intraoperative blood loss. Postresection bicarbonate level lower than 19.4 mmol/L is an adverse prognostic factor.
To examine modifications of acid-base balance in cirrhotic patients during surgery 202 hepatectomies were reviewed: hepatectomy was followed by a decrease in pH, bicarbonate and base excess in all patients, worsened by blood transfusion and severity of liver disease. A postresection bicarbonate level lower than 19.4 mmol/L was an adverse prognostic factor.
Hepatocellular carcinoma (HCC) represents one of the most common tumors worldwide and, in the majority of cases, occurs in patients with chronic underlying liver disease mainly caused by hepatitis B and C viruses.1 In cirrhotic patients, resection of HCC requires expert candidate selection, adequate skills in carrying out the surgical procedure, and optimal postoperative management.2–6
In addition to the evaluation of tumor status, a careful preoperative evaluation of liver function is mandatory to assess resectability. Several clinical and biochemical variables, namely ascites, prolonged prothrombin time (PT), increase of serum creatinine and bilirubin levels, and decrease of serum albumin are typical markers of impaired liver function; these parameters were largely used to measure hepatic functional reserve by means of the Child-Turcotte-Pugh (CTP) score7,8 and, more recently, the Model for End-stage Liver Disease (MELD).9–11 Acid-base disorders are also observed in cirrhotic patients; in particular, respiratory alkalosis has been frequently reported12–15 whereas development of metabolic acidosis has been associated with increasing severity of liver disease, bleeding, sepsis, or shock.16
We hypothesize that cirrhotic patients undergoing liver surgery may experience an acid-base disorder that reflects both the severity of the underling liver disease and the surgical injury; thus, the aims of the present study were to investigate the acid-base balance in relationship with the severity of liver disease, the surgical procedure, and the postoperative outcome of cirrhotic patients undergoing liver resection for HCC.
METHODS
Between January 1997 and March 2006, 230 patients underwent curative hepatic resection for HCC on cirrhosis at the Department of Surgery and Transplantation of the University of Bologna: the policy of our center regarding indications for hepatic resection has already been published2; however, in recent years selection criteria were enlarged, including more advanced tumors and cirrhosis, considering hepatectomy as a bridge to liver transplantation for selected patients on waiting list. Of these patients, 28 were not included in the analysis for the following reasons: incomplete clinical data (21 patients), presence of chronic renal insufficiency that did not allow a reliable measurement of both the acid-base balance and the MELD score (4 patients) and early postoperative death due to acute myocardial infarction (2 patients) and pulmonary embolism (1 patient). The final study group consisted of 202 patients with HCC and histologic-proven cirrhosis.17
General anesthesia started with intravenous midazolam (0.04 mg/kg), propofol (2–2.5 mg/kg), fentanyl (1–2 μg/kg), and vecuronium (0.1 mg/kg) administration and was maintained with sevoflurane (0.5–1% MAC) in a mixture of air and oxygen (Fio2% = 50). A peripheral venous 14G catheter, a 20-cm 14G central venous catheter (CVC) in the right internal jugular vein and a 20G catheter in the left radial artery were used for fluid infusion, hemodynamic monitoring, and acid-base parameter measurement. Arterial blood samples were collected in all patients from the radial artery, immediately before the start of parenchymal transection and at the end of resection: pH, partial pressure of carbon dioxide (pco2) and oxygen (po2), bicarbonate (HCO3−), and base excess (BE) were measured with a blood gas analyzer (Rapid Point, Bayer Healthcare, Leverkusen, Germany) in all patients.
Crystalloids, colloids, red blood cell units, fresh frozen plasma, and albumin infusions were guided by pertinent clinical signs of perfusion, mean arterial and central venous pressure (CVP) and waveform changes of the ventilatory cycle. Fluid infusion was minimal during hepatic dissection to keep CVP lower than 5 mm Hg to reduce bleeding from hepatic veins.18 Corrective actions were adopted in the event of hypotension (systolic arterial pressure <80 mm Hg or a reduction of more than 30% with respect to baseline values) and included the administration of physiologic solution, colloids in the event of poor response to crystalloids and ephedrine with atropine addiction in the event of bradycardia; if hematocrit was lower than 27% and/or hemoglobin was lower than 8 gr/dL red blood cell transfusion was started. Correction of acidosis was adopted at the end of resection and consisted of intravenous administration of sodium bicarbonate 8.4% until normalization of parameters. Patients hypothermia was prevented by warm fluid infusion, forced-air warming, and the use of warm water on the surgical field.
The endpoints of the study were as follows:
To investigate the relationship between preresection acid-base balance and the baseline characteristics of the study population; in particular, the relationship with the severity of underlying liver disease, assessed by both CTP score8 and MELD score19 was examined (for the calculation of both CTP and MELD scores only biochemical values from the laboratory of our center were used).
To examine the modification of acid-base parameters throughout surgical resection; in particular, the relationship between postresection acid-base parameters and the extent of hepatectomy, with reference to the International Hepato Pancreato Biliary Association (IHPBA) classification20; all resections were performed to achieve a tumor-free margin of at least 1 cm based on intraoperative examination and ultrasonography; major hepatic resection was defined as the removal of more than 2 segments; in addition, the relationships between postresection acid-base parameters, intraoperative blood transfusion and Pringle maneuver were also investigated.
To explore the relationship between postresection acid-base parameters and postoperative development of irreversible liver failure, defined as a growing impairment of liver function after resection that led to patient death or required transplantation; the association with postoperative complications was also explored, including occurrence of refractory ascites (requiring drainage), increase of bilirubin levels above 3 mg/dL (294 μmol/L), alteration of coagulation factors requiring fresh frozen plasma (INR above 1.50), and renal impairment (blood urea nitrogen above 2.00 g/L and/or increase of serum creatinine above 2.00 mg/dL, corresponding to 182 μmol/L) as previously reported.10 Length of in-hospital stay was computed from the day of surgery until discharge at home.
The study protocol was in accordance with the Declaration of Helsinki and subsequent amendments.
Statistical Analysis
Continuous variables were expressed as mean and standard deviation. Differences between subgroups were explored by the independent-samples t test procedure after Levene test for equality of variances and differences between preresection and postresection acid-base parameters by paired-samples t test procedure. Categorical variables were reported in number of cases and prevalence and differences between subgroups were compared using the χ2 analysis with Yates correction were necessary.
The prognostic value of postresection acid-base parameters in predicting postoperative liver failure was assessed using receiver operating characteristic (ROC) curve analysis21: the area under the curve (AUC), the sensitivity, the specificity, the positive and negative predictive values (PPV and NPV, respectively), and the positive and negative likelihood ratio (PLR and NLR, respectively) for cut-off points obtained were reported. The likelihood ratio incorporates the sensitivity and specificity of a test into a single measure, which is independent of the prevalence of the disease in the population, making the results better applicable to different series of patients. For PLR, values over 10 indicate high predictive power, whereas values between 5 and 10 moderate power. For NLR the values for high or moderate predictive power are respectively below 0.1 and between 0.1 and 0.2. Values of around 1 indicate that no useful information for ruling the diagnosis in or out was produced by the clinical findings.
A significance level of 0.05 was used in all analyses. The statistical analysis was done using SPSS Version 10.0 software for PC computer (SPSS, Chicago, IL) and ROC analysis was performed using MedCalc Version 7.2.1.0 (MedCalc Software, Mariakerke, Belgium).
RESULTS
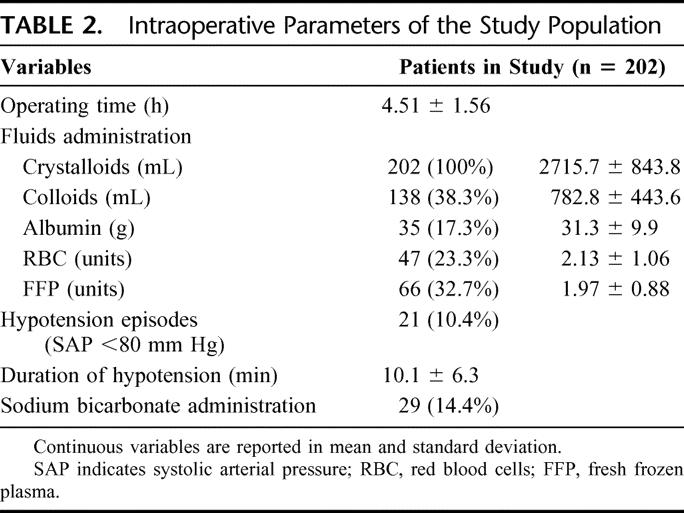
The study population included 155 men (76.7%) and 47 women (23.3%). The mean age was 63.9 ± 8.9 years ranging from 41 to 85 (Table 1). One hundred and ninety patients were classified as CTP class A (94.1%) and 12 patients as class B (7.1%): mean CTP score was 5.4 ± 0.6 ranging from 5 to 8. Mean MELD score prior to surgery was 8.8 ± 1.7 ranging from 6 to 15. Surgery consisted of minor hepatic resections in 193 cases (95.5%) and of major hepatic resections in the remaining 9 (4.5%). Data regarding operating time, crystalloids, colloids, and albumin administration (including red blood cell and fresh frozen plasma transfusions), hypotension episodes, duration of hypotension, and correction of acidosis are reported in Table 2. Fifteen of 202 patients (7.4%) developed irreversible postoperative liver failure. Of these patients, 4 were considered eligible for liver transplantation and successfully transplanted, whereas the remaining 11 had absolute contraindications for liver transplantation and died postoperatively; therefore, the mortality for postoperative liver failure was 5.4%. Mean time from surgery to liver transplantation or patient death was 59.4 ± 48.6 days (range = 14–166). Ten of 15 patients were classified as CTP class A and underwent a major hepatectomy in 3 cases; the other 5 patients, classified as CTP class B, underwent a minor hepatectomy in all cases.
TABLE 1. Baseline Characteristics of the Study Population
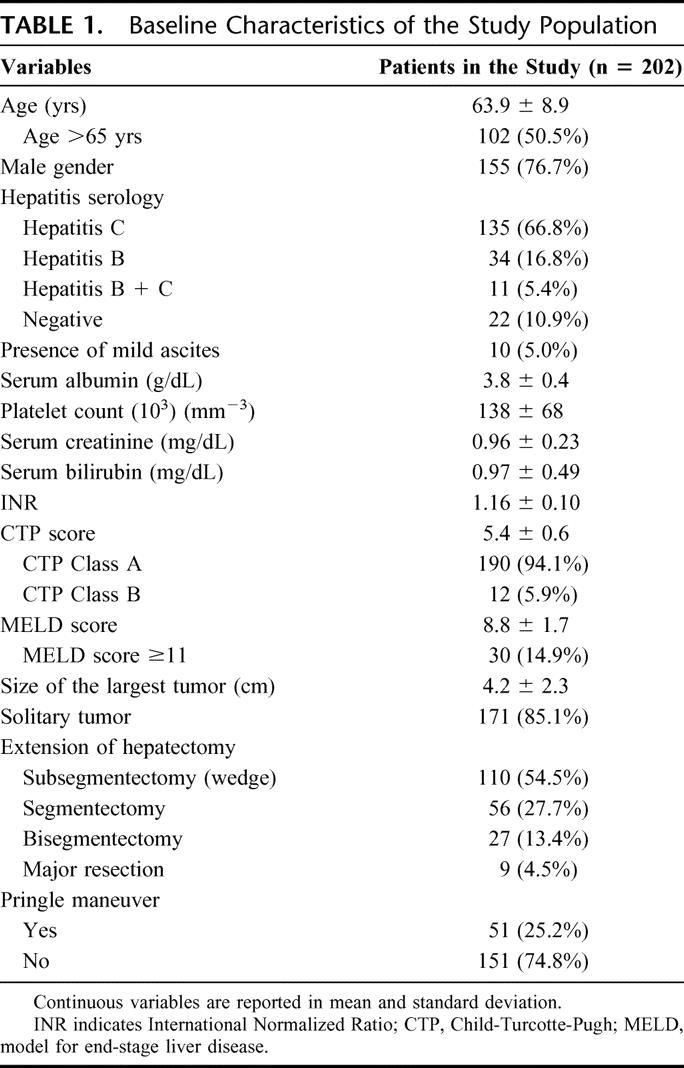
TABLE 2. Intraoperative Parameters of the Study Population
Sixty-one patients (30.2%) experienced at least 1 postoperative complication. Refractory ascites developed in 55 cases (27.2%), increase of bilirubin levels above 3 mg/dL was observed in 35 cases (17.3%), severe alteration of coagulation factors in 41 cases (20.3%), and renal impairment in 15 cases (7.4%).
Acid-Base Balance Analysis Before and After Hepatectomy
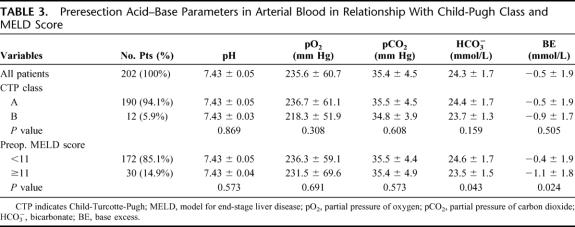
Acid-base parameters before resection are reported in Table 3. No differences were observed between CTP class A patients and class B; conversely, patients with preoperative MELD score ≥11 showed significant lower bicarbonate (P = 0.043) and BE levels (P = 0.024) compared with patients with lower MELD scores; pH, po2, and pco2 were similar. Acid-base parameters were unaffected by age, sex, and etiology of liver disease (data not shown).
TABLE 3. Preresection Acid–Base Parameters in Arterial Blood in Relationship With Child-Pugh Class and MELD Score
Considering the entire study population, a significant reduction in pH, bicarbonate, and BE levels was observed after hepatectomy: pH decreased from a mean preresection value of 7.43 ± 0.05 to a mean postresection value of 7.39 ± 0.05 (P < 0.001), bicarbonate decreased from 24.3 ± 1.7 to 22.5 ± 1.9 mmol/L (P < 0.001), and BE from −0.5 ± 1.9 to −2.5 ± 2.3 mmol/L (P < 0.001); po2 and pco2 remained similar (P = 0.236 and 0.165, respectively). Correction of acidosis was adopted in 29 patients (14.4%): these patients had a mean postresection value of pH of 7.34 ± 0.06, bicarbonate of 19.7 ± 1.9 mmol/L, and BE of −5.9 ± 2.5 mmol/L.
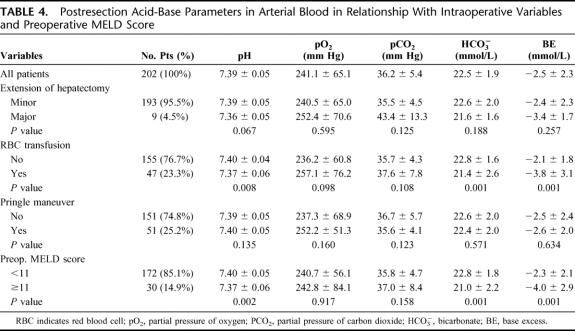
Postresection acid-base parameters (Table 4) were unaffected by the extension of hepatectomy and Pringle maneuver even if a trend toward lower pH, bicarbonate, and BE levels was observed in patients undergoing major hepatectomy. On the contrary, patients who experienced intraoperative blood loss, requiring transfusion, showed significant lower postresection pH (P = 0.008), bicarbonate (P = 0.001), and BE levels (P = 0.001) in comparison with patients who did not require transfusion; in addition, these patients had a significantly higher occurrence of hypotensive episodes (12 of 47; 25.5%) in comparison with patients who did not require transfusion therapy (9 of 155; 5.8%; P < 0.001); po2 and pco2 remained similar. Patients with preoperative MELD score ≥11 had lower postresection pH (P = 0.002), bicarbonate (P = 0.001), and BE levels (P = 0.001) in comparison with patients with lower MELD scores; po2 and pco2 remained similar. A further analysis showed that the decrease in pH, bicarbonate, and BE was more accentuated in patients with higher preoperative MELD score: the difference between preresection and postresection pH (−0.06 ± 0.03), bicarbonate (−2.6 ± 1.4 mmol/L), and BE (−3.0 ± 2.3 mmol/L) was higher than that of patients with lower preoperative MELD scores who showed a difference in pH of −0.03 ± 0.05 (P = 0.003), in bicarbonate levels of −1.7 ± 1.6 mmol/L (P = 0.004) and in BE of −1.8 ± 1.7 mmol/L (P = 0.003). Patients with higher MELD score experienced also a trend toward a higher occurrence of hypotensive episodes (5 of 30; 16.7%) in comparison with patients with lower MELD scores (16 of 172; 9.3%); however, this trend did not reach the statistical significance (P = 0.371).
TABLE 4. Postresection Acid-Base Parameters in Arterial Blood in Relationship With Intraoperative Variables and Preoperative MELD Score
Acid-Base Balance After Hepatectomy and Postoperative Outcome
The relationships of the 3 acid-base parameters modified by hepatectomy, namely pH, bicarbonate levels, and BE, with the development of irreversible liver failure were investigated by the means of ROC curve analysis. Postresection bicarbonate levels showed the highest accuracy in predicting postoperative liver failure with an AUC of 0.897 (95% CI = 0.847–0.935); BE and pH showed the lowest accuracy with an AUC of 0.790 (95% CI = 0.727–0.844) and 0.778 (95% CI = 0.715–0.834), respectively.
Figure 1 reports sensitivity, specificity, positive and negative predictive values (PPV and NPV), and positive and negative likelihood ratio (PLR and NLR) of bicarbonate levels in predicting postoperative liver failure. For bicarbonate levels higher than 22.1 mmol/L the sensitivity and the NPV were both 100%, with a NLR of 0 indicating a high predictive power in identifying patients who will experience an uneventful postoperative course; in other words, all patients who did not experience a decrease of bicarbonate below this threshold did not experience postoperative liver failure. Under this threshold, the probability to develop the event starts to increase, reaching a maximum when bicarbonate levels falls below 19.4 mmol/L with a specificity of 96.8%, a PPV of 50.0%, and a PLR of 12.47, indicating a high predictive power in identifying patients who will experience irreversible liver failure.
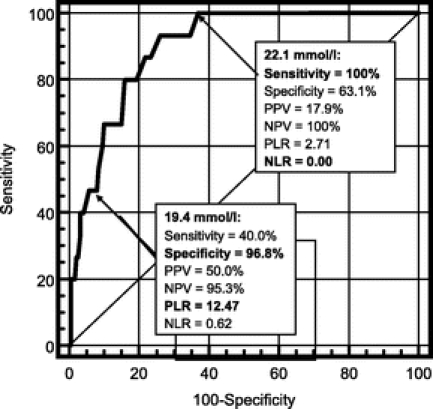
FIGURE 1. Difference in sensitivity, specificity, positive and negative likelihood ratio (PLR and NLR, respectively), positive and negative predictive values (PPV and NPV, respectively), and accuracy of different bicarbonate levels in predicting irreversible postoperative liver failure after hepatectomy in cirrhotic patients.
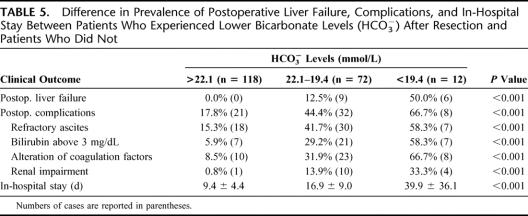
On the basis of these 2 cut-offs obtained with ROC analysis, the study population was divided into 3 groups: patients with bicarbonate levels below 19.4 mmol/L (12 cases; 5.9%), patients with bicarbonate levels between 19.4 and 22.1 mmol/L (72 cases; 35.6%), and patients with bicarbonate levels above 22.1 mmol/L (118 cases; 58.4%).
Table 5 reports the occurrence of postoperative liver failure, complications, and length of in-hospital stay in relationship to the levels of postresection bicarbonate. The prevalence of postoperative liver failure and of complications increase with the decrease of postresection bicarbonate levels (P < 0.001 in all cases); in particular, patients with bicarbonate levels above 22.1 mmol/L did not experience postoperative liver failure whereas the prevalence was very high in patients with postresection bicarbonate lower than 19.4 mmol/L (50%). A longer in-hospital stay was also related with lower postresection bicarbonate levels (P < 0.001).
TABLE 5. Difference in Prevalence of Postoperative Liver Failure, Complications, and In-Hospital Stay Between Patients Who Experienced Lower Bicarbonate Levels (HCO3−) After Resection and Patients Who Did Not
DISCUSSION
Patients with cirrhosis present several clinical and biochemical abnormalities that can potentially perturb acid-base balance: hypocapnic and hypoalbuminemic alkalosis are described in these patients12–15; however, the metabolic defects of cirrhosis could facilitate the development of metabolic acidosis in the course of complication such as sepsis, shock, or bleeding.16 Surgical resection is the treatment of choice for HCC on cirrhosis in selected patients, offering the chance for cure and providing long-term survival2–6; in this setting, acid-base disorders could be caused not only by the underlying liver disease but also by the surgical injury and the intraoperative bleeding.
The present study demonstrated that acid-base balance reflects the severity of the underlying liver disease and that a significant reduction in pH, bicarbonate levels, and BE should be expected during surgery, worsened by intraoperative blood loss. The most relevant result of this study is the relationship observed between postresection acid-base parameters and the postoperative course: postresection levels of bicarbonate lower than 19.4 mmol/L led to a very high incidence of liver failure (50%) and postoperative complications (66.7%), resulting in a significantly longer in-hospital stay. On the contrary, postresection levels of bicarbonate above 22.1 led to an uneventful postoperative course with no liver failure, lower morbidity (17.8%), and shorter in-hospital stay. At present, and to the best of our knowledge, this is the very first report that analyzes the acid-base modification during hepatectomy of cirrhotic patients and its relationship with postoperative course.
An accurate analysis of data showed that both preresection and postresection parameters were modified by the severity of the underlying liver disease. In fact, patients with a preoperative MELD score ≥11 had lower preresection bicarbonate levels and BE in comparison with patients with a lower MELD score, conversely, Child-Pugh class did not correlate. These findings are well in keeping with a recent study by Funk et al who reported a trend toward a reduction of both parameters within the 3 Child-Pugh classes but no significant differences between Child-Pugh class A and B patients12; since our study population was represented by only class A and B patients, no difference was observed but the small proportion of class B patients (5.9%) may have also biased the result. The different relationship of MELD and Child-Pugh score with preresection acid-base parameters should be explained by the fact that the MELD score can more accurately measure hepatic functional reserve in patients undergoing surgery,10,11,22,23 and thus can more accurately partition acid-base parameters into different subgroups.
Patients with a higher MELD score not only had lower preresection bicarbonate and BE but also showed lower postresection levels of both parameters and pH in comparison with patients with a lower MELD score: one could argue that, since these patients started with lower preresection bicarbonate and BE levels, it was more likely that the postresection levels would be lower; however, the differences observed between preresection and postresection values of all these parameters were much larger in patients with higher preoperative MELD scores, reflecting the fact that more severe diseased livers suffer surgical injury to a greater extent. The observation that postresection bicarbonate was related to both preoperative MELD score and postoperative liver failure is well in keeping with a previous report from our center in which preoperative MELD score ≥11 was associated with an increased risk of liver failure.10
During hepatectomy, the observed trend toward acidosis was worsened when blood transfusion was required. Hemorrhage is a well-known factor that leads to metabolic acidosis as a consequence of intracellular derangements in oxygen and substrate utilization. Intraoperative blood loss and transfusion was often associated with morbidity and mortality in the largest series,24 and so it is not surprising that postresection bicarbonate was related to both intraoperative blood transfusion and postoperative course. Patients who required intraoperative blood transfusion had also a higher occurrence of hypotensive episodes that can worse the development of metabolic acidosis; the occurrence of hypotension was increased in patients with more advanced liver disease, resulting in a further worsening of the acid-base balance. All the above situations (advanced liver disease, blood transfusion, and hypotension) are related to each other in a vicious circle that led to the development of metabolic acidosis.
After hepatectomy, a transient impairment of liver function is physiological and leads to the modification of several biochemical variables, such as prolongation of PT and increase of bilirubin levels: this impairment occurs between postoperative day 1 and 3; there is then a significant trend to return to normal values on postoperative day 5 and 7 both in cirrhotic and noncirrhotic patients. The postoperative course of these biochemical variables can help in identifying patients at a higher risk of developing irreversible liver failure. In a recent report by Balzan et al, patients with bilirubin serum levels above 50 μmol/L and PT values lower than 50% at postoperative day 5 (the so-called “50–50” criteria) were at higher risk of liver failure and postoperative death.25 A limit of the assessment of the 50–50 criteria is that one must wait 5 days until those patients at a higher risk of liver failure can be identified, which is often too late to begin intensive treatments. The present study demonstrated that postoperative mortality and morbidity can be predictable by lower postresection bicarbonate levels. The possibility of having a very early index that can accurately predict the postoperative course is of paramount importance for the immediate start of intensive treatment to avoid comorbidities, such as infections or renal impairment, that will almost certainly lead to patient death.3 Alternatively, those patients eligible for liver transplantation may enter a transplantation program.
In conclusion, acid-base parameters are strictly related to hepatic functional reserve and complexity of surgical resection. Postresection bicarbonate levels can be used as a very early indicator to identify patients who will develop postoperative morbidity and mortality, representing the trigger for beginning intensive treatment; alternatively, patients eligible for liver transplantation may enter a transplantation program.
Footnotes
Participating investigator: Matteo Ravaioli MD collected data and critically reviewed the study proposal.
Reprints: Alessandro Cucchetti, MD, Policlinico Sant'Orsola-Malpighi, University of Bologna, Via Massarenti 9, 40138 Bologna, Italy. E-mail: aleqko@libero.it.
REFERENCES
- 1.Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–285. [DOI] [PubMed] [Google Scholar]
- 2.Grazi GL, Ercolani G, Pierangeli F, et al. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg. 2001;234:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takayama T, Makuuchi M, Hirohashi S, et al. Early hepatocellular carcinoma as an entity with a high rate of surgical care. Hepatology. 1998;28:1241–1246. [DOI] [PubMed] [Google Scholar]
- 5.Poon RT, Fan ST, Lo CM, et al. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg. 2002;236:602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belghiti J, Regimbeau JM, Durand F, et al. Resection of hepatocellular carcinoma: a European experience on 328 cases. Hepatogastroenterology. 2002;49:41–46. [PubMed] [Google Scholar]
- 7.Child CG II, Turcotte JG. Surgery and portal hypertension. In: Child CG III, ed. The Liver and Portal Hypertension. Philadelphia, PA: WB Saunders, 1964:50–58. [Google Scholar]
- 8.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. [DOI] [PubMed] [Google Scholar]
- 9.Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. [DOI] [PubMed] [Google Scholar]
- 10.Cucchetti A, Ercolani G, Vivarelli M, et al. Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl. 2006;12:966–971. [DOI] [PubMed] [Google Scholar]
- 11.Teh SH, Christein J, Donohue J, et al. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of End-stage Liver Disease (MELD) score predicts perioperative mortality. J Gastrointestinal Surg. 2005;9:1207–1215. [DOI] [PubMed] [Google Scholar]
- 12.Funk GC, Doberer D, Osterreicher C, et al. Equilibrium of acidifying and alkalinizing metabolic acid-base disorders in cirrhosis. Liver Int. 2005;25:505–512. [DOI] [PubMed] [Google Scholar]
- 13.Oster JR, Perez GO. Acid-base disturbances in liver disease. J Hepatol. 1986;2:299–306. [DOI] [PubMed] [Google Scholar]
- 14.Mulhausen R, Eichenholz A, Blumentals A. Acid-base disturbances in patients with cirrhosis of the liver. Medicine (Baltimore). 1967;46:185–189. [DOI] [PubMed] [Google Scholar]
- 15.Prytz H, Thomsen AC. Acid-base status in liver cirrhosis. Disturbances in stable, terminal and portal-caval shunted patients. Scand J Gastroenterol. 1976;11:249–256. [PubMed] [Google Scholar]
- 16.Kreisberg RA. Lactate homeostasis and lactic acidosis. Ann Intern Med. 1980;92:227–237. [DOI] [PubMed] [Google Scholar]
- 17.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. [DOI] [PubMed] [Google Scholar]
- 18.Melendez JA, Arslan V, Fisher ME, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–625. [DOI] [PubMed] [Google Scholar]
- 19.Freeman RB, Wiesner RH, Harper A, et al. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8:851–858. [DOI] [PubMed] [Google Scholar]
- 20.Committee of the International Hepato-Pancreato-Biliary Association. IHPBA Brisbane. Terminology of liver anatomy and resections. Vol. 2. Abingdon, UK: Taylor & Francis 2000:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- 22.Befeler AS, Palmer DE, Hoffman M, et al. The safety of intra-abdominal surgery in patients with cirrhosis: model for end-stage liver disease score is superior to Child-Turcotte-Pugh classification in predicting outcome. Arch Surg. 2005;140:650–654. [DOI] [PubMed] [Google Scholar]
- 23.Northup PG, Wanamaker RC, Lee VD, et al. Model for End-Stage Liver Disease (MELD) predicts nontransplant surgical mortality in patients with cirrhosis. Ann Surg. 2005;242:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balzan S, Belghiti J, Farges O, et al. The “50–50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828. [DOI] [PMC free article] [PubMed] [Google Scholar]