Abstract
Background:
Experience with minimal access, transoral/transmural endoscopic drainage/debridement of walled-off pancreatic necrosis (WOPN) after necrotizing pancreatitis is limited. We sought to determine outcome using this technique.
Methods:
Retrospective analysis.
Results:
From 1998 to 2006, 53 patients underwent transoral/transmural endoscopic drainage/debridement of sterile (27, 51%) and infected (26, 49%) WOPN. Intervention was performed a median of 49 days (range, 20–300 days) after onset of acute necrotizing pancreatitis. A median of 3 endoscopic procedures/patient (range, 1–12) were performed. Twenty-one patients (40%) required concurrent radiologic-guided catheter drainage of associated or subsequent areas of peripancreatic fluid and/or WOPN. Twelve patients (23%) required open operative intervention a median of 47 days (range, 5–540) after initial endoscopic drainage/debridement, due to persistence of WOPN (n = 3), recurrence of a fluid collection (n = 2), cutaneous fistula formation (n = 2), or technical failure, persistence of pancreatic pain, colonic obstruction, perforation, and flank abscess (n = 1 each). Final outcome after initial endoscopic intervention (median, 178 days) revealed successful endoscopic therapy in 43 (81%) and persistence of WOPN in 10 (19%). Preexistent diabetes mellitus, size of WOPN, and extension of WOPN into paracolic gutter were significant predictive factors for need of subsequent open operative therapy.
Conclusions:
Successful resolution of symptomatic, sterile, and infected WOPN can be achieved using a minimal access endoscopic approach. Adjuvant percutaneous drainage is necessary in up to 40% of patients, especially when WOPN extends to paracolic gutters or pelvis. Operative intervention for failed endoscopic treatment is required in about 20% of patients.
Experience with minimal access, transoral/transmural endoscopic drainage/debridement of walled-off pancreatic necrosis (WOPN) after necrotizing pancreatitis is limited. Using this technique, 53 patients were treated; 40% required percutaneous catheter placement. Nonsurgical resolution was achieved in 43 (81%). Preexistent diabetes mellitus, size of WOPN, and extension into paracolic gutter were predictive of need for surgery.
Management and subsequent clinical outcomes of patients with necrotizing pancreatitis have changed markedly over the last 3 decades. Improvements in critical care management and nutrition, combined with a better understanding of the pathogenesis of the local and systemic complications of the necrotizing disease process, have led to major changes in the accepted treatment paradigms. Representative changes have evolved from simple peripancreatic drainage to pancreatic and peripancreatic necrosectomy, from early operative treatment to a more delayed operative intervention, from operative to nonoperative management of sterile necrosis, and most recently from open operative exploration for drainage/necrosectomy to minimal access focused approaches to drainage/necrosectomy.
Increasing interest and experience have centered on alternative, minimal access techniques of gaining access to relatively well-circumscribed areas of necrosis,1 recently referred to as walled-off pancreatic necrosis (WOPN). The term “walled-off necrosis” was introduced to us at the 2006 Digestive Disease Week during the AGA Clinical Symposium, “Problems and Pitfalls of Atlanta Classification for acute pancreatitis: AGA, APA and IAP to revisit,” chaired by Dr. Peter Banks. These techniques have included percutaneous, large-bore catheters placed via interventional radiology,2–4 percutaneous laparoscopic necrosectomy,5–8 and small incision, focused operative necrosectomy.9 The common principle among all these techniques is direct but limited access to the area of necrosis to allow drainage and some form of debridement to produce a functional necrosectomy by a minimal access approach. Peroral, endoscopic, transgastric, or transduodenal (transmural) access offers another minimal access approach for accessing the area of necrosis for drainage/debridement in selected patients, similar in principle to endoscopic drainage of pancreatic pseudocysts,10 but allowing a more aggressive technique of debridement to effect a functional necrosectomy.11–13 This approach is in some ways similar to the concept of Natural Orifice Transluminal Endoscopic Surgery.14
We have had an active interest in this minimal access, endoscopic-based intervention since first reported in 1996.15 The aim of this comprehensive report is to determine the indications, complications, and success of this approach for the management of symptomatic or infected WOPN in patients after severe necrotizing pancreatitis.
METHODS
All patients undergoing minimal access, endoscopic therapy for symptomatic or infected WOPN at the Mayo Medical Centers in Rochester, MN and Scottsdale, AZ between January 1, 1998 to May 1, 2006 were identified by searching our prospective, computerized, endoscopic databases. None of these patients has been included in prior reports of endoscopic drainage by these authors.16 The patient population consisted of patients with recent necrotizing pancreatitis referred for minimal access, endoscopic management of WOPN by all medical subspecialties, including gastroenterologists, surgeons, and hospitalists. Clinical demographics, etiology, and clinical course of acute pancreatitis, imaging studies, endoscopic, radiologic, and operative drainage procedures, complications, and long-term follow-up of these patients were determined by comprehensive review of patient medical records. A consensus of experienced physicians, including an abdominal radiologist (N.T.), a gastrointestinal surgeon (M.G.S.), and advanced endoscopists (G.I.P., T.H.B., P.C.), reviewed the medical records and all imaging studies, including computed tomography (CT) and/or magnetic resonance imaging (MRI), and agreed collaboratively that all patients truly represented cases of WOPN and not pancreatic pseudocysts. All patients had fairly well-circumscribed, pancreatic and/or peripancreatic collections of fluid and necrotic material after a well-defined episode of severe necrotizing pancreatitis.
The interval between formation of WOPN and referral for drainage/debridement varied widely; these patients were managed nonoperatively at the time of the onset of necrotizing pancreatitis until it was thought necessary to intervene by some interventional approach by the treating physician. All patients were otherwise candidates for operative necrosectomy. These patients remained systemically unwell after their initial attack of necrotizing pancreatitis and had nonresolving areas of WOPN documented by serial imaging studies. All patients had a dynamic IV and oral contrast enhanced abdominal computed tomography (CECT) and/or an abdominal MRI that documented the presence and extent of pancreatic necrosis as well as the number, size, and location of pancreatic/peripancreatic fluid collections as has been described.17 These characteristics were used to determine the appropriateness, accessibility, and method of minimal access endoscopic drainage/debridement.
Pancreatic necrosis was identified based on either CECT or MRI and clinical criteria as defined by the Atlanta International Symposium, as well as the endoscopic findings at the time of drainage/debridement.18,19 APACHE II (Acute Physiology and Chronic Health Evaluation) scores at the time of intervention at our institution were calculated.20 Specific indications for therapy were “persistent pancreatitis,” as defined by Rattner et al,21 or “persistent unwellness” manifesting as abdominal pain requiring daily narcotics, inability to eat, or failure to thrive. A clinical picture consistent with infected necrosis included fever, leukocytosis despite treatment with antibiotics, and/or positive blood cultures or cultures from the WOPN.
In addition, an experienced abdominal radiologist (N.T.) reviewed blindly all available radiographic images that were obtained prior to endoscopic drainage/debridement. This blinded review was based exclusively on the radiologic features. The features of the pancreatic/peripancreatic fluid collections included size, location, content (presence of air, debris, and septations), involvement of the pancreatic parenchyma, discontinuity and deformity of the pancreatic parenchyma, and extension to the paracolic gutters and pelvis.
Procedures
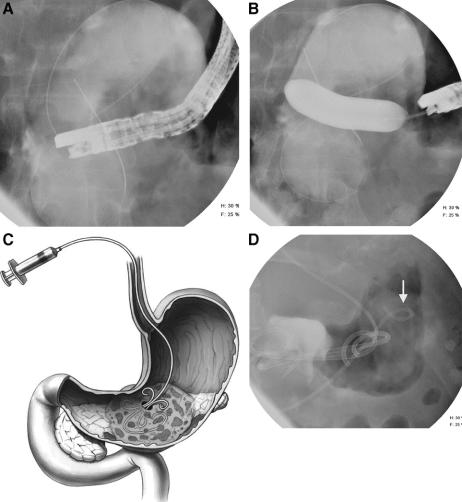
Under conscious sedation, endoscopy was performed with a therapeutic, side viewing video duodenoscope (TJF160; Olympus America, Inc., Melville, NY). Extrinsic compression of the gastric or duodenal lumen by the WOPN was determined endoscopically. A pancreatogram was performed when possible at the index endoscopy either before or after drainage to assess integrity of the pancreatic duct. A transmural puncture was performed as follows. The posterior gastric wall or the medial duodenal wall was punctured at the site of extrinsic compression as determined radiologically and endoscopically. Entry into the collection was achieved by several methods. Non EUS-guided drainage was used in the majority of patients. For non–EUS-guided drainage, either needle-knife electrocautery or needle aspiration was used as described previously.22,23 Aspiration of contents and/or injection of contrast allowed confirmation of entry into the collection. Material aspirated was sent for gram stain, culture, and amylase activity. When a “dry” aspiration was obtained, a water-soluble radiographic contrast was injected to confirm position. A 0.035" guidewire was then advanced through the needle, coiled within the collection (Fig. 1A), and the puncture tract dilated with a wire-guided hydrostatic balloon via direct endoscopic and fluoroscopic guidance until the waist of the balloon was obliterated (Fig. 1B). The color and consistency of the liquid and solid necrotic material of the contents were usually recorded. One or usually two, 10-Fr, double-pigtail stents were placed into the collection (Cook Endoscopy, Winston-Salem, NC). A 7-Fr, pigtail nasobiliary tube (Cook Endoscopy) was positioned alongside the pigtail stents into the necrotic collection to perform aggressive irrigation/debridement postprocedurally (Fig. 1C, D). Initially, this nasocystic tube was lavaged with 50 to 200 mL of 0.9% NaCl every 2 hours for the first 2 days and then every 4 to 6 hours for the ensuing 4 to 6 weeks if necessary.
FIGURE 1. Endoscopic transoral/transmural drainage of WOPN. A, A guidewire is advanced and coiled within the necrotic collection. B, Puncture tract is dilated with an 18-mm wire-guided hydrostatic balloon via direct endoscopic and fluoroscopic guidance until waist was obliterated. C, Illustration of 7-Fr, pigtail nasobiliary tube positioned alongside transmurally placed internal pigtail stents into the WOPN to perform aggressive irrigation/debridement. D, Distal tip of nasobiliary tube (arrow) is crossing the spine to area of WOPN closer to the spleen.
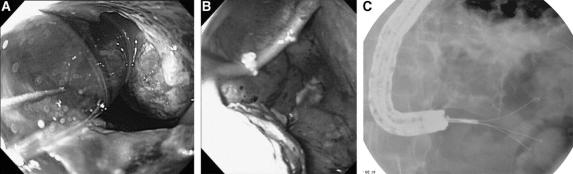
The endoscopic therapy (drainage/debridement/necrosectomy) has evolved. In the early phase of this series (1998–2001), the transmural tract was dilated with an 8-mm balloon, and larger diameter balloons (up to 20 mm) were used subsequently to dilate the tract either at the initial procedure or at the follow-up endoscopic procedures. In the middle phase of this series (2001–2004), endoscopic debridement was performed by passing extraction balloons and/or baskets into the collection through the transmural entry site. In the most recent period (2004–2006), the transmural tract was dilated (Fig. 2A), and a therapeutic upper endoscope was advanced through the gastric or duodenal wall into the collection (Fig. 2B) to allow direct endoscopic debridement/necrosectomy, as has been described recently.11 Debridement was performed under direct endoscopic vision by entering into the necrotic cavity with a standard or therapeutic channel, forward-viewing gastroscope (Olympus Corporation, Melville, NY).
FIGURE 2. Endoscopic debridement/necrosectomy of WOPN. A, Dilatation of transmural tract; necrotic debris within WOPN can be seen. B, Upper endoscope advanced through gastric wall into WOPN to allow direct endoscopic debridement/necrosectomy. C, Devitalized pancreatic tissue removed with a tripod grasper.
Devitalized pancreatic tissue was removed with the combination of several different accessories, including 15-mm biliary stone retrieval balloons (BARD, Billerica, MA), Roth retrieval net baskets (US Endoscopy, Mentor, OH), lithotripsy stone retrieval baskets (Olympus), tripod retrieval forceps, rat-toothed and pelican forceps (Olympus), and 10-Fr irrigation probes (Gold Probe, Boston Scientific Corporation, Natick, MA) (Fig. 2C).
In some patients, a percutaneous endoscopic gastrostomy (PEG) tube was placed with a “jejunal” extension tube advanced through the PEG tube into the collection to allow for irrigation to avoid the need for a nasopancreatic tube for irrigation (Fig. 3). 24
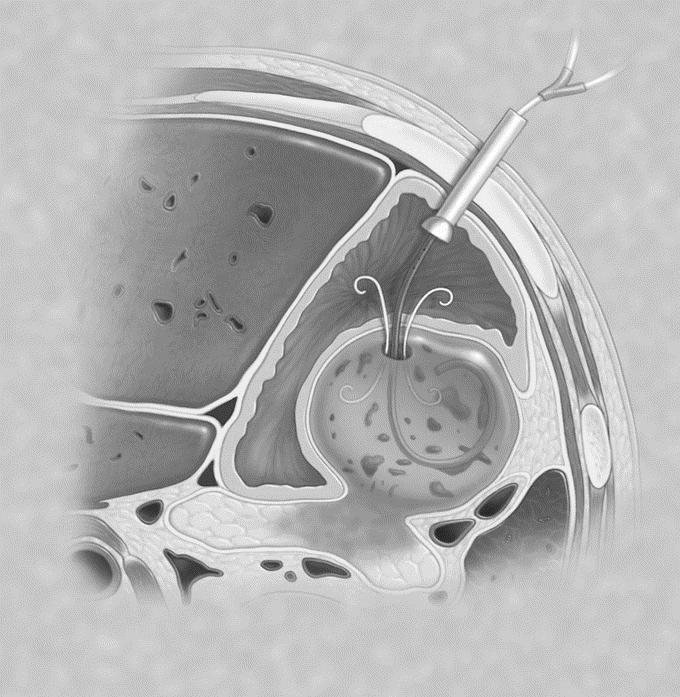
FIGURE 3. PEG tube with jejunal extension tube placed through posterior gastric wall into the WOPN to provide irrigation.
In some patients, transpapillary drainage through the pancreatic duct was performed in combination with the endoscopic transgastric or transduodenal drainage/debridement. When disruption of the pancreatic duct with leak was evident, a pancreatic duct stent was placed using standard endoscopic techniques25; the proximal tip of the pancreatic duct stent was advanced either well into the collection or bridged the site of pancreatic duct disruption. When other peripancreatic collections expanded widely to paracolic gutters, adjuvant placement of percutaneous catheters via an interventional radiologic approach was performed.
All patients received intravenous antibiotics prior to and after the procedure. When patients resumed oral intake, oral antibiotics were administered and continued until documented resolution of the WOPN. Serial abdominal CTs were obtained at 1- to 2-week intervals until resolution was established (Fig. 4). Routine endoscopic removal of stents was performed within 4 weeks of documented resolution of the collection. Endoscopic reinterventions were performed usually 2 to 4 weeks after the first procedure based on radiologic and clinical response.
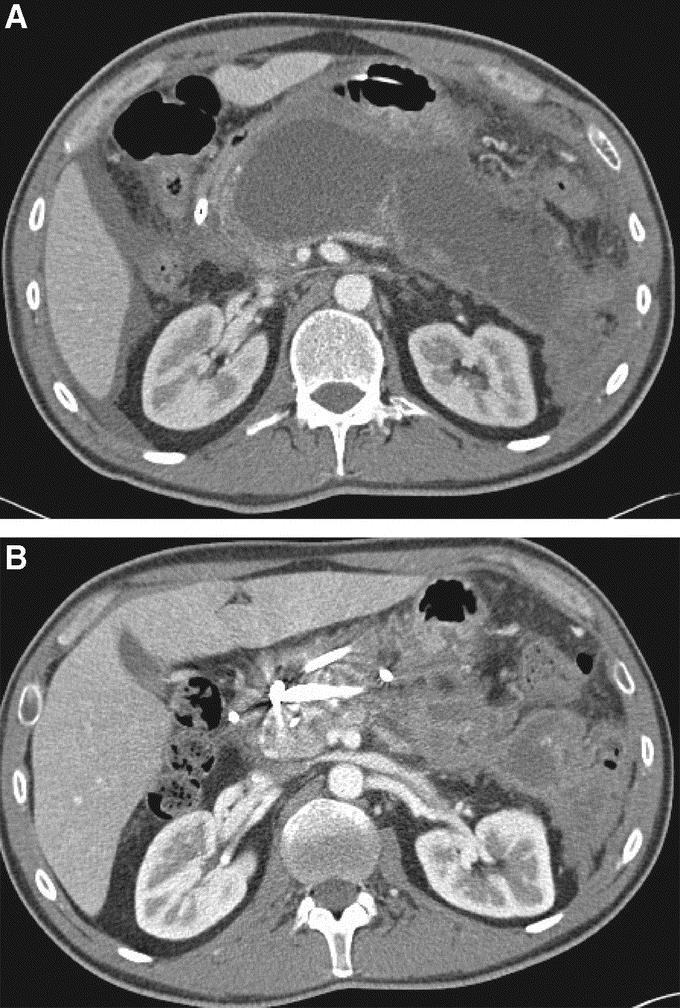
FIGURE 4. Serial abdominal CT in patient who underwent successful endoscopic drainage/debridement of WOPN. A, CT obtained 6 weeks later and prior to endoscopic drainage/debridement showing WOPN. B, CT after endoscopic drainage/debridement with presence of internal stents and significant reduction in size of WOPN.
Overall endoscopic success was defined as complete or almost complete resolution of the collection by abdominal CT in conjunction with resolution of clinical symptoms. Recurrence was defined as the same type of collection in the same location or a new collection in a different location documented by recurrent symptoms and abdominal imaging studies. Complications of endoscopic therapy were defined similarly as those for endoscopic retrograde cholangiopancreatography.26 Additionally, complications such as stent migration were included.
Comparison Between Patients Who Did and Did Not Require Operative Intervention
We aimed to identify factors that were predictive of the need for operative intervention in patients undergoing endoscopic drainage/debridement of WOPN. Clinical factors assessed included patient age, sex, and history of diabetes mellitus as comorbidity present prior to the diagnosis of acute pancreatitis. With regard to the initial episode of necrotizing pancreatitis, clinical factors evaluated were etiology of the pancreatitis (biliary vs. other), severity of episode, and duration of hospital stay. With regard to the characteristics of WOPN, presence of infection versus symptomatic sterile WOPN, APACHE II score at the time of the procedure, and timing of endoscopic drainage after onset of necrotizing pancreatitis were analyzed. With regard to radiologic features, size, location (predominantly pancreatic body vs. other), contents of WOPN, discontinuity of the pancreatic parenchyma, and extension of the collection into the paracolic gutter were compared. Endoscopic procedural factors that were analyzed included entry approach (transgastric vs.transduodenal), size of maximal balloon dilation of the tract during the initial endoscopic procedure, placement of PEG tube, culture positivity from the aspirate or by CT-guided fine needle aspiration obtained prior to the endoscopic procedure, and procedure-related complications. Finally, the total number of endoscopic procedures required and the need for concurrent radiologic drainage were assessed. Bacterial cultures taken at the time of endoscopic aspiration were only considered positive if there were no organisms considered to be oral contaminants.
Outcomes of patients were also compared specifically for transgastric versus transduodenal approach. In these patients, variables assessed included procedure-related complications, number of endoscopic procedures required, need for concurrent radiologic drainage, final outcome of endoscopic treatment (resolution, improvement or persistence of WOPN), and time for the WOPN to resolve.
Statistical Comparisons
Statistical comparisons for binary variables were performed using Pearson χ2 test. For continuous data, the rank sum test was used. Because the number of patients requiring subsequent operative intervention was small, we elected not to perform multivariate logistic regression analysis. A P value <0.05 was considered significant. The statistical analyses were performed using STATA 9.0 (StataCorp., College Station, TX).
RESULTS
A total of 53 consecutive patients (28 men, 25 women) with a median age of 61 years (range, 12–79 years) underwent attempted endoscopic drainage/debridement of WOPN. Of the 53 patients with WOPN, significant comorbidities included diabetes mellitus (27%), coronary artery disease (21%), history of cancer (13%), immunosuppression (11%), chronic obstructive pulmonary disease (11%), and chronic renal insufficiency (6%).
During this time frame, a total of 145 pancreatic/peripancreatic fluid collections were drained endoscopically at our institution (86 pseudocysts, 6 abscesses, and the 53 patients described in this series).
Etiologies of WOPN included biliary (70%), idiopathic (11%), post-ERCP (6%), medications (5%), postoperative (4%), and alcohol and pancreatic mass (2% each). The initial clinical course of necrotizing pancreatitis was “clinically persistent” in 28 patients (53%), severe requiring intensive care management in 34% (n = 18), and recurrent (defined as requiring readmission after previous discharge) in 6% (n = 3). Patients with “persistent pancreatitis” did not require prolonged intensive care management but had a prolonged illness without symptomatic improvement and could be referred to as “persistently unwell.” Recurrent pancreatitis was defined as discrete episodes of symptoms suggestive of acute pancreatitis requiring hospitalization. The median hospital stay during the initial attack of necrotizing pancreatitis was 15 days (range 0–240 days). Eighteen patients (34%) received prophylactic antibiotics in the early phases of pancreatitis. Endoscopic drainage/debridement of the WOPN was performed at a median of 49 days (range, 20–300 days) after the onset of necrotizing pancreatitis. Two patients (4%) had evidence of chronic pancreatitis on imaging studies.
The location of the WOPN involved the areas of pancreatic head in 4 patients (8%), body in 27 (51%), tail in 5 (9%), and body/tail of the pancreas in 17 (32%). The median maximal diameter of the WOPN was 16 cm (range, 3–46 cm).
Twenty-seven patients had symptomatic, clinically sterile WOPN (51%), while the remaining 26 patients had infected WOPN. The presenting symptoms at the time of endoscopic drainage were pain (68%), nausea and vomiting (55%), fever (30%), and weight loss (8%). The median APACHE II score on the day of endoscopic drainage was 6 (range, 0–22).
Drainage was performed transgastrically in 32 patients (60%), transduodenally in 37% (n = 19), transpapillary in one patient, and combined transgastric/transduodenal in 1 patient. Needle entry into the WOPN without electrocautery was used in 48 patients (91%); in 5 other patients, electrocautery using a needle-knife or fistulotome was used. In 42 patients (79%), the collection was entered with the first puncture, while 15% (n = 8) required a second puncture. In all patients, entry into the collection was achieved. In 1 patient, access was achieved via EUS-guidance after 4 unsuccessful punctures. In another, entry was achieved initially, but access was lost while attempting to balloon-dilate the tract; the procedure was aborted without placement of endoprostheses.
The material aspirated was described as brown in 25%, purulent in 15%, and turbid in 8%. Bacterial culture of the aspirate, performed in 41 patients, was positive in 20 (49%). A variety of microorganisms were identified, including primarily Enterococcus, Pseudomonas, and Klebsiella species.
The median maximal balloon dilation of the puncture site was 15 mm (range, 8–20 mm). Two 10-Fr, double pigtail plastic stents were placed in 40 patients (75%), and a single double pigtail stent was placed in 12 patients. The length of the double pigtail stents ranged from 3 to 7 cm; the majority (74%) were 5 cm. All patients had some form of irrigation catheter within the area of WOPN. A 7-Fr, nasopancreatic plastic tube was placed in 37 patients (70%), PEG placement with a “jejunal” extension tube within the collection was performed in 10 patients (19%), and 6 patients had radiologically placed percutaneous drains before endoscopic drainage/debridement that were used after endoscopic drainage/debridement for irrigation. Irrigation was performed at an average of 4 times a day. The irrigation catheter was removed at a median of 31 days (range, 9–100 days).
The median duration of the procedure was 84 minutes (range, 35–179 minutes). Endoscopic treatment was performed by 4 endoscopists, one of whom (T.H.B.) managed the majority (n = 42; 79%) of patients. EUS was used concomitantly with endoscopic drainage/debridement in 8 patients (15%). EUS was used primarily to characterize the WOPN, but on occasion to assess the presence of septations and debris and the presence of major intervening blood vessels, and to identify the distance between the gut lumen and the WOPN. The WOPN was aspirated for diagnostic purposes during EUS in 1 patient. EUS was used routinely to select and mark the optimal drainage site in all the patients (n = 3) performed by 1 endoscopist.
Successful pancreatograms were performed in 25 patients (48%) either before or after endoscopic drainage/debridement (at the time of transmural stent removal). Communication of the area of WOPN with the main pancreatic duct was demonstrated in 23 of these 25 patients.
Complications related directly to the procedure occurred in 11 patients (21%). Bleeding from the site of endoscopic drainage/debridement occurred in 9 patients; this bleeding was controlled endoscopically at the time of procedure in 4 patients, required repeat endoscopy between days 2 and 9 in 3 patients, and required blood transfusions and management in the intensive care unit in 2 patients. None of these patients with bleeding required operative intervention because of uncontrollable hemorrhage. Gallbladder puncture occurred in 1 patient while attempting transgastric puncture into the area of WOPN, which required bile duct stenting. Finally, loss of access to the collection, associated with hypotension, occurred in 1 patient. Technical complications included 2 cases of pigtail stent migration and a case of buried PEG bumper. The median hospital stay for all patients undergoing attempted endoscopic drainage of WOPN was 13 days (range, 0–90 days).
Two patients were treated solely as outpatients, had undergone placement of a nasojejunal tube because of inability to eat secondary to the WOPN, were medically quite sophisticated, and managed their care independently.
The median number of endoscopic sessions for each patient was 3 (range, 1–12). The second endoscopic drainage/debridement was performed at a median of 18 days (range, 2–86) after the initial drainage, and the third endoscopic procedure at 33 days (range, 9–138). Endoscopic necrosectomy of the WOPN cavity was performed in 22 of the 40subsequent endoscopic interventions (55%). The time of endoscopic therapy, defined as the time from the initial endoscopic drainage/debridement until the time when the last pigtail or pancreatic duct stent was removed, was 64 days (range, 9–270 days).
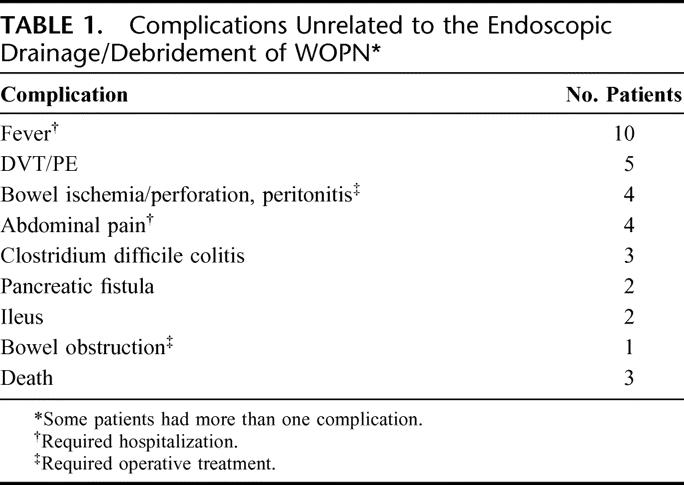
After attempted endoscopic drainage/debridement, clinical follow-up was obtained in all patients to determine the course and evolution of WOPN and the need for additional endoscopic procedures. The mean follow-up was 178 days (range, 21 days to 8 years). Complications unrelated to the endoscopic procedure occurred in 26 patients (49%) (Table 1). There were 3 deaths (6%). One patient died of unrelated cardiorespiratory arrest in the hospital 72 days after endoscopic treatment despite resolution of the area of WOPN. The second (74-year-old) never recovered despite endoscopic drainage/debridement, developed failure to thrive, comfort care measures were applied, and died finally at home on day 100. The third underwent emergent operative intervention for sigmoid perforation due to his pancreatitis unrelated to the endoscopic procedure and died 3 weeks later of multiorgan failure.
TABLE 1. Complications Unrelated to the Endoscopic Drainage/Debridement of WOPN
Twenty-one patients (40%) required concurrent radiologic placement of percutaneous drainage catheters for associated or subsequent peripancreatic fluid collections and/or nonresolution of WOPN. Overall, successful endoscopic drainage/debridement was achieved in 43 patients (81%). Imaging modalities documented complete resolution or marked improvement in the area of WOPN in 23 and 20 patients, respectively. The median time for WOPN resolution from the time of first endoscopic intervention was 81 days (range, 30–550 days). Persistence of the WOPN occurred in 10 patients (19%).
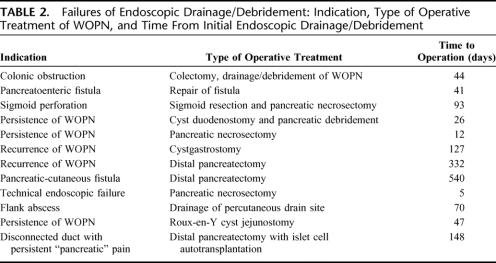
Twelve patients (23%) required operative intervention at a median of 47 days (range, 5–540 days) after initial endoscopic drainage/debridement. Need for operative intervention was required for failure of the endoscopic procedure in 9 patients with persistence of symptomatic WOPN (n = 3), recurrence of symptomatic pancreatic/peripancreatic fluid collections (n = 2), pancreatocutaneous fistula (n = 2), and technical inability to obtain endoscopic drainage/debridement and disconnected duct syndrome with persistence of pancreatic pain (n = 1 each). In 2 others, operative intervention was required for problems unrelated to the endoscopic procedure, ie, for colonic obstruction and for sigmoid colon perforation (n = 1 each) despite successful drainage/debridement of the WOPN. In 1 patient, operative intervention was required for a flank abscess associated with the radiologic placement of a percutaneous drain (Table 2).
TABLE 2. Failures of Endoscopic Drainage/Debridement: Indication, Type of Operative Treatment of WOPN, and Time From Initial Endoscopic Drainage/Debridement
In summary, endoscopic debridement alone was performed in 28 patients (53%), endoscopy and concurrent radiologic placement of percutaneous drainage catheters in 13 patients (25%), endoscopy and surgery in 5 patients (9%), and endoscopy, interventional radiology placement, and operative intervention in 7 patients (13%).
Of interest, during the same period of time, 54 patients with necrotizing pancreatitis were primarily managed with an open necrosectomy at our institution. It is also important to mention that the numbers of surgeries as the primary intervention for WOPN have been trending downwards (9 open necrosectomies in 1998, 10 in 1999, 8 in 2000, 12 in 2001, 10 in 2002, but only 2 in 2003, 3 in 2004, and 0 in 2005).
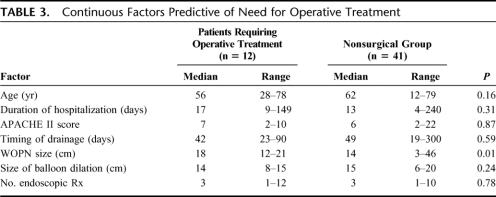
History of diabetes mellitus before the onset of pancreatitis (P = 0.035; OR 4.1; CI 1.0–19.9), size of WOPN (P = 0.01), and extension of the WOPN or other collections to one or both paracolic gutters (P = 0.003; OR 8.5; CI 1.4–52.2) were the only 3 factors predictive of the need for future operative intervention. The rest of the factors assessed were similar between the patients requiring operative intervention and those who did not (P ≥ 0.15 each) (Table 3) and included patient age, sex, etiology of necrotizing pancreatitis, severity, duration of hospital stay, infected WOPN versus symptomatic sterile WOPN, APACHE II score, timing of endoscopic intervention after onset of necrotizing pancreatitis, WOPN location, discontinuity of the pancreatic parenchyma, transgastric versus transduodenal approach, size of maximal balloon dilation of the tract, placement of PEG tube, culture positivity, procedure-related complications, total number of endoscopic interventions, and concurrent radiologic drainage.
TABLE 3. Continuous Factors Predictive of Need for Operative Treatment
The transduodenal approach appeared to be associated with an increased need for concomitant radiologic drainage (P = 0.02; OR 4.1; CI 1.1–16.5). The other factors assessed were similar between the transgastric and transduodenal groups, including procedure-related complications, number of endoscopic procedures, final outcome of endoscopic treatment, and time for the WOPN to resolve (P ≥ 0.35 each).
DISCUSSION
Many series now demonstrate that transoral/transmural endoscopic drainage of pancreatic pseudocysts is safe and effective using either a transpapillary or transmural approach.23 Because pseudocysts are composed of liquid, small-caliber stents usually allow for effective drainage. Endoscopic treatment of pancreatic necrosis differs from that of pseudocysts because of the need to evacuate solid debris. This concept applies to any intervention for necrosis, be it operative, percutaneous, or endoscopic. Thus, for endoscopic treatment of WOPN, large transmural tracts and adjuvant irrigation/debridement are required; a transpapillary approach to these collections of liquefied necrosis and solid components is usually not feasible.
Minimal access, transoral/transmural endoscopic therapy for pancreatic necrosis using the techniques described in this manuscript cannot be performed until the necrosis has become organized and walled off from the surrounding tissues. Based on our experience, this process evolving to WOPN generally requires at least 3 weeks from the onset of the acute necrotizing pancreatitis. Additionally, endoscopic therapy is best for treating central necrosis involving primarily the area of the lesser sac because this area is most accessible endoscopically, either through the stomach or duodenum.
The endoscopic methodology for evacuating the fluid and the necrosis from WOPN has evolved with our increasing experience. Initially, smaller transmural tracts were made using 8-mm balloons, similar to the approach for transmural drainage of pancreatic pseudocysts.15 Unfortunately, this approach did not allow for adequate evacuation of necrotic material from within the collection; this debris flows out of the collection around, and not through the internal stents and into the stomach or duodenum. Our initial experience evolved to a more aggressive performance of larger transmural tracts and finally by active interventional endoscopic techniques of debridement/necrosectomy.
Whether these advancements translate into improved endoscopic success rates remains unclear, although in a previous study using the less aggressive debridement techniques in a group of patients treated by the same endoscopist (T.H.B.), the success rate was 31 of 43 (72%), lower than in the current series.16
Several factors may be predictive of successful endoscopic therapy. As one might expect, extension of the necrosis into the paracolic gutters is predictive for failure of endoscopic therapy because these areas, although usually in continuity with central necrosis, are remote and possibly not contiguous from the transgastric or transduodenal entry areas. We think that selected patients should not be excluded from consideration of endoscopic therapy, but rather that an aggressive percutaneous therapy should be considered as adjuvant therapy concomitantly with endoscopic therapy as a complementary approach. The best results with percutaneous therapy appear to be achieved when aggressive upsizing of drains to large calibers (30-Fr) with aggressive, frequent irrigation and active debridement methods, such as basket extraction, are used. An alternative might be to use either a percutaneous laparoscopic/nephroscopic approach7 or a small incision/focused necrosectomy.9
Size of the collection was also predictive of need for operative intervention. This finding may be indicative of more extensive underlying necrotic debris, more severe ductal disruption, or both. Of the 9 patients who required operative intervention for a nonresolving WOPN, all failures occurred in patients with a WOPN >15 cm, although we were also able to achieve complete endoscopic resolution in 11 of 17 patients whose collections were ≥15 cm in diameter. The third factor we found to be predictive of endoscopic failure was the presence of underlying diabetes mellitus as a comorbid medical illness. This association is difficult to explain but may relate to poor wound healing or other as yet undefined factors. Thus, we think that a size of the WOPN of >15 cm in the presence of paracolic gutter involvement may be patients that are best treated either by operative necrosectomy or by early, aggressive adjuvant percutaneous drain placement into the paracolic gutters in conjunction with endoscopic debridement/necrosectomy. In the very least, counseling patients about expected outcomes is mandatory.
Advantages of a minimal access, transoral/transmural endoscopic approach are the avoidance of the morbidity and convalescence of open necrosectomy, including the development of abdominal wall hernias and external fistulae.27 Endoscopic therapy can also be used in poor operative candidates. Disadvantages, however, include the limited ability to evacuate large areas of less well-liquefied necrotic debris and the need for repeat procedures. Additionally, endoscopic intervention cannot be used “early” in the necrotizing process; however, early necrosectomy is being performed less commonly with the advent of improved medical therapy in patients with pancreatic necrosis. Finally, it is possible that the outcome after operative intervention for patients who have failed endoscopic therapy for WOPN is worse than those who underwent operative intervention as initial therapy, as has been suggested by others for pancreatic pseudocysts.28
Our results show that successful endoscopic therapy for WOPN can be achieved in about 80% of patients in whom therapy is attempted. These results are likely not generalizable to nonexperienced endoscopists, similar to the results of operative and percutaneous therapies, where better outcomes have been reported from tertiary medical centers. It needs to be acknowledged that patients with WOPN have greater complication rates and lower success rates after endoscopic therapy than those with pancreatic pseudocysts and the need for the multidisciplinary availability of expert surgeons and interventional endoscopists and radiologists.
The exact role of minimal access, transoral/transmural drainage/debridement in the management of patients with necrotizing pancreatitis compared with operative and percutaneous methods remains uncertain. Comparative trials between disciplines are lacking and unlikely to be performed because of need for expertise in the areas of operative necrosectomy, interventional radiology, and endoscopy as well as the sample size required to find a significant difference. Additionally, some patients may not be candidates for each therapy based on location of the WOPN and patient health status.
We think that the future of endoscopic management of WOPN after necrotizing pancreatitis depends on the development of better endoscopic debridement tools and techniques for this minimal access approach. As with operative techniques for these patients, less invasive strategies are being developed for these patients as well.
Footnotes
Presented at the combined American Pancreatic Association/International Pancreatic Association meeting, Chicago, November 2, 2006.
Reprints: Todd H. Baron, MD, 200 First Street SW, Charlton 8A, Rochester, MN 55905. E-mail: baron.todd@mayo.edu.
REFERENCES
- 1.Connor S, Raraty MG, Howes N, et al. Surgery in the treatment of acute pancreatitis: minimal access pancreatic necrosectomy. Scand J Surg. 2005;94:135–142. [DOI] [PubMed] [Google Scholar]
- 2.Zorger N, Hamer OW, Feuerbach S, et al. Percutaneous treatment of a patient with infected necrotizing pancreatitis. Nat Clin Pract Gastroenterol Hepatol. 2005;2:54–57. [DOI] [PubMed] [Google Scholar]
- 3.Cheung MT, Ho CN, Siu KW, et al. Percutaneous drainage and necrosectomy in the management of pancreatic necrosis. Aust NZ J Surg. 2005;75:204–207. [DOI] [PubMed] [Google Scholar]
- 4.Endlicher E, Volk M, Feuerbach S, et al. Long-term follow-up of patients with necrotizing pancreatitis treated by percutaneous necrosectomy. Hepatogastroenterology. 2003;50:2225–2228. [PubMed] [Google Scholar]
- 5.Castellanos G, Pinero A, Serrano A, et al. Translumbar retroperitoneal endoscopy: an alternative in the follow-up and management of drained infected pancreatic necrosis. Arch Surg. 2005;140:952–955. [DOI] [PubMed] [Google Scholar]
- 6.Risse O, Auguste T, Delannoy P, et al. Percutaneous video-assisted necrosectomy for infected pancreatic necrosis. Gastroenterol Clin Biol. 2004;28:868–871. [DOI] [PubMed] [Google Scholar]
- 7.Carter CR, McKay CJ, Imrie CW. Percutaneous necrosectomy and sinus tract endoscopy in the management of infected pancreatic necrosis: an initial experience. Ann Surg. 2000;232:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ammori BJ. Laparoscopic transgastric pancreatic necrosectomy for infected pancreatic necrosis. Surg Endosc. 2002;16:1362. [DOI] [PubMed] [Google Scholar]
- 9.Horvath KD, Kao LS, Wherry KL, et al. A technique for laparoscopic-assisted percutaneous drainage of infected pancreatic necrosis and pancreatic abscess. Surg Endosc. 2001;15:1221–1225. [DOI] [PubMed] [Google Scholar]
- 10.Hookey LC, Debroux S, Delhaye M, et al. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006;63:635–643. [DOI] [PubMed] [Google Scholar]
- 11.Seewald S, Groth S, Omar S, et al. Aggressive endoscopic therapy for pancreatic necrosis and pancreatic abscess: a new safe and effective treatment algorithm (videos). Gastrointest Endosc. 2005;62:92–100. [DOI] [PubMed] [Google Scholar]
- 12.Binek J, Fretz C, Meyenberger C. Endoscopic transgastric debridement and drainage for splenic necrosis following an acute episode in chronic alcoholic pancreatitis. Endoscopy. 2006;38:639–640. [DOI] [PubMed] [Google Scholar]
- 13.Charnley RM, Lochan R, Gray H, et al. Endoscopic necrosectomy as primary therapy in the management of infected pancreatic necrosis. Endoscopy. 2006;38:925–928. [DOI] [PubMed] [Google Scholar]
- 14.Rattner D, Kalloo A. ASGE/SAGES Working Group: ASGE/SAGES Working Group on Natural Orifice Transluminal Endoscopic Surgery, October 2005. Surg Endosc. 2006;20:329–333. [DOI] [PubMed] [Google Scholar]
- 15.Baron TH, Thaggard WG, Morgan DE, et al. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology. 1996;111:755–764. [DOI] [PubMed] [Google Scholar]
- 16.Baron TH, Harewood GC, Morgan DE, et al. Outcome differences after endoscopic drainage of pancreatic necrosis, acute pancreatic pseudocysts, and chronic pancreatic pseudocysts. Gastrointest Endosc. 2002;56:7–17. [DOI] [PubMed] [Google Scholar]
- 17.Morgan DE, Baron TH, Smith JK, et al. Pancreatic fluid collections prior to intervention: evaluation with MR imaging compared with CT and US. Radiology. 1997;203:773–778. [DOI] [PubMed] [Google Scholar]
- 18.Bradley EL. A clinically based classification system for acute pancreatitis: summary of the Atlanta symposium. Arch Surg. 1993;128:586–590. [DOI] [PubMed] [Google Scholar]
- 19.Balthazar EJ, Freeny PC, vanSonnenberg E. Imaging and intervention in acute pancreatitis. Radiology. 1994;193:297–306. [DOI] [PubMed] [Google Scholar]
- 20.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 21.Rattner DW, Legermate DA, Lee MJ, et al. Early surgical debridement of symptomatic pancreatic necrosis is beneficial irrespective of infection. Am J Surg. 1992;163:105–110. [DOI] [PubMed] [Google Scholar]
- 22.Monkemuller KE, Baron TH, Morgan DE. Transmural drainage of pancreatic fluid collections without electrocautery using the Seldinger technique. Gastrointest Endosc. 1998;48:195–200. [DOI] [PubMed] [Google Scholar]
- 23.Baron TH. Endoscopic drainage of pancreatic fluid collections and pancreatic necrosis. Gastrointest Endosc Clin North Am. 2003;13:743–764. [DOI] [PubMed] [Google Scholar]
- 24.Baron TH, Morgan DE. Endoscopic transgastric irrigation tube placement via PEG for debridement of organized pancreatic necrosis. Gastrointest Endosc. 1999;50:574–577. [DOI] [PubMed] [Google Scholar]
- 25.Kozarek RA, Patterson DJ, Ball TJ, et al. Endoscopic placement of pancreatic stents and drains in the management of pancreatitis. Ann Surg. 1989;209:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jimenez H, Aldrete JS. Clinical implications derived from the morphological classification of 89 patients with acute pancreatitis. J Clin Gastroenterol. 1983;5:137–142. [DOI] [PubMed] [Google Scholar]
- 27.Tsiotos GG, Smith CD, Sarr MG. Incidence and management of pancreatic and enteric fistulas after surgical management of severe necrotizing pancreatitis. Arch Surg. 1995;130:48–52. [DOI] [PubMed] [Google Scholar]
- 28.Nealon WH, Walser E. Surgical management of complications associated with percutaneous and/or endoscopic management of pseudocyst of the pancreas. Ann Surg. 2005;241:948–957. [DOI] [PMC free article] [PubMed] [Google Scholar]