Abstract
Objective:
To assess the impact of microsteatosis (MiS) and macrosteatosis (MaS) on major hepatectomy.
Summary Background Data:
While steatosis of a liver graft is an established risk factor in transplantation, its impact on major hepatectomy remains unclear.
Methods:
Fifty-eight steatotic patients who underwent major hepatectomy were matched 1:1 with patients with normal liver according to age, gender, ASA score, diagnosis, extent of hepatectomy, and need of hepaticojejunostomy. Steatosis was evaluated quantitatively and qualitatively. Primary endpoints were mortality and complications.
Results:
Pure MaS and MiS were present in only 10 and 3 patients, respectively, while mixed steatosis was noted in 45 patients. Forty-four patients had mild (10%–30%) and 14 moderate/severe (>30%) steatosis. Steatotic patients had significantly higher serum transaminase and bilirubin levels, and lower prothrombin time. Blood loss (P = 0.04) and transfusions (P = 0.03), and ICU stay (P = 0.001) were increased in steatotic patients. Complications were higher in steatotic patients when considered either overall (50% vs. 25%, P = 0.007) or major (27.5% vs. 6.9%, P = 0.001) complications. Patients with pure MaS had increased mortality (MaS: 20% vs. MiS: 6.6% vs. mixed: 0%; P = 0.36) and major complications (MaS: 66% vs. MiS: 50% vs. mixed: 24%; P = 0.59), but not significantly. Preoperative cholestasis was a highly significant risk factor for mortality in patients with hepatic steatosis.
Conclusion:
Steatosis per se is a risk factor for postoperative complications after major hepatectomy and should be considered in the planning of surgery. Caution must be taken to perform major hepatectomy in steatotic patients with preexisting cholestasis.
We designed a case-matched study in noncirrhotic patients to assess the impact of macrosteatosis and microsteatosis on major hepatectomy. We demonstrated that steatosis per se regardless of the microscopic type is a risk factor for complications with a high risk of mortality when associated with cholestasis.
High-volume centers have convincingly reported a dramatic decrease in perioperative mortality after liver resection over the past 2 decades.1,2 As a result, indications for liver surgery and the extent of liver resection have loosened, and currently major liver resections are routinely performed despite underlying liver disorders. While extensive hepatic resection (up to 70%) can be safely performed on healthy livers,3 the risk of such resection in patients with underlying hepatic disease remains unclear. Data correlating the extent of resection and outcome are almost exclusively available in patients with established cirrhosis,4–6 but are sparse in those with other conditions such as steatosis. In a retrospective study of a large series of hepatic resections covering a decade (1991–2001), liver steatosis was found to be the most common underlying hepatic abnormality.1 Only a few studies have focused on liver steatosis as a risk factor for postoperative complications after liver resection.7,8
Whether the type of liver steatosis such as microsteatosis (MiS) and macrosteatosis (MaS) affects the postoperative outcome in patients undergoing liver resection is unknown. Experimental data suggest that both types of steatosis can have deleterious effects on ischemic injury and regeneration,9 although in one study MaS caused worse injury.10 To address this issue, we designed a case-matched study in noncirrhotic patients to assess how hepatic MaS versus MiS may influence postoperative outcome following liver resection.
MATERIALS AND METHODS
Study Design
All patients who underwent liver resection for malignant or benign diseases in the University Hospital of Zurich from May 2000 to May 2004 were included in a prospectively collected database. Fifty-eight patients with steatosis in the nontumoral liver specimen were identified and included in the study based on the following inclusion criteria: liver resection ≥3 segments, acceptable clotting profile (platelet count >100 × 109/L and prothrombin activity > 60%) and minimum of 6 months follow-up. Liver steatosis was defined as diffuse accumulation of fat droplets affecting >10% of the hepatocytes. Exclusion criteria included underlying liver cirrhosis, patients receiving additional ablation therapies (cryosurgery or radiofrequency), preoperative planning of total vascular exclusion based on tumor location, laparoscopic resection, and simultaneous major extrahepatic procedures (ie, simultaneous colorectal resection or pancreatoduodenectomy). A control group of patients with nonsteatotic, noncirrhotic liver parenchyma who meet the inclusion/exclusion criteria were randomly selected from the large pool of patient available in the database in a blinded fashion regarding intraoperative and postoperative outcome. Patients were selected by a one-to-one case-matched methodology, where each patient with steatosis was matched with a patient displaying normal hepatic parenchyma (control group) according to the following matching criteria: age (classified according to decades of ages), gender, ASA (American Society of Anesthesiologists) score, diagnosis (benign or malignant), extent and type of liver resection, and need of a bilioenteric reconstruction.
The extent and type of liver resection were defined according to the Brisbane 2000 Terminology of Liver Anatomy & Resections.11,12 Major liver resection was defined as resection of 3 or more liver segments (minimal criterion for inclusion in this study). To properly match the extent and type of liver resection, major liver resections were categorized in 2 groups: less than hemi-hepatectomy and hemi-hepatectomy or more.
Histologic Evaluation
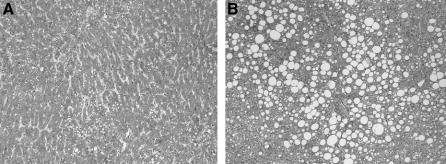
Liver resection specimens were evaluated by a single pathologist (W.J.) for the presence of steatosis using hematoxylin and eosin-stained sections. The degree of total steatosis was graded as mild (10%–30%), moderate (30%–60%), or severe (>60%) based on the percentage of hepatocytes with fat droplets.13 To investigate a possible impact of steatosis type, steatotic liver tissue was further characterized for the percentage of hepatocytes with macrovesicular (MaS) and microvesicular (MiS) steatosis (Fig. 1). Pure steatosis was considered when only one type of steatosis was present. Mixed steatosis was categorized as equivalent MaS/MiS, when the difference between the degree of MaS and MiS was <10%. Dominant MaS or dominant MiS was diagnosed when the difference between the degree of MaS and MiS was >10%. Liver fibrosis was quantified according to the METAVIR score using Sirius red stained sections14: absent (F0), portal fibrosis without septa (F1), portal fibrosis with rare septa (F2), numerous septa (F3), and cirrhosis (F4). Furthermore, histologic evaluation included the screening for the hallmarks of steatohepatitis (steatosis, hepatocyte ballooning, lobar inflammation, Mallory's hyaline). Histologic analysis was performed without knowledge of the perioperative outcome in patients.
FIGURE 1. Hematoxylin and eosin-stained sections from the liver specimen of patients with mixed pattern of liver steatosis with predominant microsteatosis (A) and macrosteatosis (B) (original magnification ×40).
Preoperative Patient Data
Demographic data included age, gender, ASA score, body mass index (BMI), and diagnosis. Other relevant clinical conditions, such as diabetes and obesity (defined as BMI ≥ 30 kg/m2), were also recorded. Preoperative chemotherapy was defined as chemotherapy used for downstaging or neoadjuvant treatment of liver tumors. In patients with a future remnant left liver of <25% of total liver volume, a right portal vein embolization was indicated 4 to 6 weeks before hepatectomy. The following biochemical blood variables were assessed preoperatively: prothrombin time, total bilirubin, alanine aminotransferase (AST), aspartate aminotransferase (ALT), alkaline phosphatase (AP), albumin, and creatinine. Cholestasis was defined as serum bilirubin levels ≥35 μmol/L (≥2 mg/dL).
Surgical Procedure
An abdominal approach with a bilateral subcostal incision was used in most of the patients, using a midline extension only when was needed. Each patient underwent an intraoperative ultrasound to define the localization of the tumor in relation to major vascular and biliary structures. Anatomic hemi-hepatectomy was performed in a standardized manner with a selective devascularization of the resected specimen as previously described.15 All resection were performed under a low central venous pressure anesthesia (0–5 mm Hg). A stapler device was used for the transection of the hepatic veins, only.
Continuous portal triad clamping (hepatic artery and portal vein through a Pringle maneuver) was used depending on the liver transection technique: systematically in association with clamp crushing technique and selectively (ie, inflow occlusion used only in the presence of significant bleeding during liver parenchyma transection) when other transection devices such as cavitron ultrasonic surgical aspirator (CUSA, Tyco Healthcare, Mansfield, MA), Hydrojet (Hydro-Jet, Erbe, Tubingen, Germany), and dissecting sealer (TissueLink, Dover, NH) were used according to a previous study.16 Liver resections were performed under supervision of one senior surgeon (P.-A.C.). Prophylactic postoperative drainage was only used when a bilioenteric anastomosis was performed.
Postoperative Course
Patients undergoing major liver resection or having intraoperative adverse events such as major bleeding or cardiac arrhythmia were admitted to the Intensive Care Unit (ICU). Fresh frozen plasma or benzodiazepines were not administered after liver resection to monitor liver function. Postoperative surveillance included clinical examinations during hospitalization and daily laboratory tests during the first week including prothrombin time, total bilirubin, AST, ALT, AP and creatinine. After the first week, blood analysis was done according to the clinical outcome. Abdominal ultrasound or computed tomography was performed in cases of suspicion of postoperative intra-abdominal fluid collection and systematically after 3 months of the operation.
Outcome Measures
All patients were followed for a minimum of 6 months. Primary end points were mortality and postoperative complications. Mortality was defined as death within 90 days after surgery and complications were graded according to a therapy-oriented severity system.17 Special emphasis was placed on the occurrence of postoperative bile leaks, abdominal fluid collections and postoperative liver failure defined according to the 50-50 criteria at 5th postoperative day (POD).18 Bilioma was defined as a symptomatic biliary fluid collection requiring external drainage.
Secondary end points were intraoperative variables such as operative time, blood loss and transfusion requirement. The indications for blood transfusion were massive hemorrhage (>1500 mL) during surgery or a hemoglobin level <7 g/dL within 24 hours following surgery. In addition, demographic data including BMI, change in biochemical profiles, ICU, and hospital stay were analyzed. Change in biochemical profiles was defined as the difference between the postoperative peak and the preoperative value.
Statistical Analysis
Data are expressed as mean ± SEM or median (range). All variables were included into a univariate analysis using the two-tailed Mann-Whitney and χ2 test. Fisher exact test was used where appropriate. Significant variables in the univariate analysis were taken forward to a stepwise logistic regression analysis (multivariate analysis) to identify independent factors that were associated with postoperative morbidity. To adjust variables for multiple comparisons, the Bonferroni correction was used. Correlations were assessed using Spearman test. P value less than 0.05 indicated statistical significance. Statistically analysis was performed by SPSS 11.5 for Windows computer software (SPSS Inc., Chicago, IL).
RESULTS
Were the Steatotic and Lean Groups Comparable?
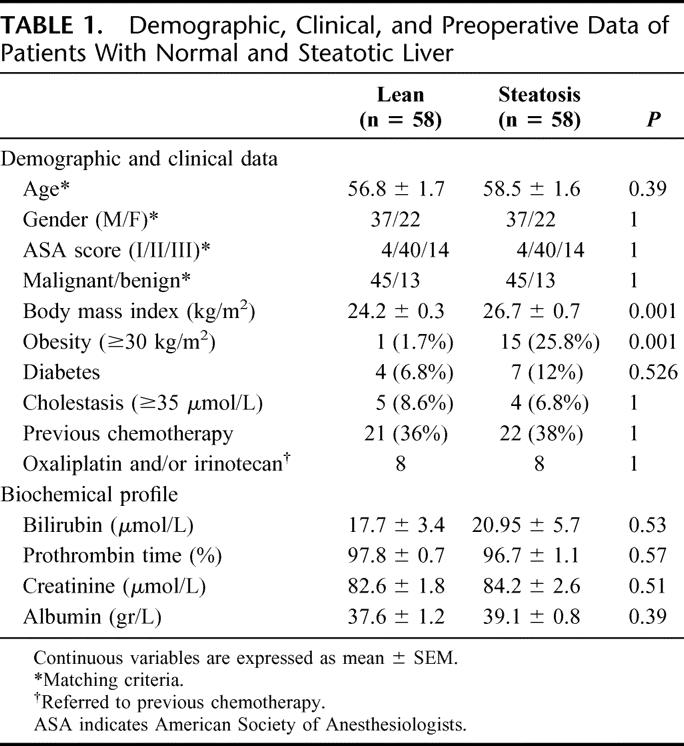
The preoperative performance status did not differ between both groups in terms of demographics, preoperative liver and kidney function as well as nutritional status. As expected, BMI was significantly higher in patients with liver steatosis compared with those with normal liver (24.2 kg/m2 vs. 26.7 kg/m2, P < 0.001). Although obesity was more frequent in patients with liver steatosis (1.7% vs. 25.8%, P < 0.001), there was no strong correlation between the degree (total amount, r2 = 0.09) and the quality (MiS, r2 = 0.17; MaS, r2 = 0.43) of steatosis and BMI. In 8 patients (3 in steatotic and 5 in lean group, P = 0.71), a right portal vein embolization was used 4 to 6 weeks before hepatectomy to increase the left remnant liver. Other clinical conditions such as preoperative cholestasis, diabetes, as well as the presence and type of previous chemotherapy were similar between the 2 groups (Table 1).
TABLE 1. Demographic, Clinical, and Preoperative Data of Patients With Normal and Steatotic Liver
Was the Surgical Strategy Different in Patients With Liver Steatosis?
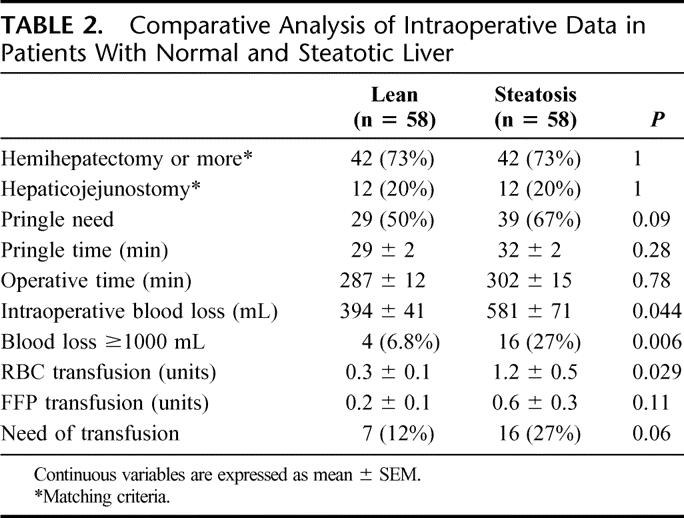
The extent and type of liver resection were comparable in the steatotic and control group as described under matching criteria. Both groups were also comparable regarding operative time and use of a Pringle maneuver (67% vs. 50%, respectively; P = 0.09) with similar hepatic ischemia times. Therefore, the ischemic insult to the liver was similar in both groups (Table 2).
TABLE 2. Comparative Analysis of Intraoperative Data in Patients With Normal and Steatotic Liver
What Underlying Type of Steatosis Was Present in the Steatotic Livers?
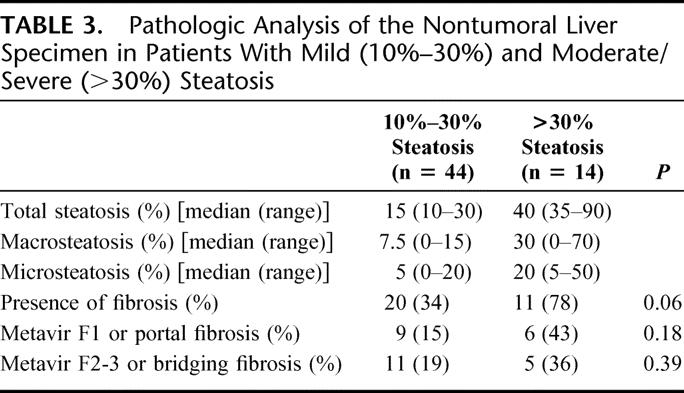
Among the 58 patients with liver steatosis, 44 patients had mild and 14 patients had marked steatosis (moderate in 10 and severe in 4 patients) (Table 3). Although the presence (45% vs. 78%, P = 0.06) as well as the severity of fibrosis based on the METAVIR score were higher in the group with moderate/severe steatosis, this difference did not reach statistical significance.
TABLE 3. Pathologic Analysis of the Nontumoral Liver Specimen in Patients With Mild (10%–30%) and Moderate/Severe (>30%) Steatosis
Pure MiS and MaS were present in only 3 and 10 patients, respectively, while a mixed pattern of steatosis (combined MiS and MaS) was noted in the other 45 patients (77%). Patients with mixed steatosis were also grouped in those with dominant MiS (7 patients), equivalent MiS/MaS (19 patients), and dominant MaS (19 patients) (Fig. 1). Although some patients had portal inflammation suggesting unspecific hepatitis, no patient had steatohepatitis. No association was detected between liver steatosis and diabetes, obesity, and chemotherapy.
Did the Presence of Liver Steatosis Influence Transfusion Requirement, Postoperative Hepatocyte Injury, and Liver Function?
Mean intraoperative blood loss (581 mL vs. 395 mL, P= 0.04) and red blood cells (RBC) units transfused (1.28 units vs. 0.33 units, P = 0.02) were statistically higher in patients with steatotic livers compared with the control group (Table 2). Although there was a trend toward a higher transfusion rate in the steatotic group, this difference failed to reach statistical significance (27% vs. 12%, P = 0.06).
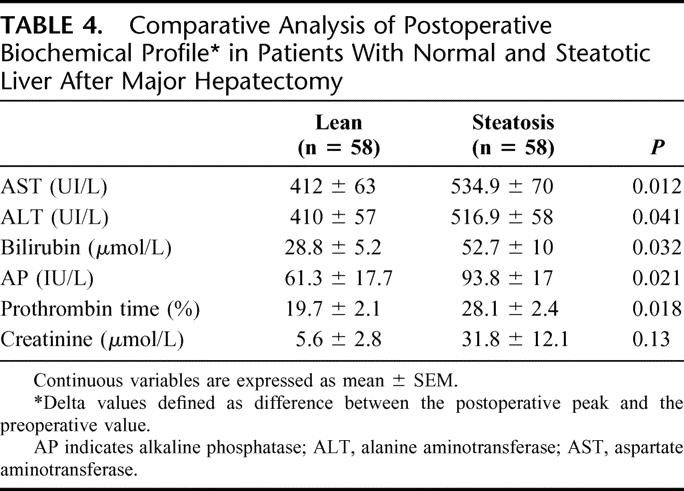
The degree of hepatic injury in patients with fatty liver assessed by postoperative serial measurements of serum AST, ALT, and AP was significantly increased compared with the control group (Table 4). Additionally, postoperative hepatocyte function assessed by prothrombin time and bilirubin was significantly impaired in patients with steatotic livers undergoing liver resection. At the 5th POD, a prothrombin time <50% (15% vs. 1.7%; P = 0.02) and a serum bilirubin >50 μmol/L (48% vs. 18%; P = 0.002) were more likely to occur in patients with a steatotic liver compared with the control group. However, the combination of these 2 parameters, ie, 50-50 criteria for definition of postoperative liver failure,18 did not reach statistical differences (8.6% in steatotic and 1.7% in the lean group, P = 0.21).
TABLE 4. Comparative Analysis of Postoperative Biochemical Profile in Patients With Normal and Steatotic Liver After Major Hepatectomy
How Did the Presence of Liver Steatosis Impact on Postoperative Outcome?
Six patients died within 1 month following surgery: one in the lean group (mortality, 1.7%) and 5 in the steatotic group (mortality, 8.5%). While different, these figures did not reach statistical significance (P = 0.21) (Table 5). The death in the lean group occurred on the 16th POD due to multiorgan failure (MOF) in an obese patient who underwent an extended liver resection. In the steatotic group, 1 patient developed a mesenteric infarction at the 5th POD, 3 others died from sepsis and MOF (on 6th, 7th, and 11th POD), and the fifth patient died due to cardiac failure and eventually MOF on the 53rd POD. Patients with moderate/severe steatosis had a higher mortality rate compared with the others (moderate/severe, 14%; vs. mild, 6.8%; vs. lean, 1.7%), but without reaching statistical significance (P = 0.13). Although the mortality rate was higher in patients with pure MaS compared with those with mixed steatosis and pure MiS (pure MaS, 20%; vs. pure MiS, 6.6%; vs. mixed, 0%), this difference was also not significant (P = 0.36).
TABLE 5. Postoperative Complications in Patients With Normal and Steatotic Liver After Major Hepatectomy
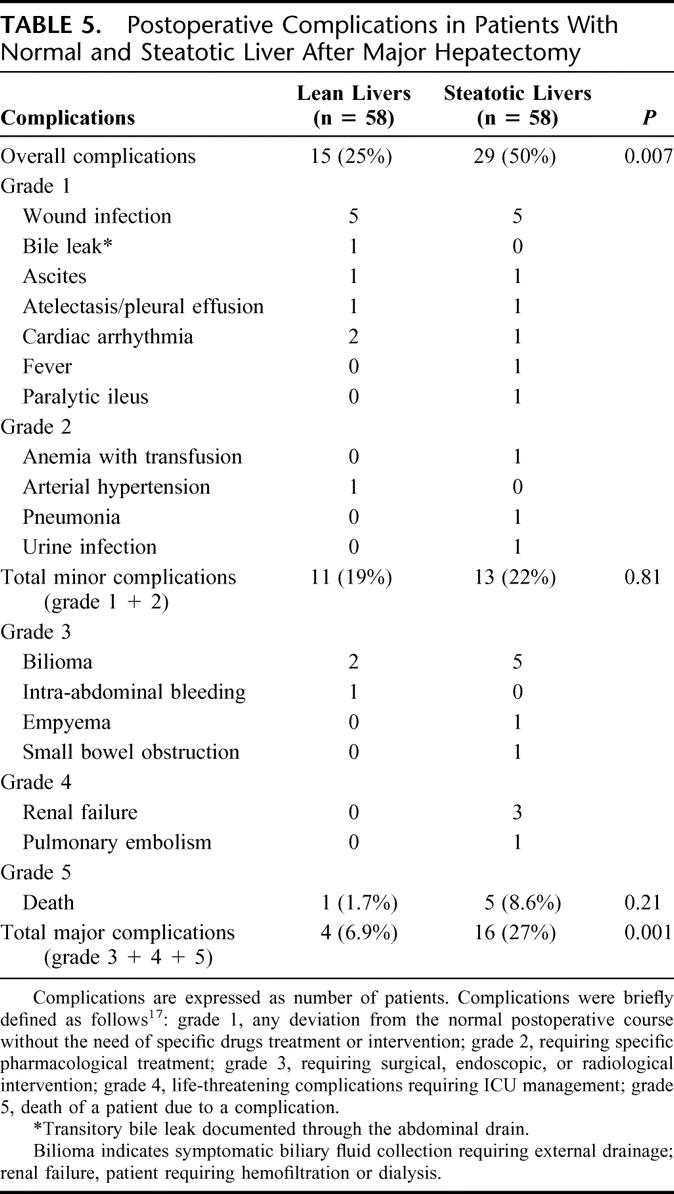
Complications were ranked according to our recently reported classification system of surgical complications17 (Table 5). The overall complication rate was higher in the group of steatotic livers compared with the lean group (50% vs. 24%, P = 0.007). Although there were no significant differences regarding minor complications between the 2 groups (5% in steatotic vs. 19% in control; P = 0.31), major complications (ie, grades 3–5) were more frequent in patients with a fatty liver (27% vs. 5%; P = 0.001). Although mean hospital stay was comparable between both groups (15.1 days in steatotic group versus 12.5 days in control group; P = 0.31), patients with liver steatosis had a longer mean ICU stay (3 vs. 1.5 days; P = 0.01). When steatosis was quantitatively evaluated, patients with mild and moderate/severe steatosis had equal incidence of overall complication (50% vs. 50%) as well as major postoperative complications (29% vs. 21%; P= 0.97). The qualitative analysis demonstrated that patients with pure MaS had increased overall (pure MaS, 70%; vs. pure MiS, 66%; vs. mixed, 44%; P = 0.28) and major complication rates (pure MaS, 66%; vs. pure MiS, 50%; vs. mixed, 24%), but these differences were not significant (P = 0.09). Among patients with mixed steatosis, the incidence of overall complications was 42% for dominant MaS, 42% for equivalent MaS/MiS, and 57% for those with dominant MiS (P = 0.84). The incidence of major complications was comparable among the 3 groups: 21% in patients with dominant MaS, 26% in equivalent MaS/MiS, and 28% in those with dominant MiS (P = 0.89). There was no significant linear correlation between the degree and quality of mixed steatosis as well as the severity of liver fibrosis with the presence and severity of postoperative complications.
The overall incidence of postoperative abdominal complications was not statistically different in patients with lean and steatotic livers (8.6% vs. 18.9%; P = 0.1). Despite a more frequent occurrence of bilioma and bile leak after liver resection in steatotic patients (15.5% vs. 5.1%; P = 0.1), the difference did no reach statistical significance. Other complications such as intra-abdominal abscess and postoperative bleeding were equally distributed between the groups (data not shown).
Is Liver Steatosis an Independent Risk Factor of Morbidity and Mortality Following Major Hepatectomy?
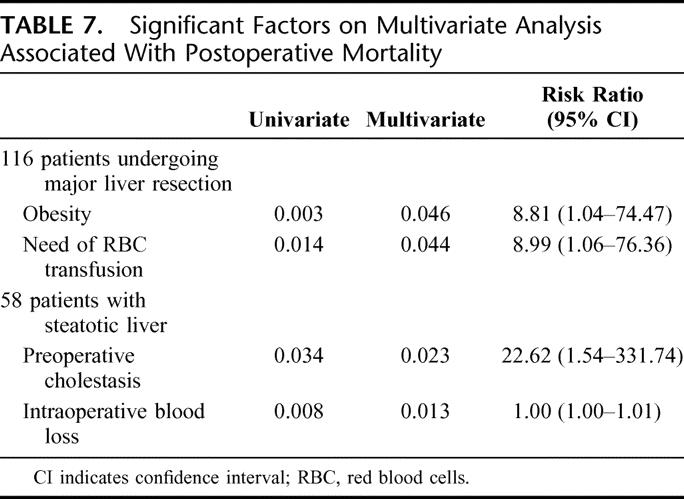
Statistical analysis was performed in all 116 patients to identify independent prognostic factors that were associated with postoperative morbidity and mortality. Many parameters were evaluated, including demographics and clinical, operative, histologic, and preoperative and postoperative biochemical variables (Tables 1–4). The multivariate analysis for postoperative morbidity revealed that the association of a hepaticojejunostomy, the requirement of RBC transfusions and the presence of liver steatosis were independent risk factors for postoperative complications (Table 6). Only the presence of obesity and the need of RBC transfusion were independent risk factors for mortality in these patients (Table 7).
TABLE 6. Significant Factors on Multivariate Analysis Associated With Postoperative Complications
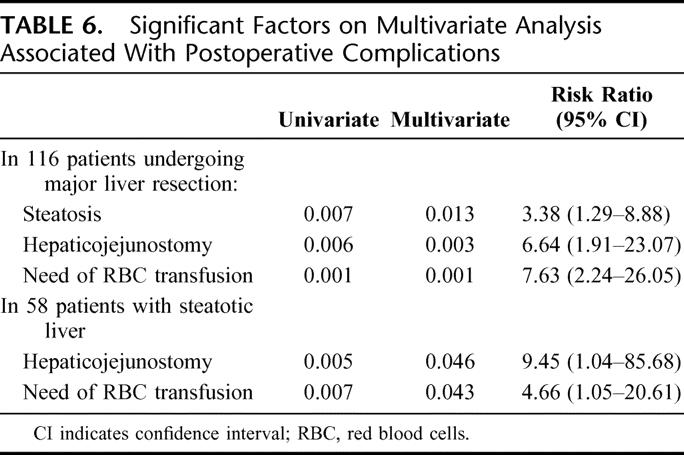
TABLE 7. Significant Factors on Multivariate Analysis Associated With Postoperative Mortality
Which Are the Risk Factors/Postoperative Complications in Patients With Liver Steatosis?
Among the group of patients with liver steatosis (n = 58), additional histologic factors such as the degree and type of liver steatosis and the severity of fibrosis were also included in the univariate and multivariate analysis. The multivariate analysis revealed that preoperative cholestasis and the need for RBC transfusions were independent risk factors for the development of postoperative complications (Table 6), while preoperative cholestasis and intraoperative blood loss were independent predictors of mortality (Table 7).
DISCUSSION
As convincing clinical data correlating liver steatosis with postoperative morbidity after major liver resection are sparse, we designed a case-matched study in a noncirrhotic population comparing patients with steatotic and lean livers. We found that the presence of liver steatosis per se regardless of the quantitative and qualitative types of steatosis is the most significant preoperative risk factor for postoperative complications after major hepatectomy. We propose that the presence of steatosis rather than MiS or MaS alone should be taken into account in the preoperative evaluation prior to extended liver resections.
The study protocol was designed with careful attention to minimize potential bias. To properly select a population with a significant underlying liver steatosis, patients with minimal steatosis, ie, <10%, and/or focal fatty changes were excluded from the analysis. We also excluded patients with underlying cirrhosis, simultaneous major extrahepatic procedures, and liver ablation procedures to secure homogeneity of the study population. The steatotic group was compared with an identical number of control patients who were carefully matched one to one for important factors in terms of demographic data, operative risk, extent of liver resection, and need of hepaticojejunostomy. Furthermore, the analysis of the data indicated not only an adequate matching but also comparable parameters of nutritional status, the use of previous chemotherapy, kidney function, cholestasis, and liver function between both groups. Finally, an experienced pathologist, who was blinded for the perioperative outcome and has a large experience in the field of liver pathology (W.J.), performed all histologic assessments.
However, such study design is inherently associated with some limitations. First, due to the retrospective nature of the study, we failed to provide accurate data regarding a reliable history of alcohol consumption, which is a well-known causal factor for the development of steatosis and other liver dysfunction. Second, only hematoxylin and eosin-stained liver tissue was available for the histologic evaluation of steatosis. Although hematoxylin and eosin staining is the most popular staining, which enables an adequate detection of MaS, this technique could underestimate the presence of MiS.19 Third, meaningful conclusions of severe steatosis are limited due to the small number of such patients who underwent major liver resection.
Although the negative impact of liver steatosis remains unclear, there is currently a consensus that steatosis increases the risk of postoperative complications following major liver resection.20 Behrns et al reported in a series of 135 patients undergoing hepatic resection that patients with >30% mixed steatosis developed more complications but had a similar mortality rate compared with patients with mild steatosis or normal liver parenchyma.7 This retrospective series, however, did not address complications according to their severity, and the number of patients with moderate/severe steatosis was very limited. Although the presence of steatosis was not an independent risk factor for postoperative complications in a large single center series of liver resections,1 another large series from France disclosed that patients with mixed steatosis involving >30% of hepatocytes have increased postoperative morbidity compared with those with lean livers (22% vs. 8%).2 Perhaps the most relevant study comes from the Memorial Sloan-Kettering group, who designed a case-match study with postoperative outcome as primary endpoint in patients with liver steatosis.8 Interestingly, the authors showed that steatosis was a predictor of postoperative complications, but mainly due to minor complications. Major complications, eg, requiring major therapeutic intervention or mortality, were not affected by the presence of steatosis. Information regarding the presence of concomitant risk factors, such as nutritional parameters, kidney function, and cholestasis, were not included. Furthermore, the histologic analysis did not differentiate between MiS and MaS or other histologic features such as steatohepatitis or fibrosis.
In agreement with others,1,2,7,8 we failed to demonstrate that liver steatosis is an independent risk factor for postoperative mortality after liver resection. The overall mortality rate of 5.1% is in the range of previous reports from large centers reporting on major hepatectomies,1,2,21 and was related, in all but 1 case, to a combination of major liver resection involving more than one hemiliver in the presence of steatosis. Of note, our study population was composed of patients requiring a hemi-hepatectomy or more in about three fourths of the cases, with the use of a bilioenteric reconstruction in a fifth of them.
Postoperative complications were assessed according to a novel therapy-oriented severity grading system17 and liver failure was defined based on the proposed 50-50 criteria at the 5th POD.18 In contrast to previous studies,7,8,22 not only overall but also major postoperative complications (grades 3–5) were significantly affected by the presence of steatosis regardless of the quantitative and qualitative features on histology (Table 5). Interestingly, patients with pure MaS appear to have the highest mortality and major complication rates compared with those with mixed and pure MiS. Although various animal models are currently available to study fatty liver, none of them offers a pure model of MaS or MiS, since they all contain both types of steatosis.10 Probably, the development of novel animal models or future studies in human with a larger sample size may provide additional insight regarding the impact of pure MaS on postoperative outcome.
We and others found that not only steatosis but also transfusion requirement and preexisting cholestasis were independent predictors of postoperative complications.1,2,23 Interestingly, these last 2 factors were also independent predictors for postoperative complications in the steatotic group. In addition, intraoperative blood loss and cholestasis were independent predictors of mortality after major hepatectomy. While the parenchyma of steatotic liver is softer and more friable making transection with control of biliary and vascular structures more difficult, we did not observe increased blood loss or local complications such as bile leak, intra-abdominal abscess, or bleeding. This finding may be attributed to a wide experience of a HPB center using modern surgical techniques.16
Hepatic resection with inflow occlusion is associated with both tissue loss and ischemic injury, which are potential factors responsible for postoperative liver failure in steatotic patients. We have experimentally demonstrated that liver regeneration is markedly impaired in the remnant fatty liver after hepatectomy.24 Impaired microcirculation has been proposed as another important factor contributing to a decreased ischemic tolerance of the steatotic liver.9,20 Although experimental studies indicate that livers with predominant MaS have a higher vulnerability to ischemic injury than those with MiS,10 this clinical study suggests that hepatic injury as well as liver function were significantly impaired in patients with mixed steatotic livers. The 2 biochemical parameters at the 5th POD defining liver failure (ie, serum bilirubin and prothrombin time) were more likely to occur in steatotic patients,18 although postoperative liver failure with combined 50-50 criteria were similar in both groups of patients.
The epidemiology of obesity is associated with a significant increase in obesity-related health problems.25 In contrast to previously published autopsy series,22 we failed to demonstrated any linear correlation between BMI and the quantity/quality of steatosis. However, the mean BMI as well as the proportion of obese patients in the group with steatotic livers were significantly higher compared with controls. In contrast to a previous series of general surgery procedures in obese patients,26 we found that obesity is an independent risk factor for mortality after major hepatectomy. Therefore, preoperative liver biopsies may be useful in obese patients considered for major liver resection. It has been suggested that conservative management of obesity improves liver steatosis.27,28 A recent study showed that a short-term combination therapy for diet, exercise, and drugs effectively reduced liver steatosis in potential living donor liver transplant candidates.29 This might be an effective preoperative strategy to reduce steatosis-associated complications after major liver surgery.
While obesity is a well-known risk factor for steatosis, the role of chemotherapy is not well defined.8,30,31 In patients with preexisting liver steatosis, however, chemotherapy associated steatohepatitis (CASH) may develop after aggressive neoadjuvant treatment using novel agents such as oxaliplatin or irinotecan.32,33 A recent study in patients with colorectal liver metastases demonstrated that the occurrence of CASH is rare (8%) but, when present, it is associated with an increased risk of mortality after liver surgery.34 Although we included many patients with previous systemic chemotherapy, we failed to show significant data since the type and duration of chemotherapy regimens were quite heterogeneous in our series. Probably because only a small number of steatotic patients received oxaliplatin, irinotecan, or both before liver resection, none of them developed steatohepatitis in our series.
Most of the surgeons agree that preoperative biliary drainage must be performed to avoid severe cholestasis at the time of major hepatectomy (eg, serum bilirubin >100 μmol/ L or >6 mg/dL). However, cholestasis can be often reduced before surgery but sometimes does not reach normal bilirubin levels at time of hepatectomy despite a successful drainage. We found that the presence of preoperative jaundice in patients with steatosis is an independent risk factor for mortality. Consequently, efforts should be made either to postpone surgery until normalization of bilirubin or to minimize the extent of liver resection in those patients (eg, economic hepatectomy combined with ablation therapies, two-stage hepatectomy). Another useful strategy may be the increment of the future remnant liver volume through preoperative portal vein embolization, as successfully reported in patients with liver fibrosis or cirrhosis.4,35 Future prospective trials using preoperative and postoperative volumetric assessment of remnant liver volume will define a safe volume after major hepatectomy in patient with liver steatosis, particularly in those with preexisting jaundice.
CONCLUSION
Liver steatosis, regardless of the presence of MiS or MaS, is the most significant preoperative risk factor for postoperative complications after major liver resection and should be considered for the planning of surgery. Further randomized trials should focus on the evaluation of novel preoperative strategies, such as short-term intensive medical treatment of hepatic steatosis and preoperative portal vein embolization, to minimize risk in these patients. Concomitant cholestasis in patients with underlying liver steatosis should alert surgeons for the high risk of postoperative mortality.
ACKNOWLEDGMENTS
The authors thank Paul Harpes, PhD, Department of Biostatistics, University of Zurich, for his assistance in the statistical analysis.
Footnotes
Reprints: Pierre-Alain Clavien, MD, PhD, Department of Visceral and Transplantation Surgery, Zurich University Hospital, Rämistrasse 100, CH-8091 Zürich, Switzerland. E-mail: clavien@chir.unizh.ch.
REFERENCES
- 1.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406; discussion 406–407. [DOI] [PMC free article] [PubMed]
- 2.Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. [DOI] [PubMed] [Google Scholar]
- 3.Vauthey JN, Chaoui A, Do KA, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512–519. [DOI] [PubMed] [Google Scholar]
- 4.Azoulay D, Castaing D, Krissat J, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1022. [DOI] [PubMed] [Google Scholar]
- 6.Lam CM, Fan ST, Lo CM, et al. Major hepatectomy for hepatocellular carcinoma in patients with an unsatisfactory indocyanine green clearance test. Br J Surg. 1999;86:1012–1017. [DOI] [PubMed] [Google Scholar]
- 7.Behrns KE, Tsiotos GG, DeSouza NF, et al. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg. 1998;2:292–298. [DOI] [PubMed] [Google Scholar]
- 8.Kooby DA, Fong Y, Suriawinata A, et al. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–1044. [DOI] [PubMed] [Google Scholar]
- 9.Selzner M, Rudiger HA, Sindram D, et al. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32:1280–1288. [DOI] [PubMed] [Google Scholar]
- 10.Selzner N, Selzner M, Jochum W, et al. Mouse livers with macrosteatosis are more susceptible to normothermic ischemic injury than those with microsteatosis. J Hepatol. 2006;44:694–701. [DOI] [PubMed] [Google Scholar]
- 11.Terminology Committee of the International Hepato-Pancreato-Biliary Association. Brisbane 2000 Terminology of Liver Anatomy & Resections. HPB. 2000;2:333–339. [Google Scholar]
- 12.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351–355. [DOI] [PubMed] [Google Scholar]
- 13.D'Alessandro A, Kalayoglu M, Sollinger H, et al. The predictive value of donor liver biopsies for the development of primary nonfunction after orthotopic liver transplantation. Transplantation. 1991;51:157–163. [DOI] [PubMed] [Google Scholar]
- 14.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C: the METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. [DOI] [PubMed] [Google Scholar]
- 15.Clavien PA, Selzner M, Rudiger HA, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843–850; discussion 851–852. [DOI] [PMC free article] [PubMed]
- 16.Lesurtel M, Selzner M, Petrowsky H, et al. How should transection of the liver be performed? A prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann Surg. 2005;242:814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balzan S, Belghiti J, Farges O, et al. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urena M, Ruiz-Delgado F, Gonzales E, et al. Hepatic steatosis in liver transplant donors: common feature of donor population? World J Surg. 1998;22:837–844. [DOI] [PubMed] [Google Scholar]
- 20.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–113. [DOI] [PubMed] [Google Scholar]
- 21.Poon RT, Fan ST, Lo CM, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708; discussion 708–710. [DOI] [PMC free article] [PubMed]
- 22.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. [DOI] [PubMed] [Google Scholar]
- 23.Kooby DA, Stockman J, Ben-Porat L, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869; discussion 869–870. [DOI] [PMC free article] [PubMed]
- 24.Selzner M, Clavien PA. Failure of regeneration of the steatotic rat liver: disruption at two different levels in the regeneration pathway. Hepatology. 2000;31:35–42. [DOI] [PubMed] [Google Scholar]
- 25.Jonas MM. Nonalcoholic fatty liver disease. Adolesc Med Clin 2004;15:159–173. [DOI] [PubMed] [Google Scholar]
- 26.Dindo D, Muller MK, Weber M, et al. Obesity in general elective surgery. Lancet. 2003;361:2032–2035. [DOI] [PubMed] [Google Scholar]
- 27.Powell EE, Jonsson JR, Clouston AD. Steatosis: co-factor in other liver diseases. Hepatology. 2005;42:5–13. [DOI] [PubMed] [Google Scholar]
- 28.Hickman IJ, Clouston AD, Macdonald GA, et al. Effect of weight reduction on liver histology and biochemistry in patients with chronic hepatitis C. Gut. 2002;51:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamuta M, Morizono S, Soejima Y, et al. Short-term intensive treatment for donors with hepatic steatosis in living-donor liver transplantation. Transplantation. 2005;80:608–612. [DOI] [PubMed] [Google Scholar]
- 30.Parikh AA, Gentner B, Wu TT, et al. Perioperative complications in patients undergoing major liver resection with or without neoadjuvant chemotherapy. J Gastrointest Surg. 2003;7:1082–1088. [DOI] [PubMed] [Google Scholar]
- 31.Karoui M, Penna C, Amin-Hashem M, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez FG, Ritter J, Goodwin JW, et al. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845–853. [DOI] [PubMed] [Google Scholar]
- 33.Fong Y, Bentrem DJ. CASH (Chemotherapy-Associated Steatohepatitis) costs. Ann Surg. 2006;243:8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. [DOI] [PubMed] [Google Scholar]
- 35.Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]