Abstract
Objective:
To define the prognostic significance of surgical center case volume on outcome for soft tissue sarcoma (STS).
Methods:
STS cases registered in the Florida Cancer Data System (FCDS) between 1981 and 2001 were analyzed. Medical facilities were ranked by STS operative volume. Facilities above the 67th percentile for volume were defined as high-volume centers (HVCs).
Results:
Of the 4205 operative cases of STS identified, 68.1% were treated at low-volume centers (LVCs) and 31.9% at HVCs. A larger proportion of high-grade tumors (53.8% vs. 44.3%) and lesions over 10 cm (40.7% vs. 28.7%) were resected at HVC (P < 0.001). The 30-day mortality was 0.7% for HVC and 1.5% for LVC (P = 0.028), and mortality rates at 90 days were 1.6% and 3.6%, respectively (P = 0.001). Median survival was 40 months at HVC and 37 months at LVC (P = 0.002). Univariate analysis demonstrated significantly improved survival at HVC for high-grade tumors (median 30 months vs. 24 months, P = 0.001), lesions over 10 cm (28 months vs. 19 months, P = 0.001) and truncal or retroperitoneal sarcomas (39 months vs. 31 months, P = 0.011). Limb amputation rate was lower (9.4% vs. 13.8%, P = 0.048) and radiation and chemotherapy were more frequently administered at HVC (OR = 1.54). On multivariate analysis, treatment at a HVC was a significant independent predictor of improved survival (OR = 1.292, P = 0.047).
Conclusions:
STS patients treated at HVC have significantly better survival and functional outcomes. Patients with either large (>10 cm), high-grade or truncal/retroperitoneal tumors should be treated exclusively at a high-volume center.
Using a large prospective cancer registry, the effects of surgical volume on patient outcomes for soft tissue sarcoma were examined. A significantly increased overall short-term and long-term survivals as well as improved limb preservation were observed at high-volume centers. This study suggests that soft tissue sarcoma should be treated at high-volume centers.
Soft tissue sarcomas (STSs) are an uncommon, heterogeneous group of neoplasms of mesenchymal origin that occur in all body sites. Approximately 9420 cases were diagnosed in the United States in 2005, accounting for less than 1% of all new malignancies.1–3
Traditionally, the standard of care for management of STS has been complete surgical resection with tumor-free margins. Treatment of STS has improved significantly in the last 2 decades with the advent of limb-, tissue-, and function-sparing procedures, which adjuvant or neoadjuvant chemoradiotherapy have made possible.1,4,5 Most of these therapeutic advances have been reported from individual institutions, primarily major tertiary cancer treatment centers.
The low incidence and heterogeneity of these neoplasms are directly responsible for marked disparities in case volumes between large tertiary cancer centers with well-established local, state, and national referral patterns and smaller or community-based institutions. These lower volume institutions have less experience and/or fewer resources to deal with these rare and often complicated tumors. We postulate that treatment of STS at institutions with higher sarcoma case volumes eventuates in superior outcomes in terms of survival and preservation of function. This hypothesis was addressed in an analysis of STS cases from a large, population-based state cancer registry.
PATIENTS AND METHODS
The Florida Cancer Data System (FCDS) is a prospective database of all cancer cases in the state of Florida since 1981, and currently includes over 2.7 million records.6 The FCDS is an incident cancer registry, and the legislation through which it was established requires it to be inclusive of all cancer cases in the state of Florida, home to about 6% of the U.S. population. In 1994, the FCDS became part of the National Program of Cancer Registries administered by the Centers for Disease Control. Over 96,000 invasive, reportable cancer incidence cases are abstracted annually, following the North American Association of Central Cancer Registries procedure guidelines for which it has earned “gold” certification for quality, timeliness, and completeness (eg, >95% capture of all incidence cancers within 1 year of diagnosis). All cancers in the database are coded using the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3).7 The FCDS is wholly supported by the State of Florida Department of Health, the National Program of Cancer Registries of the Centers for Disease Control and Prevention and the Sylvester Comprehensive Cancer Center at the University of Miami Miller School of Medicine.
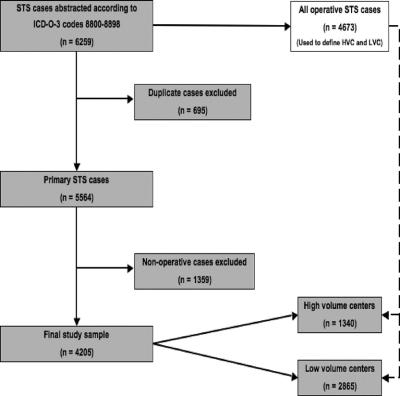
The most current 2005 FCDS data set was used to identify all incident cases of soft tissue sarcoma diagnosed in the state of Florida from 1981 to 2001 using ICD codes 8800-8898. A total of 6259 cases of STS were extracted for analysis (Fig. 1). Removal of duplicate cases (ie, sarcoma recurrences, patients seen at more than one institution, etc) resulted in 5564 unique, primary presentations of STS. All nonoperative entries were excluded for a final sample size of 4205 surgical cases. Tumor type was classified as follows: fibrosarcoma, liposarcoma, malignant fibrous histiocytoma (MFH), and leiomyosarcoma. These 4 subtypes were chosen as together they account for approximately 89% of all malignant soft tissue sarcomas.3 The FCDS registry only collects malignant cases of mesenchymal tumors and does not record neoplasms of indeterminate or benign biologic behavior.
FIGURE 1. Selection of study sample. ICD-O-3, International Classification of Diseases for Oncology, 3rd ed. STS indicates soft tissue sarcoma; HVC, high-volume center; LVC, low-volume center.
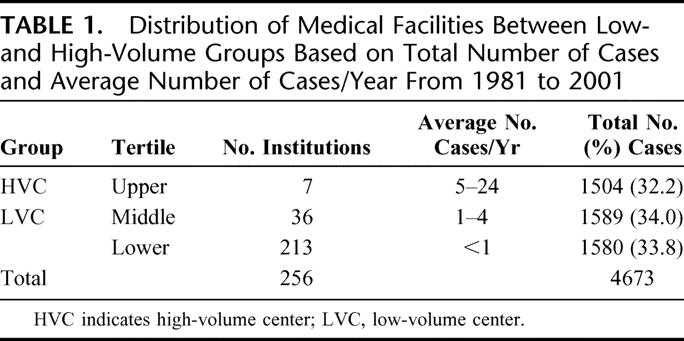
The FCDS database was queried to determine the number of all STS surgical procedures performed at each institution in the state of Florida during the study period (Table 1). Medical facilities were grouped into 3 balanced percentile ranges by surgical procedure volume. The upper third of institutions were classified as high-volume centers (HVC) and the lower two-thirds as low-volume centers (LVC). The percentile cutoff of approximately 67% for comparison of outcomes was established as the break point for this analysis as this produced 2 groups of institutions, which were very similar in their surgical volumes to tertiary referral centers (HVC) or community hospitals (LVC). Total number of cases rather than number of cases per year was used to make this determination to prevent skewing of the data by facilities that may not have been in existence over the entire 20-year study period.
TABLE 1. Distribution of Medical Facilities Between Low- and High-Volume Groups Based on Total Number of Cases and Average Number of Cases/Year From 1981 to 2001
Patient demographics were extracted from the FCDS database. Complete data on tumor grade and tumor size at initial presentation were available for only 2239 and 1374 cases, respectively, due to reporting omissions. Tumors characterized as low-grade included well-differentiated and moderately differentiated lesions (ICD-O Grades 1 and 2), while high-grade tumors were comprised of poorly differentiated, undifferentiated, and anaplastic lesions (ICD-O grades 3 and 4).7
Statistical analysis was performed with SPSS Statistical Package version 13.0 (SPSS Inc., Chicago, IL). Correlations between categorical variables were made using the χ2 or Fisher exact test, where appropriate. Median, 5- and 10-year survivals were calculated by the Kaplan-Meier method. Survival data are regularly collected by the FCDS on an ongoing basis. Since the FCDS does not record complete information on cause of death, disease-specific survival could not be determined. Patients lost to follow-up were censored at the time of last contact. Overall survival was calculated from the time of the initial sarcoma diagnosis. The effects of demographic, clinical, pathologic, and treatment variables on survival were examined using the log-rank test for categorical values. A multivariate analysis using the Cox proportional hazards model was used to further test prognostic factors found to be significant in the univariate analysis. Statistical significance was set at a P value of <0.05.
RESULTS
Hospital Volume
A total of 256 institutions in Florida performed at least one resection of a STS from 1981 to 2001 (Table 1). There were 4673 surgical procedures recorded, including both initial resections and repeat procedures. By the case-volume stratification previously described, 7 institutions performed 1504 cases (32.2% of total) and were classified as HVC. The remaining two thirds of the institutions performed 3169 cases (67.8% of total) and were classified as LVC.
Patient Demographics and Clinical Data
During the 20-year study period, there were a total of 4205 primary operative cases of soft tissue sarcoma reported in the FCDS database. Repeat surgeries were excluded from the analysis (n = 468). LVC case volume was 2865 (68.1%) while 1340 (31.6%) patients were treated at HVC. Table 2 summarizes the demographic, tumor, and treatment characteristics of the study population. Briefly, 57.4% were males with a median age of 67 years, and 3219 (76.6%) of patients were over the age of 50. The study population was predominantly white (90.7%). In patients for whom both tumor size and grade were available, 1077 (48.1%) tumors were high-grade and 455 (33.1%) were more than 10 cm in greatest dimension at diagnosis. MFH was the most common histology (n = 2273, 54.1%), followed by liposarcoma (n = 1447, 34.4%), fibrosarcoma (n = 433, 10.3%), miscellaneous histologies (n = 45, 1.1%), and leiomyosarcoma (n = 7, 0.2%). The majority of cases were situated in the extremities (n = 1937, 46.7%), 1722 (41.5%) were truncal or retroperitoneal, and 490 (11.8%) arose in the head and neck.
TABLE 2. Association of Volume of Medical Facility With Demographic and Clinical Variables for All Patients Undergoing Surgical Resection for Soft Tissue Sarcoma
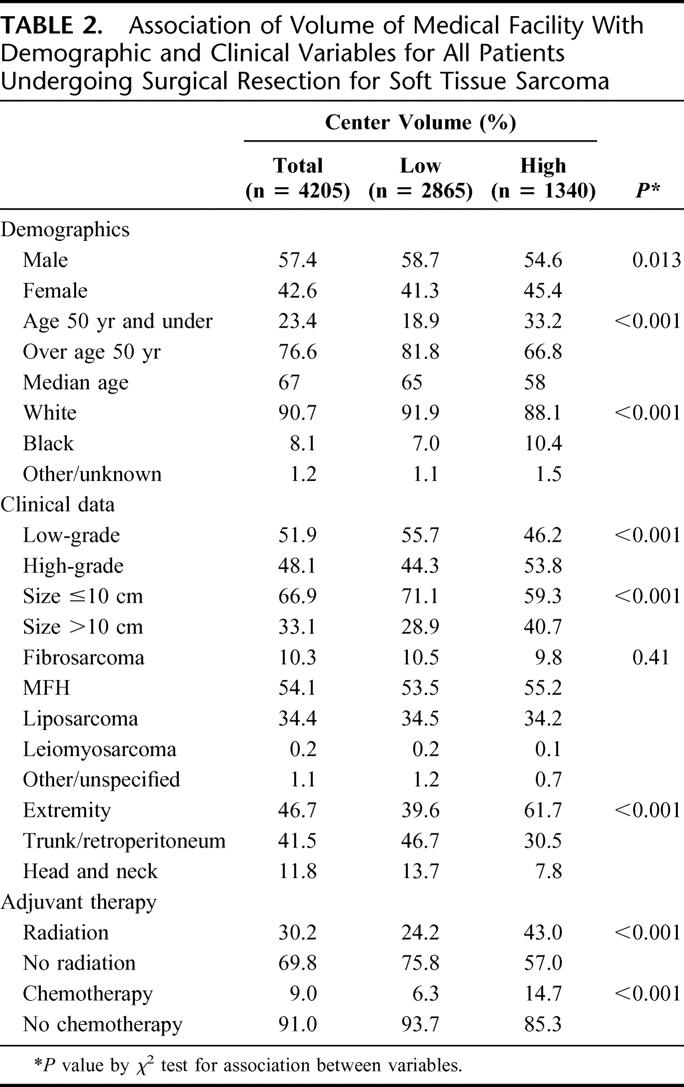
Demographic and clinical variables by institutional treatment volume are given in Table 2. Patients at HVC were generally younger (median age 58 years vs. 65 years at LVC, P < 0.001), with a higher proportion of females (45.4% vs. 41.3%, P = 0.013) and non-white race/ethnicity (10.4% vs. 7.0%, P < 0.001). Patients at HVC were more likely to have high-grade tumors (53.8% vs. 44.3%, P < 0.001) or tumors of over 10 cm (40.7% vs. 28.9%, P < 0.001) than those at LVC. There was no significant difference in the distribution of histologic types between the HVC and LVC. HVC treated a significantly larger proportion of extremity sarcomas than did LVC (61.7% vs. 39.6%, P < 0.001). So, too, a greater proportion of patients treated at HVC received radiation therapy (43.0% vs. 24.2%, P < 0.001) and chemotherapy (14.7% vs. 6.3%, P < 0.001).
Outcome and Choice of Treatment Facility
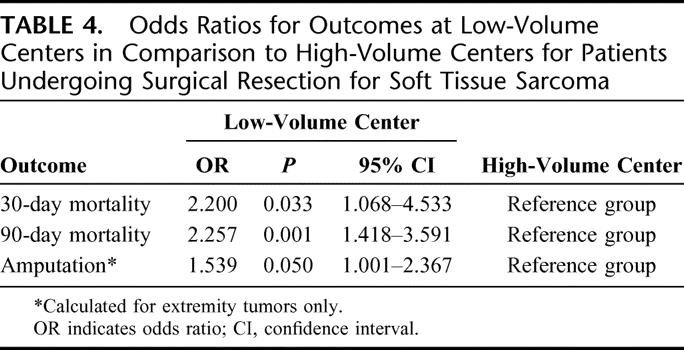
Short-term outcomes are shown in Table 3. The 30-day mortality rate was twice as high in LVC in comparison to HVC (1.5% vs. 0.7%, P = 0.028), with a similar disparity in the 90-day mortality rate (3.6% in LVC vs. 1.6% in HVC, P = 0.001). Analysis of patients with extremity tumors (n = 1937) showed a higher amputation rate at LVC (13.8%) than at HVC (9.4%, P = 0.048). Odds ratios for unadjusted outcomes, summarized in Table 4, show similar results.
TABLE 3. Outcomes for Patients Following Surgical Resection for Soft Tissue Sarcoma According to Volume of Medical Facility
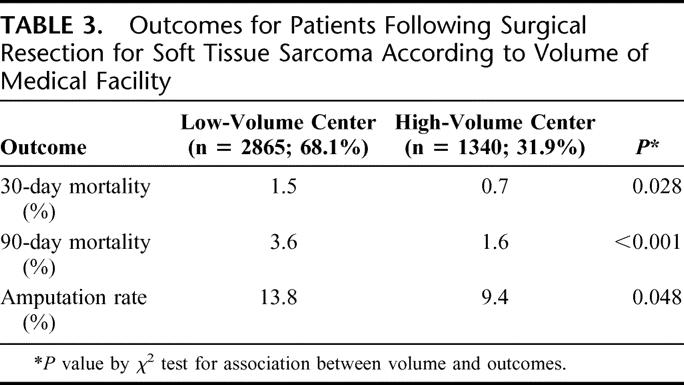
TABLE 4. Odds Ratios for Outcomes at Low-Volume Centers in Comparison to High-Volume Centers for Patients Undergoing Surgical Resection for Soft Tissue Sarcoma
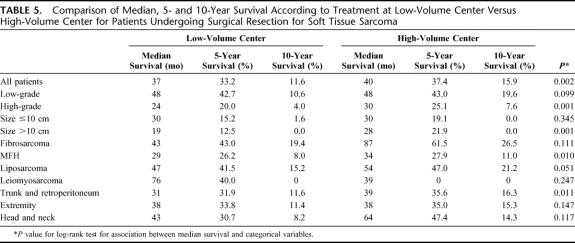
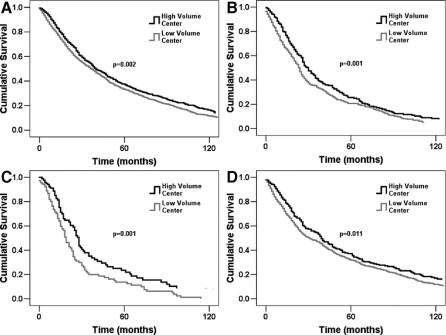
Median, 5- and 10-year survivals are summarized in Table 5. Median survival for patients treated at HVC was 40 months, significantly better than LVC (37 months, P = 0.002). Surgical resection at a HVC eventuated in superior median survival in high-grade tumors (30 vs. 24 months, P < 0.001) and in lesions over 10 cm in size (28 vs. 19 months, P < 0.001). This was also true for truncal and retroperitoneal sarcomas (39 vs. 31 months, P = 0.011). A trend toward improved survival for head and neck STS treated in a HVC was observed (median survival, 64 vs. 43 months, P = 0.117). Median survival for extremity sarcomas was equal in both groups, although a trend toward improved 5- and 10-year survival was observed in the HVC group (35.0% and 15.3% vs. 33.8% and 11.4%, respectively, P = 0.147). Analysis by histologic subtype revealed improved survival in HVC for MFH and trends in favor of HVC for liposarcoma and fibrosarcoma. Kaplan-Meier survival curves are shown in Figure 2.
TABLE 5. Comparison of Median, 5- and 10-Year Survival According to Treatment at Low-Volume Center Versus High-Volume Center for Patients Undergoing Surgical Resection for Soft Tissue Sarcoma
FIGURE 2. Overall survival comparison between low-volume centers and high-volume centers for (A) all patients, (B) high-grade tumors, (C) tumors >10 cm, and (D) truncal and retroperitoneal tumors (P value for log-rank test for association between median survival and categorical variables).
Limb Preservation and Choice of Treatment Facility
Patients with extremity tumors were studied to determine if there was any correlation between the volume of the treating facility and functional outcome as reflected in amputation rates. A total of 1937 extremity tumors were analyzed and demographic and clinical variables according to the volume of treatment facility outlined in Table 6. Among extremity tumors resected at HVC, 90.6% of these procedures were limb-sparing operations as compared with 86.2% at LVC (P = 0.048). Patients with extremity tumors who were treated at HVC were younger (median age, 59 years vs. 69 years at LVC, P < 0.001).
TABLE 6. Association of Volume of Medical Facility With Demographic and Clinical Variables for Patients Undergoing Surgical Resection for Soft Tissue Sarcoma of the Extremity
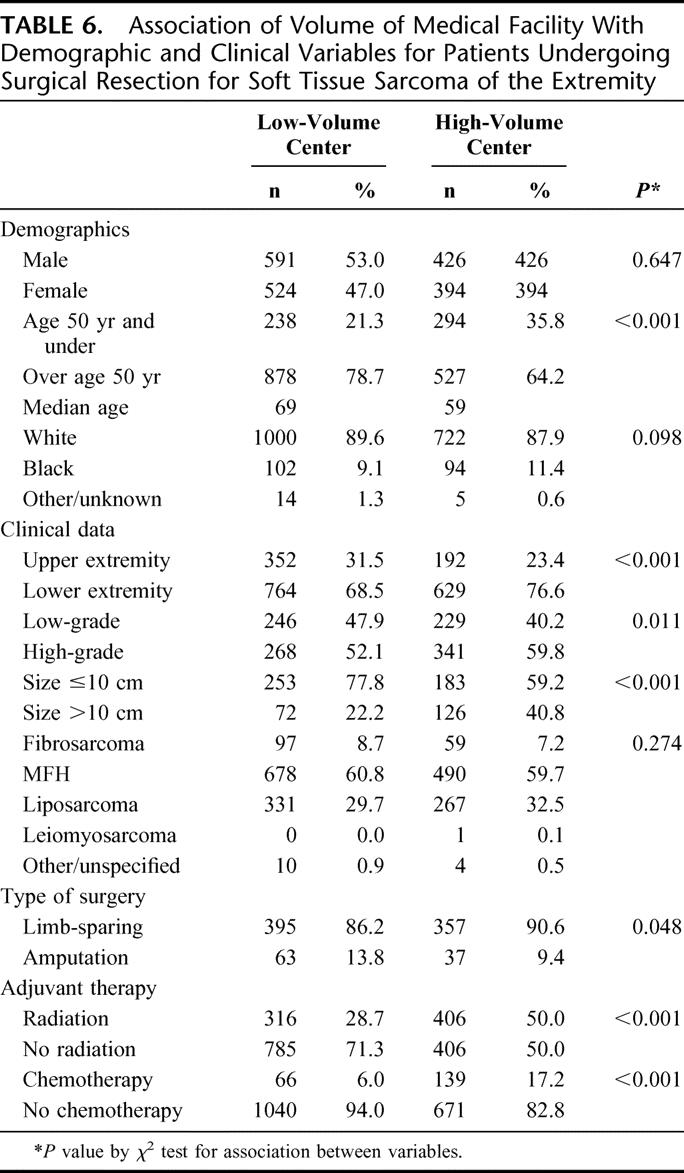
There were no significant differences between the 2 groups in gender or race. Lower extremity sarcomas accounted for 76.6% of cases treated at HVC as compared with 68.5% at LVC (P < 0.001). Extremity tumors resected at HVC were of higher grade (59.8% vs. 52.1%) and larger size (40.8% over 10 cm vs. 22.1%) than those at LVC (P = 0.02). There were no significant case-volume differences in histologies between HVC and LVC patients. More patients with extremity sarcomas received radiation therapy (50.0% vs. 28.7%) and chemotherapy (17.2% vs. 6.0%) at HVC than at LVC (P < 0.001).
Multivariate Analysis
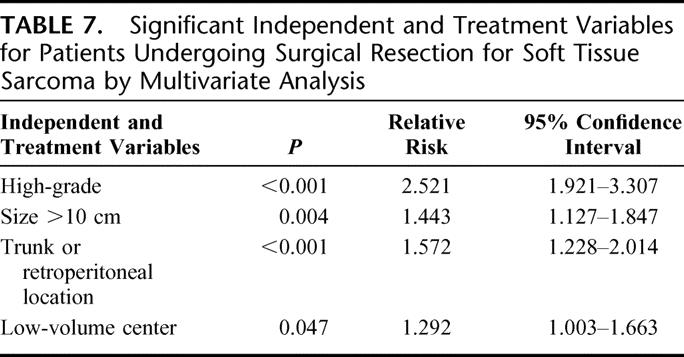
Stepwise multivariate analysis of variables identified as significant in univariate analysis was undertaken using the Cox regression method. High tumor grade, size over 10 cm, and truncal or retroperitoneal location were independent predictors of lower overall survival. Treatment at a HVC was also an independent predictor of good outcome (Table 7).
TABLE 7. Significant Independent and Treatment Variables for Patients Undergoing Surgical Resection for Soft Tissue Sarcoma by Multivariate Analysis
DISCUSSION
The medical literature is replete with studies showing a direct correlation between procedural volume and treatment outcome, the earliest studies dating from the early 1980s.8,9 A systematic MEDLINE review conducted by Halm et al in 2000 revealed 135 such studies, 71% of studies of hospital volume and 69% of studies of physician volume reporting statistically significant associations between higher volume and better outcomes.10 Many of these reports focus on cancer-related procedures. A positive correlation between hospital volume and improved outcome was reported for surgical resection for pancreatic cancer in 198411 and corroborated in subsequent reports.12–18 Similar findings have been reported for colorectal,19–25 esophageal,11,23,26 lung,25,27 gastric,21,23,25 and breast cancer.28
STSs are rare.29 This paucity leaves most healthcare institutions with low case volumes and outdated or inadequate resources, which impede the ability to offer optimal treatment of these rare and often complicated tumors. Our analysis of 20 years’ surgical management of STS in Florida included 4673 operations performed at 256 institutions (Table 1). Volumes in 213 facilities amounted to less than 1 case per year, and less than 2 cases per year were managed at an additional 79 healthcare institutions. The 7 institutions in the HVC group, only 3% of the state's hospitals, treated 32.2% of all sarcoma cases in Florida since 1981. A larger proportion of patients received radiation therapy (43.0% vs. 24.2%) and chemotherapy (14.7% vs. 6.3%) at HVC as compared with LVC (P < 0.001). Over the 2 decades studied, there has been a small minority of institutions providing the bulk of multimodality treatment of STS, and a large number of hospitals with only occasional exposure to these tumors.
The 30-day mortality rate in LVC was twice that of HVC, and this difference persisted at 90 days. Median survival for patients treated at HVC was slightly better than that of those at LVC. The trends in 5-year and 10-year survival rates were similar between the 2 groups. High case volume was an independent predictor of good outcome in multivariate analysis. Although more retroperitoneal lesions were treated at LVCs, both tumor location and treatment center volume were each independently significant in multivariate analysis. It therefore appears that tumor location does not explain the increased short-term mortality observed in LVCs.
Several differences between the 2 patient populations are apparent. Patients seen at HVC were younger and a higher proportion was female (45.4% vs. 41.3%, P = 0.013) and of minority race/ethnicity. Sarcomas managed at HVC were higher grade and larger size than those at LVC. HVC treated the largest, most aggressive sarcomas in Florida's population. Despite this case selection bias in favor of LVC, HVC achieved superior outcomes in patients with high-grade lesions and those with tumors over 10 cm in size. Age, gender, and race were not significant factors in this analysis.
Proportionately, more truncal and retroperitoneal lesions were treated at LVC than at HVC. Nonetheless, patients with truncal and retroperitoneal sarcomas fared better when treated at a HVC than at a LVC. A recent analysis of FCDS data showed that retroperitoneal tumors are larger and of higher grade at presentation than those at other anatomic locations (unpublished observation). These 2 characteristics, and the frequent involvement of adjacent organs and other vital structures, make accurate diagnosis and appropriate treatment of these lesions difficult and complex.30–33 The data reported here support the premise that the management of truncal and retroperitoneal tumors is best carried out at high-volume, regional referral centers with the resources and expertise needed to achieve optimal outcomes.
An association between procedural volume and outcomes for extremity sarcoma has previously been established, especially in patients with residual disease following incomplete resection.34–37 Although there was no difference in median survival of extremity sarcomas between HVC and LVC, a trend toward improved 5-year and 10-year survivals for those treated at a HVC was apparent. The amputation rate for extremity sarcomas was significantly lower among patients treated at a HVC.
Limited data availability for grade and size prevented a formal multivariate logistic regression analysis for extremity sarcomas, but univariate analysis showed that extremity tumors at HVC tended to be of higher grade and larger size. The higher amputation rate at LVC provides one possible explanation for the surprisingly similar survival rates; limb amputation is a relatively simple procedure that reliably and easily achieves tumor-free margins of resection, albeit with devastating functional consequences and reduced quality of life. It is noteworthy that this higher amputation rate may be attributable to some extent to the older age of patients with extremity sarcomas treated at LVC. That said, the higher proportion of limb-sparing procedures at HVC is most readily explained by depth of surgical experience and immediate availability of multidisciplinary and multimodality therapy.
The FCDS, although an excellent database for comparative outcomes analysis, is nonetheless not without limitations. Many of the limitations in this study are similar in type and scope to those of other similar large cancer registries. Omissions in data reporting by individual institutions to the FCDS have resulted in incomplete information on tumor size and grade. Additionally, information on patients’ comorbidities is not included in the FCDS registry and thus was not included in our analysis. The FCDS provides only passive follow-up for registered patients. Individuals residing outside of the state of Florida following their cancer surgery may actually be deceased and not recorded as such. Because of this problem survival may be overestimated by as much as 5% to 10% (FCDS, personal communication). Further, as the database does not record information on cause of death, we were unable to include disease-specific survival in our examination.
Potential pitfalls in analysis of volume-outcomes relationships are well characterized.38 Despite these, this registry provides critical information regarding treatment of patients with STS. The use of Medicare databases is limited in having data only from patients over the age of 65 years. Thus, although the FCDS set is not perfect, it provides an excellent database for assessment of volume-outcome relationship in the management of patients with STS particularly as the majority of sarcoma patients are under 65 years.
CONCLUSION
This analysis reveals a direct correlation between hospital surgical volume and both short-term and long-term treatment outcomes for STS. While the observations reported here require confirmation with additional independent data sets, they argue persuasively for exclusive referral of patients with STS to high-volume specialized centers for optimal treatment, survival, and functional outcome.
Footnotes
Reprints: Leonidas G. Koniaris, MD, Alan Livingstone Chair in Surgical Oncology, University of Miami School of Medicine, 3550 Sylvester Comprehensive Cancer Center, 1475 NW 12th Ave., Miami, FL 33136. E-mail: lkoniaris@med.miami.edu.
REFERENCES
- 1.Borden EC, Baker LH, Bell RS, et al. Soft tissue sarcomas of adults: state of the translational science. Clin Cancer Res. 2003;9:1941–1956. [PubMed] [Google Scholar]
- 2.Zahm SH, Fraumeni JF Jr. The epidemiology of soft tissue sarcoma. Semin Oncol. 1997;24:504–514. [PubMed] [Google Scholar]
- 3.Cameron JL. Current Surgical Therapy, 8th ed. Philadelphia: Elsevier Mosby, 2004. [Google Scholar]
- 4.Mack LA, Crowe PJ, Yang JL, et al. Preoperative chemoradiotherapy (modified Eilber protocol) provides maximum local control and minimal morbidity in patients with soft tissue sarcoma. Ann Surg Oncol. 2005;12:646–653. [DOI] [PubMed] [Google Scholar]
- 5.Stojadinovic A, Leung DH, Allen P, et al. Primary adult soft tissue sarcoma: time-dependent influence of prognostic variables. J Clin Oncol. 2002;20:4344–4352. [DOI] [PubMed] [Google Scholar]
- 6.Florida Cancer Data System (FCDS), 2005. University of Miami Miller School of Medicine and the Florida Department of Health; Accessed September, 2005.
- 7.Fritz AG. International Classification of Diseases for Oncology, 3rd ed. Geneva: World Health Organization, 2000. [Google Scholar]
- 8.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364–1369. [DOI] [PubMed] [Google Scholar]
- 9.Luft HS. The relation between surgical volume and mortality: an exploration of causal factors and alternative models. Med Care. 1980;18:940–959. [DOI] [PubMed] [Google Scholar]
- 10.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511–520. [DOI] [PubMed] [Google Scholar]
- 11.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–1751. [DOI] [PubMed] [Google Scholar]
- 12.Gordon TA, Burleyson GP, Tielsch JM, et al. The effects of regionalization on cost and outcome for one general high-risk surgical procedure. Ann Surg. 1995;221:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birkmeyer JD, Warshaw AL, Finlayson SR, et al. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery. 1999;126:178–183. [PubMed] [Google Scholar]
- 14.Glasgow RE, Mulvihill SJ. Hospital volume influences outcome in patients undergoing pancreatic resection for cancer. West J Med. 1996;165:294–300. [PMC free article] [PubMed] [Google Scholar]
- 15.Imperato PJ, Nenner RP, Starr HA, et al. The effects of regionalization on clinical outcomes for a high risk surgical procedure: a study of the Whipple procedure in New York State. Am J Med Qual. 1996;11:193–197. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman MD, Kilburn H, Lindsey M, et al. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg. 1995;222:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sosa JA, Bowman HM, Gordon TA, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg. 1998;228:429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simunovic M, To T, Theriault M, et al. Relation between hospital surgical volume and outcome for pancreatic resection for neoplasm in a publicly funded health care system. CMAJ. 1999;160:643–648. [PMC free article] [PubMed] [Google Scholar]
- 19.Simons AJ, Ker R, Groshen S, et al. Variations in treatment of rectal cancer: the influence of hospital type and caseload. Dis Colon Rectum. 1997;40:641–646. [DOI] [PubMed] [Google Scholar]
- 20.Schrag D, Cramer LD, Bach PB, et al. Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA. 2000;284:3028–3035. [DOI] [PubMed] [Google Scholar]
- 21.Hannan EL, O'Donnell JF, Kilburn H Jr, et al. Investigation of the relationship between volume and mortality for surgical procedures performed in New York State hospitals. JAMA. 1989;262:503–510. [PubMed] [Google Scholar]
- 22.Riley G, Lubitz J. Outcomes of surgery among the Medicare aged: surgical volume and mortality. Health Care Financ Rev. 1985;7:37–47. [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon TA, Bowman HM, Bass EB, et al. Complex gastrointestinal surgery: impact of provider experience on clinical and economic outcomes. J Am Coll Surg. 1999;189:46–56. [DOI] [PubMed] [Google Scholar]
- 24.Harmon JW, Tang DG, Gordon TA, et al. Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Ann Surg. 1999;230:404–411; discussion 411–413. [DOI] [PMC free article] [PubMed]
- 25.Hannan EL, Radzyner M, Rubin D, et al. The influence of hospital and surgeon volume on in-hospital mortality for colectomy, gastrectomy, and lung lobectomy in patients with cancer. Surgery. 2002;131:6–15. [DOI] [PubMed] [Google Scholar]
- 26.Patti MG, Corvera CU, Glasgow RE, et al. A hospital's annual rate of esophagectomy influences the operative mortality rate. J Gastrointest Surg. 1998;2:186–192. [DOI] [PubMed] [Google Scholar]
- 27.Romano PS, Mark DH. Patient and hospital characteristics related to in-hospital mortality after lung cancer resection. Chest. 1992;101:1332–1337. [DOI] [PubMed] [Google Scholar]
- 28.Roohan PJ, Bickell NA, Baptiste MS, et al. Hospital volume differences and five-year survival from breast cancer. Am J Public Health. 1998;88:454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. [DOI] [PubMed] [Google Scholar]
- 30.Lewis JJ, Leung D, Woodruff JM, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linehan DC, Lewis JJ, Leung D, et al. Influence of biologic factors and anatomic site in completely resected liposarcoma. J Clin Oncol. 2000;18:1637–1643. [DOI] [PubMed] [Google Scholar]
- 32.Cormier JN, Huang X, Xing Y, et al. Cohort analysis of patients with localized, high-risk, extremity soft tissue sarcoma treated at two cancer centers: chemotherapy-associated outcomes. J Clin Oncol. 2004;22:4567–4574. [DOI] [PubMed] [Google Scholar]
- 33.Windham TC, Pisters PW. Retroperitoneal sarcomas. Cancer Control. 2005;12:36–43. [DOI] [PubMed] [Google Scholar]
- 34.Karakousis CP, Proimakis C, Walsh DL. Primary soft tissue sarcoma of the extremities in adults. Br J Surg. 1995;82:1208–1212. [DOI] [PubMed] [Google Scholar]
- 35.Noria S, Davis A, Kandel R, et al. Residual disease following unplanned excision of soft-tissue sarcoma of an extremity. J Bone Joint Surg Am. 1996;78:650–655. [DOI] [PubMed] [Google Scholar]
- 36.Shiu MH, Hilaris BS, Harrison LB, et al. Brachytherapy and function-saving resection of soft tissue sarcoma arising in the limb. Int J Radiat Oncol Biol Phys. 1991;21:1485–1492. [DOI] [PubMed] [Google Scholar]
- 37.Simon MA, Enneking WF. The management of soft-tissue sarcomas of the extremities. J Bone Joint Surg Am. 1976;58:317–327. [PubMed] [Google Scholar]
- 38.Christian CK, Gustafson ML, Betensky RA, et al. The volume-outcome relationship: don't believe everything you see. World J Surg. 2005;29:1241–1244. [DOI] [PubMed] [Google Scholar]