Abstract
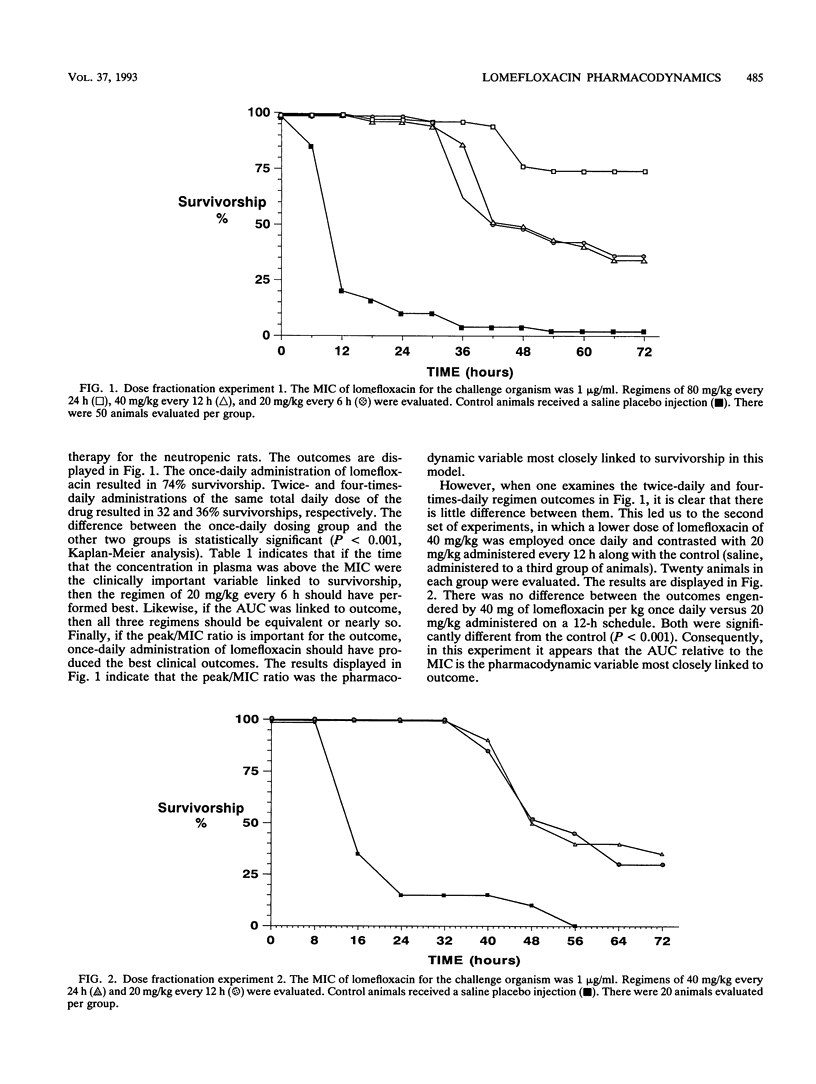
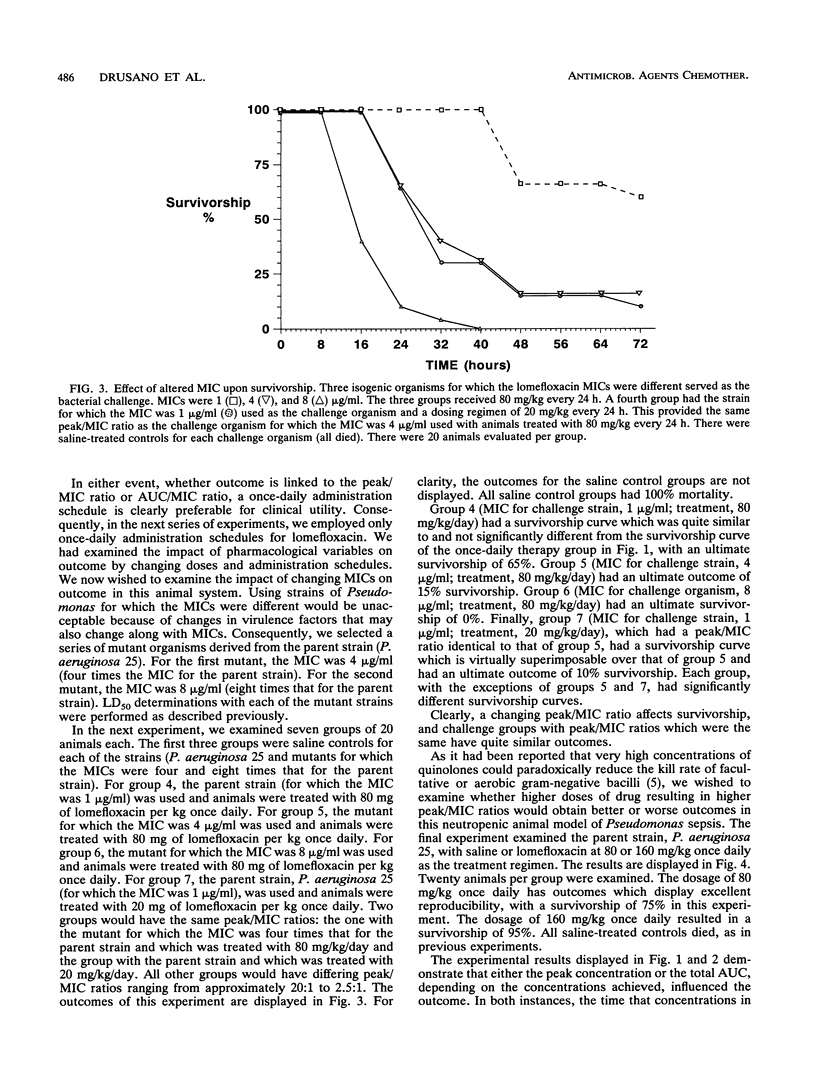
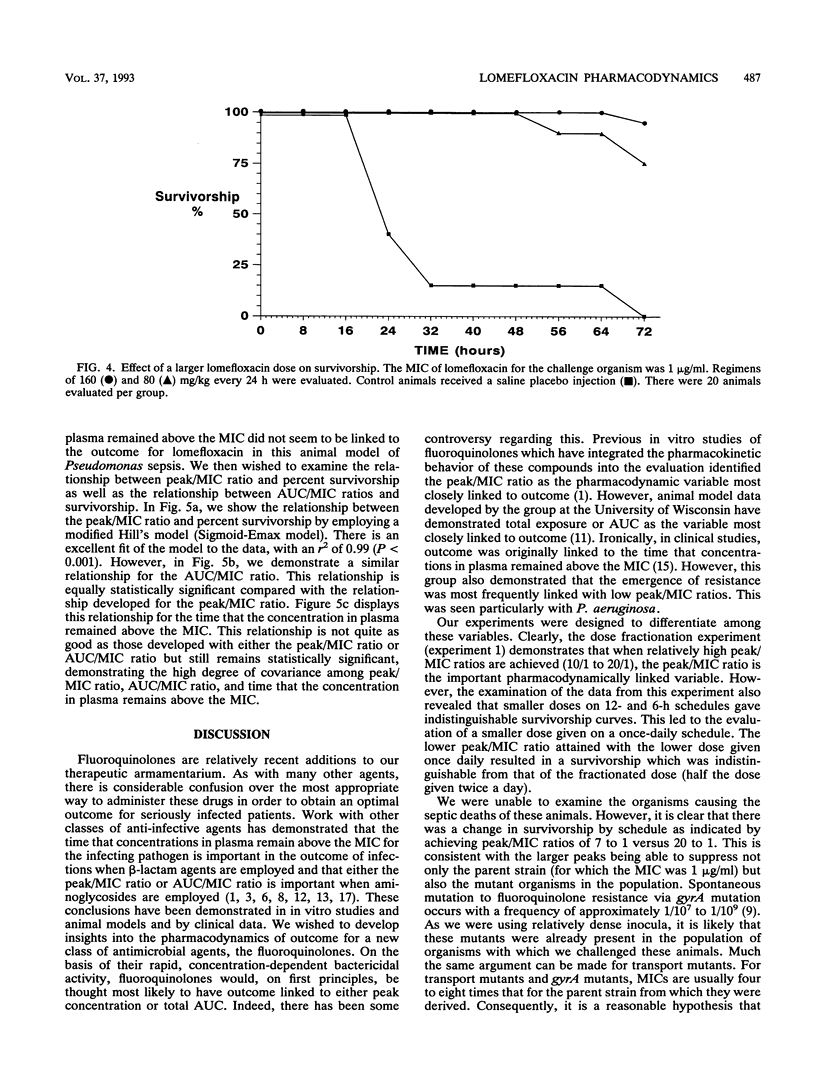
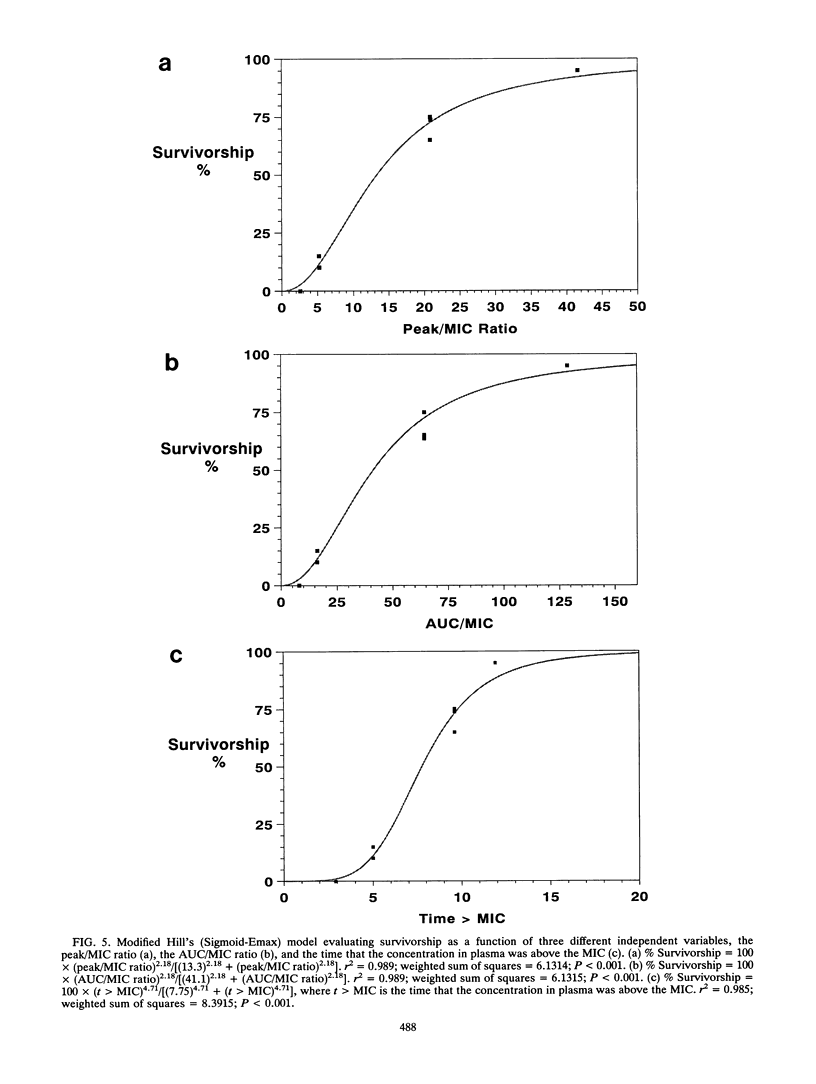
We examined the impact of dose fractionation and altered MICs on survivorship in a neutropenic rat model of Pseudomonas aeruginosa sepsis employing the new fluoroquinolone antibiotic lomefloxacin. Once-daily administration of a drug dose which produced a high peak concentration/MIC (peak/MIC) ratio (ca. 20/1) produced significantly better survivorship compared with regimens employing the same daily dose but on a more fractionated schedule. The use of a smaller dose, producing lower (< 10/1) peak/MIC ratios, did not show this effect, as once-daily and twice-daily regimens produced equivalent results (the area under the concentration-time curve/MIC ratio was linked to survivorship). Challenge with resistant mutants selected for altered MICs of fluoroquinolones (two and four times the MIC for the parent strain, respectively) resulted in markedly diminished survivorship. Challenge with the parent strain and use of a drug dose which produced a peak/MIC ratio identical to that for animals challenged with the mutant for which the MIC was four times that for the parent strain and treated with the larger drug dose produced survivorship curves which were not different. For this animal model, peak/MIC ratio was linked to survivorship, particularly when high ratios (10/1 to 20/1) were obtained. At lower doses, producing peak/MIC ratios < 10/1, the area under the concentration-time curve relative to the MIC appeared to be most closely linked to outcome. The time that levels in plasma exceeded the MIC did not influence survivorship. The hypothesis most likely to explain these findings is that higher peak/MIC ratios can suppress the parent strain and mutant organisms (gyrA and transport mutants) for which the MIC is higher but limited (no more than eight times that for the parent strain).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser J., Stone B. B., Groner M. C., Zinner S. H. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother. 1987 Jul;31(7):1054–1060. doi: 10.1128/aac.31.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G. L. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemother. 1988 Mar;32(3):289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G. L., Yuen G. J., Lambert J. S., Seidlin M., Dolin R., Valentine F. T. Relationship between dideoxyinosine exposure, CD4 counts, and p24 antigen levels in human immunodeficiency virus infection. A phase I trial. Ann Intern Med. 1992 Apr 1;116(7):562–566. doi: 10.7326/0003-4819-116-7-562. [DOI] [PubMed] [Google Scholar]
- Dudley M. N., Mandler H. D., Gilbert D., Ericson J., Mayer K. H., Zinner S. H. Pharmacokinetics and pharmacodynamics of intravenous ciprofloxacin. Studies in vivo and in an in vitro dynamic model. Am J Med. 1987 Apr 27;82(4A):363–368. [PubMed] [Google Scholar]
- Dudley M. N. Pharmacodynamics and pharmacokinetics of antibiotics with special reference to the fluoroquinolones. Am J Med. 1991 Dec 30;91(6A):45S–50S. doi: 10.1016/0002-9343(91)90311-k. [DOI] [PubMed] [Google Scholar]
- Gerber A. U., Craig W. A., Brugger H. P., Feller C., Vastola A. P., Brandel J. Impact of dosing intervals on activity of gentamicin and ticarcillin against Pseudomonas aeruginosa in granulocytopenic mice. J Infect Dis. 1983 May;147(5):910–917. doi: 10.1093/infdis/147.5.910. [DOI] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Ng E. Y., Swartz M. N. Mechanisms of action of and resistance to ciprofloxacin. Am J Med. 1987 Apr 27;82(4A):12–20. [PubMed] [Google Scholar]
- Johnson D. E., Thompson B., Calia F. M. Comparative activities of piperacillin, ceftazidime, and amikacin, alone and in all possible combinations, against experimental Pseudomonas aeruginosa infections in neutropenic rats. Antimicrob Agents Chemother. 1985 Dec;28(6):735–739. doi: 10.1128/aac.28.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett J. E., Ebert S., Fantin B., Craig W. A. Comparative dose-effect relations at several dosing intervals for beta-lactam, aminoglycoside and quinolone antibiotics against gram-negative bacilli in murine thigh-infection and pneumonitis models. Scand J Infect Dis Suppl. 1990;74:179–184. [PubMed] [Google Scholar]
- Moore R. D., Lietman P. S., Smith C. R. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987 Jan;155(1):93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- Moore R. D., Smith C. R., Lietman P. S. Association of aminoglycoside plasma levels with therapeutic outcome in gram-negative pneumonia. Am J Med. 1984 Oct;77(4):657–662. doi: 10.1016/0002-9343(84)90358-9. [DOI] [PubMed] [Google Scholar]
- Peloquin C. A., Cumbo T. J., Nix D. E., Sands M. F., Schentag J. J. Evaluation of intravenous ciprofloxacin in patients with nosocomial lower respiratory tract infections. Impact of plasma concentrations, organism, minimum inhibitory concentration, and clinical condition on bacterial eradication. Arch Intern Med. 1989 Oct;149(10):2269–2273. [PubMed] [Google Scholar]
- Schentag J. J., Smith I. L., Swanson D. J., DeAngelis C., Fracasso J. E., Vari A., Vance J. W. Role for dual individualization with cefmenoxime. Am J Med. 1984 Dec 21;77(6A):43–50. doi: 10.1016/s0002-9343(84)80074-1. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Nakagawa T., Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978 Apr;6(2):165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]



