Abstract
Objective:
The aims of this study were to present evidence to develop and validate the Japanese Tumor-Node-Metastasis (TNM) staging system for primary liver cancer and to compare its discriminatory ability and predictive power with those of Vauthey's simplified staging, which was adopted as the TNM staging system of the American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC).
Summary Background Data:
Among many staging systems for hepatocellular carcinoma, the Japanese TNM staging system and the AJCC/UICC staging system were developed based on a survival analysis of surgical patients. These 2 staging systems have not been compared in large series.
Methods:
The Liver Cancer Study Group of Japan (LCSGJ) prospectively collected clinicopathologic data of 63,736 patients with primary liver cancer from 1995 to 2001. Among them, 13,772 patients received curative hepatic resection. Based on univariate and multivariate survival analyses, the Japanese TNM staging system was developed. The accuracy of the Japanese TNM staging system for predicting patient survival was compared with that of the AJCC/UICC staging system using the cross-validation method.
Results:
The independent prognostic factors (relative risk; 95% confidence interval) were vascular or bile duct invasion (1.36;1.29–1.43), liver cirrhosis (1.26;1.20–1.32), diameter (≤2 cm or >2 cm) (1.21;1.14–1.28), alpha-fetoprotein (1.20;1.15–1.25), single/multiple (1.18;1.12–1.23), liver damage (1.15;1.10–1.20), hepatic involvement (1.14;1.09–1.19), histologic differentiation (1.14;1.08–1.20), gross classification (1.13;1.08–1.18), and esophageal varices (1.07;1.02–1.13). Based on these results, 3 criteria (vascular or bile duct invasion, diameter, and single/multiple) were selected. Patients with none of these 3 factors were considered T1, and those with 1, 2, and 3 factors were T2, T3, and T4, respectively. The number of patients and 5-year survival rates for T1, T2, T3, and T4 were 2078, 70%; 6853, 58%; 3021, 41%; and 582, 24% (P < 0.0001), respectively, while those for the AJCC-T were 8457, 61% in T1, 2888, 46% in T2, and 1189, 30% in T3 (P < 0.0001). While both the LCSGJ-T and the AJCC-T had good discriminating ability, the former was significantly superior (P = 0.0007).
Conclusions:
Our findings support the development of LCSG stage. While both staging systems allow for the clear stratification of patients into prognostic groups, the LCSGJ staging may be more appropriate for stratifying patients with early-stage HCC.
There are currently two Tumor-Node-Metastasis (TNM) classifications for hepatocellular carcinoma: the Japanese TNM and AJCC/UICC TNM (Vauthey simplified stage). We present evidence to develop and validate the Japanese TNM staging system and compare it with the latter system in 13,772 patients.
Over the past 20 years, great progress has been made in the diagnosis of hepatocellular carcinoma (HCC); high-risk groups for this disease can be established, and the number of patients with resectable HCC and small-sized HCC is increasing. Under these circumstances, liver transplantation, hepatic resection, radiofrequency ablation, and transarterial chemoembolization have all been used in these patients according to their clinicopathologic characteristics and hepatic functional reserve, but the optimal management for these patients remains controversial.1,2 As a result, there is an increasing need for a staging system that can reflect the prognosis and permit the stratification of these patients for clinical trials. Several staging systems have been proposed: Okuda staging, the Cancer of the Liver Italian Program (CLIP), the Barcelona Clinic Liver Cancer staging, the Japan Integrated Staging Score (JIS), the Chinese University Prognostic Index, and the French Score.3–8 All of these staging systems include liver function parameters, and the percentages of patients who received hepatic resection among all of the patients used to develop the stages were 18.5% (Okuda), 10.4% (Chinese University Prognostic Index), 6% (CLIP), and 7% (French). In an attempt to standardize the staging of HCC, the American Hepatico-Pancreatico-Biliary Association organized a consensus conference that was cosponsored by the American Joint Committee on Cancer (AJCC) in 2002. The consensus panel made important observations regarding the purposes of various staging systems and noted that 2 types of staging systems were required to adequately stage the spectrum of HCC: a medical staging system that covered all patients with HCC and a surgical staging system that was designed for patients who were operable.9 The staging systems described above are considered medical staging systems.
There are currently 2 surgical staging systems, which were developed based on the analysis of patients who received hepatic resection: one from the Liver Cancer Study Group of Japan (LCSGJ) and another from the AJCC/International Union Against Cancer (UICC). In 1983, the LCSGJ first introduced an HCC Tumor-Node-Metastasis (TNM) scheme, which has subsequently been revised, most recently from the third to 4th edition in 2000.10,11 Vauthey et al developed a simplified staging system for HCC in 2002,12 which was adopted as the TNM staging system of AJCC/UICC after minor changes.13 The prognostic power and stratification ability of the Japanese TNM Staging System has been verified in Japanese and Chinese patients,14–16 and it has been compared with the AJCC/UICC staging system.16 These 2 staging systems have some similarities; for example, parameters of liver function are not included, patients with distant metastasis are assigned to the highest stage, and those with hepatic lymph node metastasis are assigned to the second highest stage. In contrast, they use different methods for determining the T classification (Figs. 1, 2). In this paper, we present evidence for the development of the Japanese TNM system, validate the system, and compare its discriminatory ability and predictive power to those of the AJCC/UICC staging system in 13,772 patients who received curative hepatic resection.
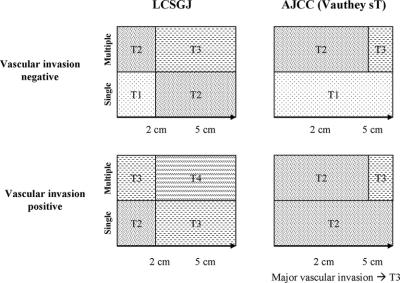
FIGURE 1. Comparison of the T classification in LCSGJ and AJCC/UICC.
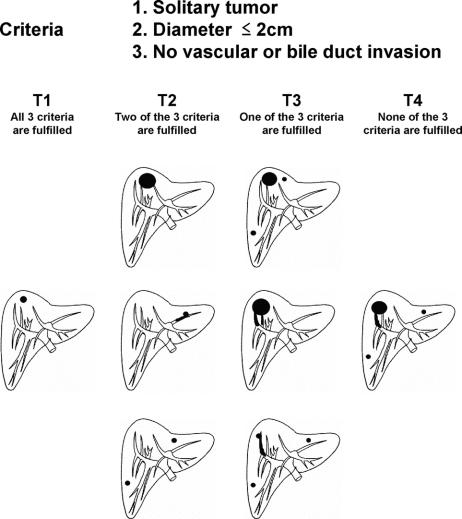
FIGURE 2. The T category of LCSGJ is determined on the basis of the “number,” “size,” and “vascular or bile duct invasion.” All multiple tumors, including multicentric tumors and intrahepatic metastatic tumors, are equally counted.
MATERIALS AND METHODS
Source of Data
LCSGJ determined the classification and handling methods of primary liver cancer in 1965 and started a nationwide registration of clinicopathologic and prognostic data of patients with primary liver cancer.17–23 Questionnaires that included 178 items of clinicopathologic data were mailed to all of the LCSGJ-approved hospitals in Japan, and these data were entered into a computer, once every 3 years from 1970 (first) to 1979 (4th), and once every 2 years after 1981 (fifth). The status of the presence of recurrence, additional treatment, and final prognosis of the registered patients were also followed until confirmation of death at every survey. Micropathologic data of liver tumor were requested on the form from the 12th survey. Accordingly, the data from the 12th to 15th surveys were used in this study. The number of patients and hospitals in each survey are shown in Table 1. Of the total 66,007 patients with primary liver cancer, the clinical diagnosis of 63,736 patients (96.6%) was HCC, and 18,948 (29.7%) received hepatic resection. Of these, 1189 patients without pathologic data, 956 with incomplete survival data, and 1881 without data on operative curability, distant metastasis, or hepatic lymph node metastasis were excluded, which meant that eventually 14,922 patients were included in this study (hepatectomy-cohort). Of these 14,922 patients, the operations were not curative in 1150, and 13,772 received curative hepatic resection (curative-hepatectomy-cohort). Among these patients, 76 had distant metastasis, 147 had hepatic lymph node metastasis, and 17 had both. The 13,566 remaining patients were included in the curative-hepatectomy-N0M0 cohort.
TABLE 1. Number of Registered Patients and Clinical Diagnosis
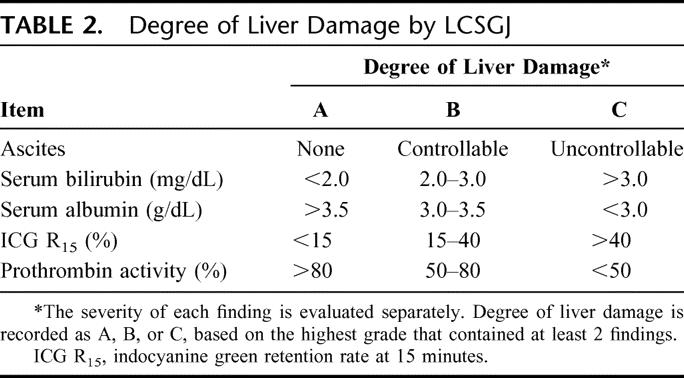
The prognosis was examined in February 2001, and was categorized as alive, dead, or unknown. Death was subclassified according to the direct cause: death by HCC, liver failure, gastrointestinal bleeding, rupture of HCC, operative death, and other. All deaths were counted as events and living patients were censored to the date of the last follow-up. Curative resection was defined as that in which the entire tumor could be removed macroscopically. Lymph node involvement and distant metastasis were based on macroscopic inspection and palpation at the time of surgery. Tumor size was based on the largest dimension of the tumor specimen. Portal, hepatic venous, and bile duct invasion were defined by macroscopic examination of resected specimens. The number of HCCs was defined by the total number of nodules, including intrahepatic metastasis, in the resected specimen. Hepatic involvement means the number of segments in which liver tumors are present. The degree of liver damage as a guide to liver function was defined by LCSGJ based on ascites, serum bilirubin, serum albumin, indocyanine green retention rate at 15 minutes, and prothrombin activity (Table 2). 10,11,22 The serologic presence of hepatitis B surface antigen was considered to be positive evidence of hepatitis B serology, and hepatitis C antibody was considered to be positive for hepatitis C serology. A history of alcohol consumption of 86 g of ethanol per day over a 10-year period was defined as positive.24
TABLE 2. Degree of Liver Damage by LCSGJ
Statistical Analysis
Survival was measured from the time of hepatic resection, and death was the endpoint. Survival curves were constructed using the Kaplan-Meier product-limit method and compared using a log-rank test. Significant prognostic factors in a univariate analysis were entered into a Cox proportional hazards model using stepwise selection to identify independent predictors of death. Statistical significance was defined as a P value <0.05. Statistical Analysis System, version 8 (SAS Institute Inc., Cary, NC) was used for statistical analyses. Based on these survival analyses, the LCSGJ T classification was developed and the effects of liver cirrhosis and degree of liver damage as defined by LCSGJ were evaluated because these variables were components of other tumor classification scheme, such as CLIP or JIS.
The abilities of the LCSGJ T classification and the AJCC T classification to accurately predict survival were verified and compared by the cross-validation method. Patients were randomly divided into 2 groups: a training sample and a validation sample. In the training sample, 2 predictive models were constructed using the Cox proportional hazards model, which included either the LCSGJ T classification or the AJCC T classification as a covariate. In the validation sample, each estimated model from the test sample was used to predict the survival for each patient. The predicted survival curves, plotted based on each model, were compared with the observed survival curves in the validation sample plotted by the Kaplan-Meier method. The predictive accuracies were compared in terms of the residual, which was the difference between the observed survival time and the predicted survival time in the validation sample. An analysis of variance using the Generalized Estimating Equation method was used to compare the absolute values of the residuals between the 2 T classifications.25
RESULTS
Of the 14,922 patients who underwent hepatic resection for HCC, 10,259 were alive and 4663 had died. The direct cause of death was HCC in 2886 patients, liver failure in 844, gastrointestinal bleeding in 81, rupture of esophageal varices in 112, rupture of HCC in 38, operative death in 186, and other in 516.
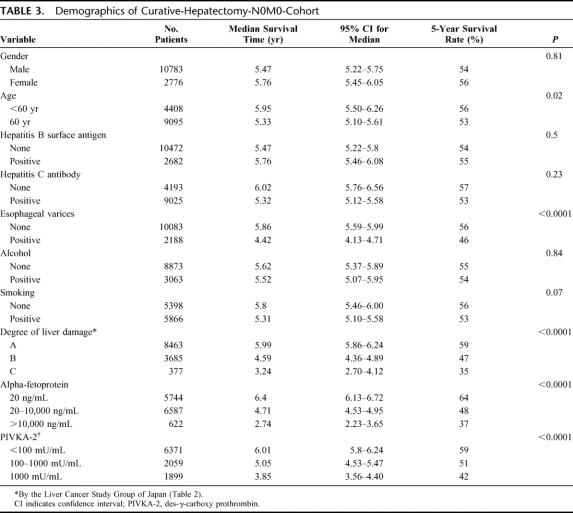
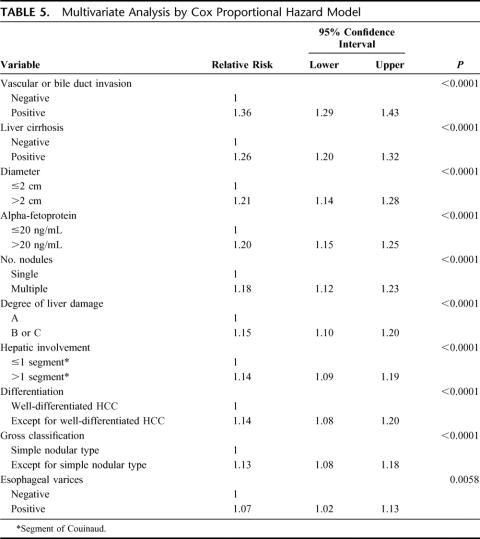
The demographic and clinicopathologic characteristics of the 13,566 patients in the curative-hepatectomy-N0M0 cohort are shown in Tables 3 and 4. The survival curves according to the number of HCCs are shown in Figure 3a. Single HCC showed the best prognosis, and the survival time gradually decreased with an increase in the number of HCCs. The diameter of the largest nodule significantly influenced survival (Fig. 3b). Patients with HCC ≤2 cm had a significantly longer duration of survival than those with HCC of 2 to 5 cm (P < 0.0001). The survival curves according to the location of portal invasion are shown in Figure 3c. The significant factors identified by a multivariate analysis are shown in Table 5. Vascular or bile duct invasion (portal vein, hepatic vein, or bile duct invasion) had the greatest impact on survival, followed by the presence of liver cirrhosis in the background liver, diameter of HCC, alpha-fetoprotein, number of HCCs, degree of liver damage, hepatic involvement, grade of differentiation, gross classification, and presence of esophageal varices.
TABLE 3. Demographics of Curative-Hepatectomy-N0M0-Cohort
TABLE 4. Pathologic Factors of Curative-Hepatectomy-N0M0-Cohort
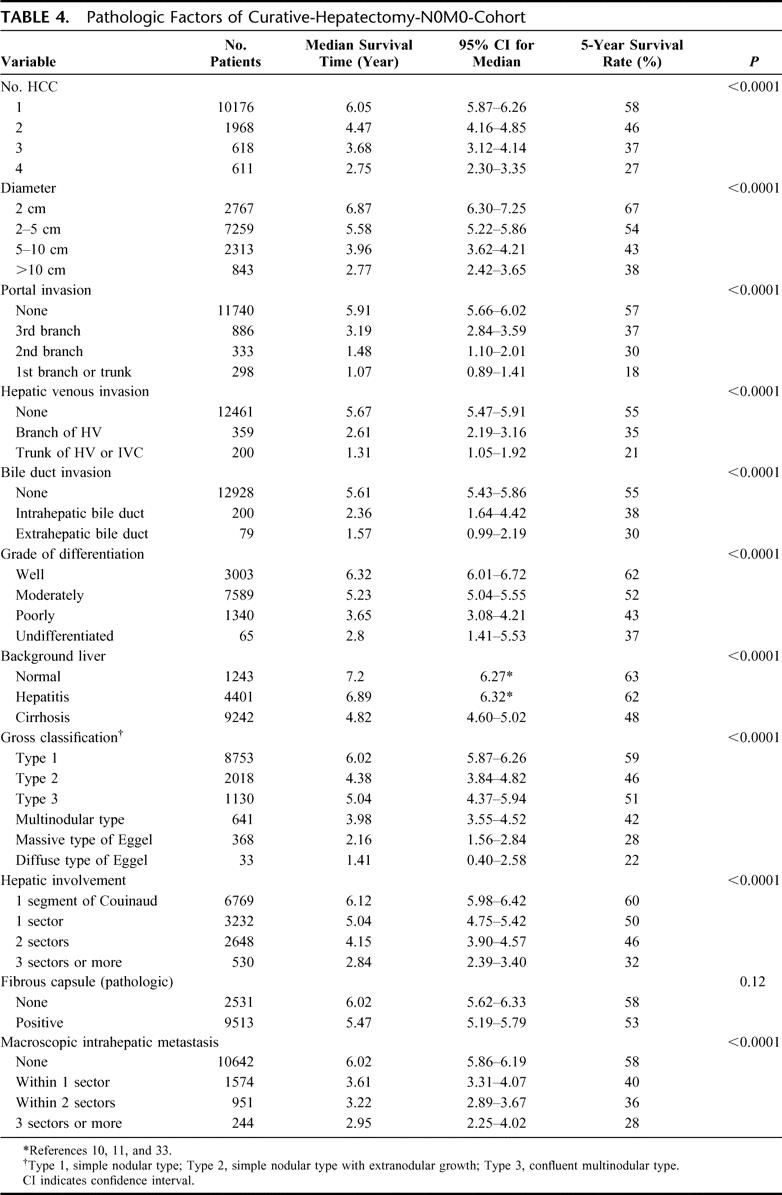
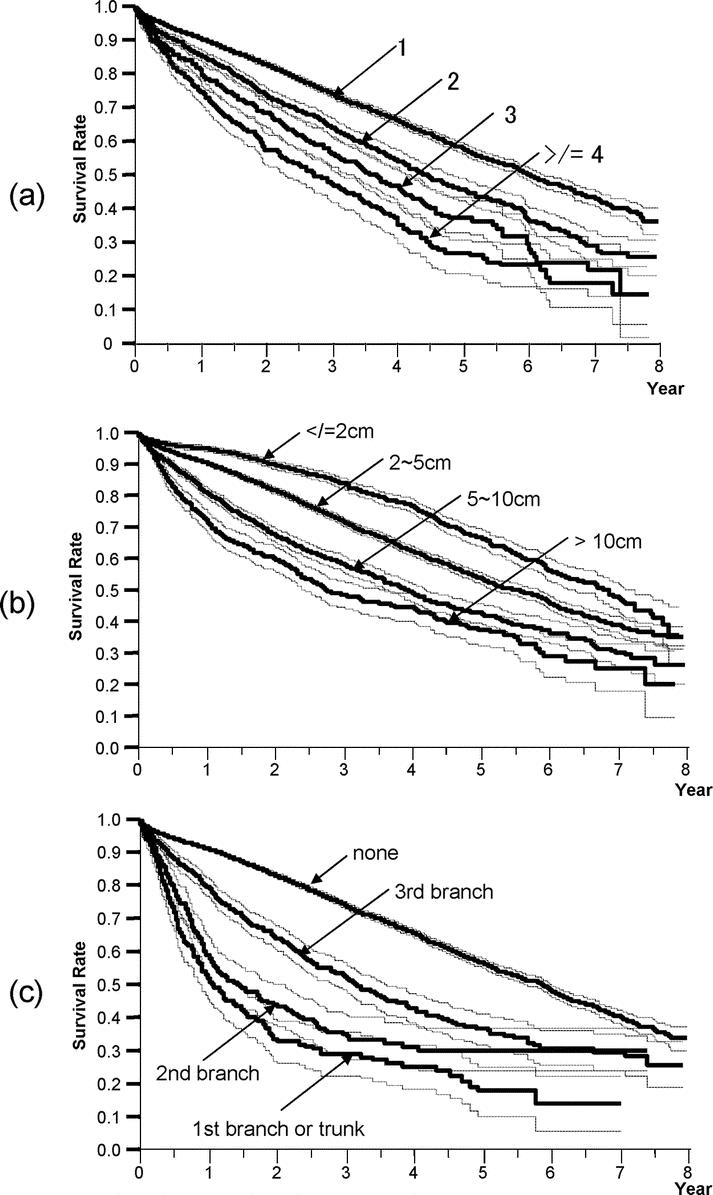
FIGURE 3. Kaplan-Meier survival analysis (solid line) with 95% confidence interval (dotted line) for patients in the curative-hepatectomy-N0M0 cohort stratified according to the number of liver nodules (a), the maximum diameter of liver nodules (b), and the location of portal invasion (c). The number, median survival time (95% CI), and 5-year survival rate of patients are described in Table 4.
TABLE 5. Multivariate Analysis by Cox Proportional Hazard Model
Development of T Classification
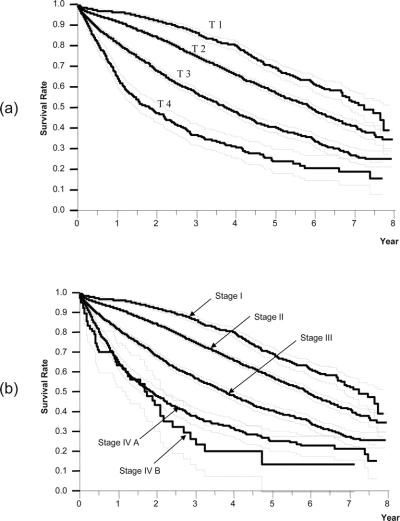
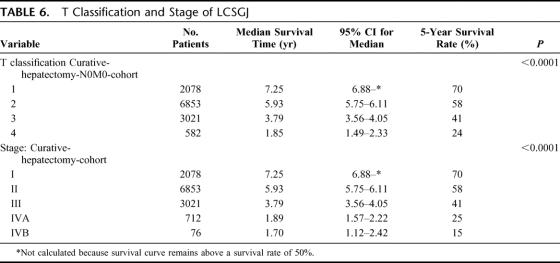
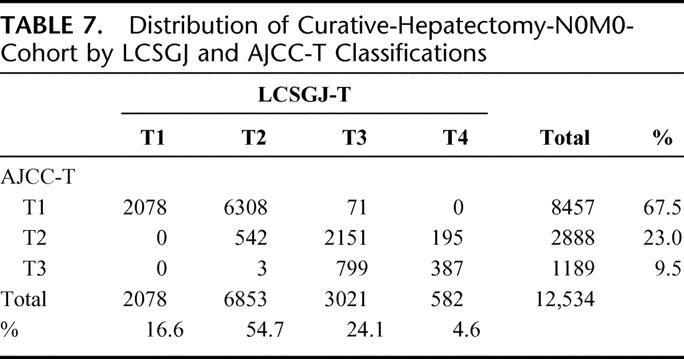
Based on the results of the multivariate analysis, we stratified patients into 4 groups according to the presence of vascular or bile duct invasion, and the number of HCCs (single or multiple). Because these factors had the first and fifth highest relative risk for death in stepwise Cox proportional model (Table 5), and were used in the previous LCSGJ-TNM scheme, third edition. The survival curves of these 4 groups were clearly separate, and the differences in survival between any 2 of these groups were significant; the P values of all combinations were less than 0.0001 (Fig. 4a). The impact of tumor size on survival was analyzed within each group because it had third highest relative risk for death in stepwise Cox proportional model, and were used in the previous LCSGJ-TNM scheme, third edition. In all of the groups, tumor size significantly affected survival (Fig. 4b–e). In particular, patients with tumors that were 2 cm or smaller in diameter had a more favorable prognosis in all of the groups. The median survival time (95% CI) and the 5-year survival rate of patients with HCCs of 2 cm or smaller were as follows: 7.3 years (6.9-*) and 70% in patients with single tumor without vascular or bile duct invasion (Fig. 4b), 5.4 years (4.5–6.0) and 54% in patients with multiple tumors without vascular or bile duct invasion (Fig. 4c), 5.8 years (4.9-*) and 64% in patients with single tumor with vascular or bile duct invasion (Fig. 4d), and years (1.9-*) and 52% in patients with multiple tumors with vascular or bile duct invasion (Fig. 4e). (*Not calculated because survival curve remains above a survival rate of 50%.) Patients with tumors 2 cm or smaller in diameter had a more favorable prognosis regardless of vascular or bile duct invasion or growth pattern (single or multiple). Therefore, these criteria [growth pattern (single or multiple), vascular or bile duct invasion, and size (≤2 cm or >2 cm)] were used to determine T classification. Patients who had none of these 3 factors were assigned to T1, and those with 1, 2, and 3 factor(s) were considered T2, T3, and T4. The survival of patients according to this T classification is shown in Figure 5a. The median survival time (95% CI) and 5-year survival rate of patients with T4 HCC in the curative-N0M0-cohort was 1.85 years (1.49–2.33 years) and 24%, which was similar to the values in patients with hepatic lymph node metastasis in the curative-hepatectomy-cohort (any T N1 M0); 1.9 years (1.3–2.5 years) and 32%. This prompted us to combine these patients into a single group; stage IVA. Patients with extrahepatic metastasis (any T any N M1) showed a median survival time (95% CI) of 1.7 years (1.1–2.4 years) and a 5-year survival rate of 15%, and these patients were assigned to stage IVB. Patients with T1N0M0, T2N0M0, and T3N0M0 HCC were assigned to stage I, stage II, and stage III, respectively (Table 6). The survival curves are shown in Figure 5b. The distribution of curative-hepatectomy-N0M0-cohort by LCSGJ-T and AJCC T is shown in Table 7.
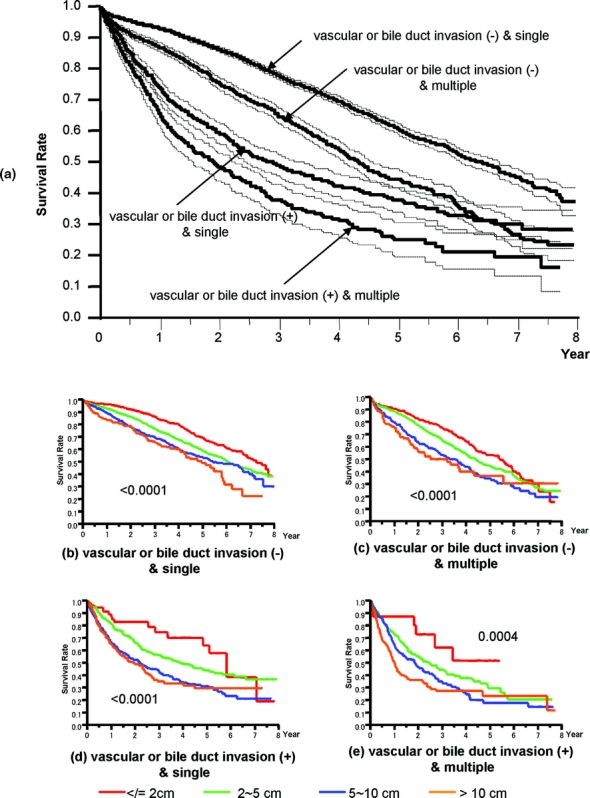
FIGURE 4. Kaplan-Meier survival analysis (solid line) with 95% confidence interval (dotted line) for patients in the curative-hepatectomy-N0M0 cohort stratified according to vascular or bile duct invasion, and growth pattern (single or multiple). The number, median survival time (95% CI), and 5-year survival rate of patients with vascular or bile duct invasion (−) and single, vascular or bile duct invasion (−) and multiple, vascular or bile duct invasion (+) and single, and vascular or bile duct invasion (+) and multiple were 8565, 6.41 years (6.08–6.71), 61%; 2461, 4.37 years (4.12–4.61), 45%; 1163, 2.90 years (2.48–3.23), 38%; and 615, 1.92 years (1.56–2.28), 25% (P < 0.0001) (a). Influence of tumor size in the 4 groups (b, c, d, e). Tumor size significantly influenced survival in all of the groups.
FIGURE 5. Kaplan-Meier survival analysis (solid line) with 95% confidence interval (dotted line) for patients in the curative-hepatectomy-N0M0 cohort stratified according to the T classification (a) and the stage (b) of LCSGJ.
TABLE 6. T Classification and Stage of LCSGJ
TABLE 7. Distribution of Curative-Hepatectomy-N0M0-Cohort by LCSGJ and AJCC-T Classifications
The effects of liver cirrhosis and degree of liver damage as defined by LCSGJ on patients with T1, T2, T3, and T4 HCC are shown in Figure 6 because these variables are components of other tumor classification scheme, such as CLIP or JIS. The presence of liver cirrhosis in the background liver was a negative prognostic factor in patients with all of the T-classes of HCC. The degree of liver damage significantly influenced the prognosis of patients with T1, T2, or T3 tumor (P < 0.0001), but did not affect the prognosis of patients with T4 HCC (P = 0.509).
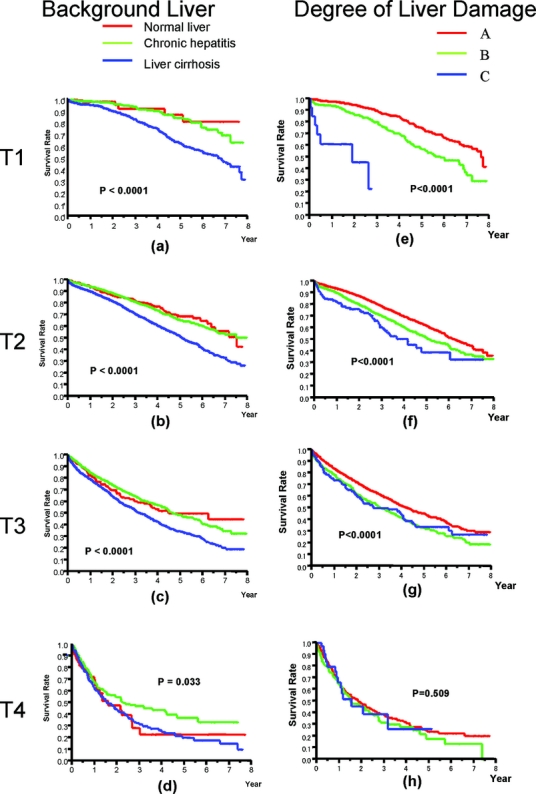
FIGURE 6. Influence of liver cirrhosis, chronic hepatitis (a, b, c, d) and the degree of liver damage (e, f, g, h) in T1, T2, T3, and T4.
Although patients with T1 or T4 HCC are identical with regard to the presence of 3 factors [growth pattern (single or multiple), vascular or bile duct invasion, and size (≤2 cm or >2 cm)], patients with T2 or T3 tumor can be subclassified into 3 groups according to combinations of these 3 factors. While no survival difference was observed in patients with T2 tumor (P = 0.220), among patients with T3 tumor, those with vascular or bile duct invasion showed a significantly worse outcome than those without. When patients with T3 HCC were stratified according to negative factors, the number, median survival time (95% CI), and 5-year survival rate of those with HCCs of 2 cm or smaller, single HCCs, and HCCs without vascular or bile duct invasion were 25, *years (1.9-*), 52%; 1,086, 2.7 years (2.4–3.2), 37%; and 1,917, 4.1 year (3.8-4.5), 42% (P < 0.0001).
Validation of T Classification and Comparison With AJCC T
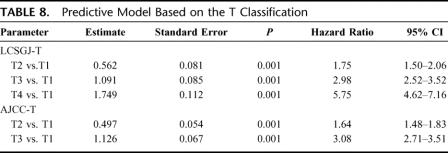
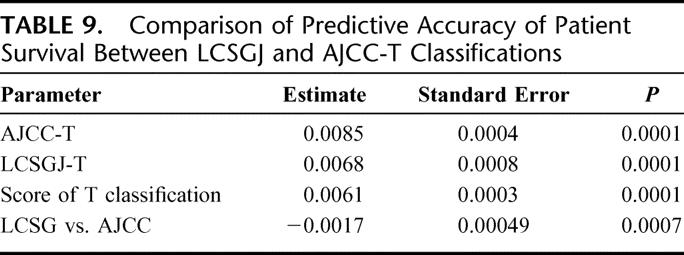
A total of 13,566 patients in the curative-hepatectomy-N0M0-cohort were randomly assigned to either a training sample (n = 6819 patients with 1964 deaths) or a test sample (n = 6747 patients with 1943 deaths) to validate its prognostic significance. Table 8 shows the prediction models constructed by a Cox proportional hazards model in the training sample based on the LCSGJ T classification and the AJCC T classification. In the training sample, both of these models had good discriminating ability. Figure 7 shows the results of the application of each predictive model to the validation sample. The results of an analysis of variance using the Generalized Estimating Equation method for 2 residual sum of squares of each patient are shown in Table 9. The negative estimate of LCSGJ T versus AJCC T (−0.0017) indicated that the difference between the estimated survival time and actual survival time with LCSGJ-T was significantly smaller than that with AJCC T after adjusting for stage (P = 0.0007).
TABLE 8. Predictive Model Based on the T Classification
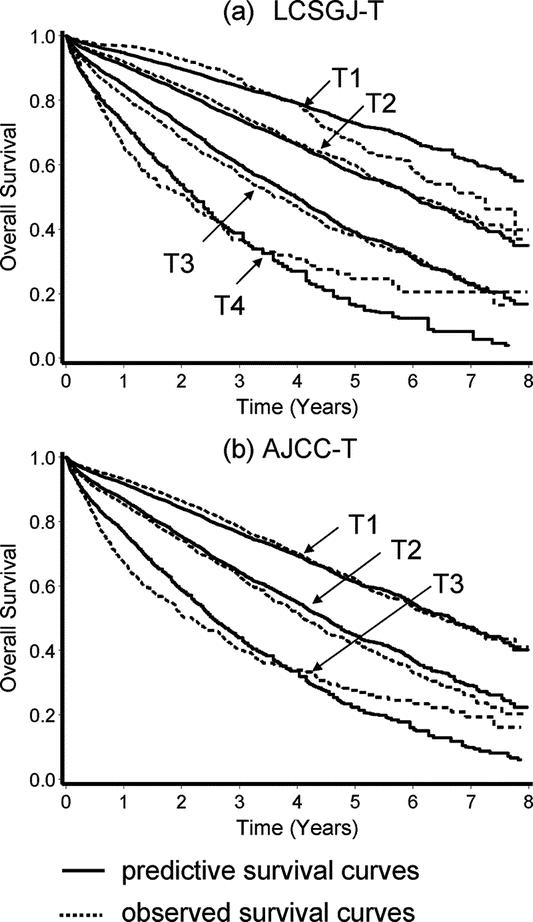
FIGURE 7. Comparison of the predictive survival curves (solid line) and the observed survival curves (broken line) in the validation sample. Prediction based on the LCSGJ-T classification (a), and AJCC T classification (b).
TABLE 9. Comparison of Predictive Accuracy of Patient Survival Between LCSGJ and AJCC-T Classifications
DISCUSSION
LCSGJ published The General Rules for the Clinical and Pathologic Study of Primary Liver Cancer, 4th edition, which described the present staging system, in November 2000.10 The previous AJCC/UICC TNM staging system, introduced in 1988, was derived from the TNM classification third edition of LCSGJ.26 This system required information on the growth pattern (single or multiple), size (≤2 cm or >2 cm), vascular invasion, and location (unilateral or bilateral) to determine the stage. It has been suggested that this system does not stratify patients adequately with respect to prognosis, and might be unnecessarily complex.12,27–30 Izumi et al modified this system by eliminating the size and location (unilateral or bilateral).27 The Izumi-TNM system was developed based on an analysis of 104 patients who underwent hepatic resection at a single center. Its prognostic value was demonstrated in 53 patients who received hepatectomy at an Italian center.29,30 Influenced by this system, Vauthey et al13 proposed a scoring system that included size (≤5 cm or >5 cm), vascular invasion, and growth pattern (single or multiple): patients with 0, 1, 2, and all 3 of these factors were assigned to T1, T2, T3, and T4, respectively.31 They applied this T-stage to 97 patients and demonstrated its ability to stratify patients. It was also validated in 323 patients who received curative hepatic resection in Taiwan.32 In 2002, Vauthey et al developed a simplified staging system based on a survival analysis of 557 patients who received hepatic resection at 4 hepatobiliary centers in the United States, France, and Japan.12 It was adopted as the AJCC Cancer Staging Manual, 6th edition, in 2002.13
The major differences between LCSGJ 4th TNM and AJCC/UICC 6th TNM are the cutoff value for tumor size and its application in prognostic classification (Fig. 1). In the revision from the fifth to sixth edition of the AJCC/UICC TNM staging, the cutoff value for tumor size in the prognostic classification was shifted from 2 cm to 5 cm, based on the results of Vauthey et al.12 In their series, tumor size had no effect on survival in patients with no vascular invasion or microvascular invasion, and had a significant effect on survival only in patients with multiple HCCs.12 The lack of a significant difference may be related to the small sample size. In the AJCC/UICC staging system, a single tumor without vascular invasion is assigned to T1, while a tumor with vascular invasion is assigned to T2 regardless of its size. In our series, the prognosis of patients with a single tumor without vascular invasion showed a wide range according to its size (Fig. 4b): patients with tumors of 2 cm or smaller showed a 5-year survival rate of 70%, while those with tumors of 10 cm or larger had a 5-year survival rate of 49%. The prognosis of patients with vascular invasion is also greatly influenced by the tumor size (Fig. 4d), and that of patients with multiple tumors gradually worsened with increasing tumor size in our data (Fig. 4c, e). Patients with tumors of 2 cm or smaller showed a significantly favorable prognosis regardless of vascular invasion or growth pattern (single or multiple) (Fig. 4b–e). Among tumors 2 cm or smaller, about 22% are early HCCs, which are defined as tumors that have Glisson's triad and show hypercellularity of over 2-fold with minimal cellular or nuclear atypia (Edmondson's grade 1).33,34 These tumors showed a remarkably favorable prognosis: a 5-year survival rate of 93% was reported.34 Ideally, patients with these tumors should be graded to the earliest stage, but the definition of these tumors requires a pathologic examination of a resected specimen. To simplify the diagnosis, a single HCC of 2 cm or smaller without vascular invasion should be graded as the earliest stage.
In the current study, the presence of liver cirrhosis in the background liver and the degree of liver damage10,22 were also independent prognostic factors (P < 0.0001) (Table 5; Fig. 6). This factor significantly influenced the prognosis in the T1, T2, and T3 subsets. The influence of liver damage on survival decreases with T stage, until it is no longer significant for patients with T4 HCC (Fig. 6h). This may be a consequence of the fact that tumor factors govern the prognosis of patients with advanced HCC and liver function plays an important role in patients with relatively early HCC. Thus, a scoring system that uniformly assigns the tumor stage and liver function, for example, the score proposed by CLIP or JIS, may be limited in its ability to stratify patients with an advanced score.4,8,35,36 Accordingly, a staging system that is composed only of tumor factors and is additionally subclassified by liver cirrhosis in the background liver or liver function, like AJCC stage or LCSG stage, may be simple and suitable for patients who will undergo curative treatment such as hepatectomy, radiofrequency ablation, or liver transplantation.
A weakness of this LCSGJ staging system may be a result of the assumption of equal weight for growth pattern (single or multiple), size, and vascular or bile duct invasion. In our data, vascular or bile duct invasion had the strongest impact on survival: its relative risk (RR) was 1.36, followed by liver cirrhosis (RR 1.26), diameter (RR 1.21), alpha-fetoprotein (RR 1.20), and tumor number (RR 1.18) (Table 5). Therefore, patients with T2 or T3 tumor can be divided into 3 subgroups according to these factors, and it is logical to hypothesize that the subgroup with a more heavily weighted prognostic factor, ie, vascular or bile duct invasion, will have a worse prognosis. In our data, no survival differences were observed among patients with T2 tumor, although patients with T3 tumor were layered with regard to their prognosis: those with vascular or bile duct invasion showed a significantly worse prognosis. Interestingly, patients with T3 tumor accompanied by vascular or bile duct invasion had a longer survival than those with T4 tumor, and patients with T3 tumor that was not accompanied by vascular or bile duct invasion had a worse outcome than those with T2 tumor. Accordingly, the cohort of T3 tumor should be subdivided according to the presence of vascular or bile duct invasion.
The preferred treatment of small HCC (hepatic resection, radiofrequency ablation, or transplantation) has been controversial for many years.1 In the AJCC T classification, 8457 of 12,534 patients (67.5%) were assigned to T1, while in the LCSGJ T classification 16.6% were assigned to T1 (Table 7). Of the 8457 patients with T1 HCC in the AJCC T classification, 2078 were classified as T1, 6308 were T2, and 71 were T3 in the LCSGJ T classification. As expected, patients with T1 tumor in AJCC T classification had a 5-year survival rate of 61%, which was similar to that of patients with T2 tumor in the LCSGJ T classification (58%). These results suggest that the LCSGJ-T classification may make it possible to classify patients with early-stage HCC more precisely than with AJCC T.
Both the LCSGJ-stage and the AJCC-stage were developed based on a survival analysis of patients who underwent hepatic resection. Thus, these staging systems are appropriate for patients who will undergo hepatic resection. The applicability of these surgical staging systems to other local therapies such as transplantation and radiofrequency ablation has been a matter of dispute. The previous version of the AJCC TNM classification was shown by Llovet et al to not have prognostic power in patients who were treated by liver transplantation,37 although Peck-Radosavljevic et al showed that stage IVA predicted recurrence in these patients.38 The current AJCC TNM system 6th edition has been shown to be a significant predictor of tumor recurrence, but not of survival in liver transplantation.39 Machi et al studied 65 patients with unresectable HCC who underwent radiofrequency ablation and showed that TNM stage 6th edition was a significant predictor of survival.40 The applicability of LCSGJ TNM stage to liver transplantation or radiofrequency ablation has not been fully evaluated. Thus, it may be preferable to apply these surgical staging systems only in patients who will receive liver resection.
All staging systems represent a compromise between simplicity and discriminatory ability. Although we tried to reduce the over-fitting bias by internal cross-validation, a further independent external validation of the LCSGJ-TNM stage is needed.
ACKNOWLEDGMENTS
The authors thank all members of the Liver Cancer Study Group of Japan who completed the questionnaires. The authors also thank Shigeki Arii, MD, PhD, from the Department of Hepato-Biliary-Pancreatic Surgery, Tokyo Medical and Dental University, Graduate School of Medicine, Tokyo, Japan; Takafumi Ichida, MD, PhD, from the Department of Gastroenterology, Juntendo University School of Medicine, Tokyo, Japan; Kiwamu Okita, MD, PhD, from the Department of Gastroenterology and Hepatology, Yamaguchi University School of Medicine, Ube, Japan; Masao Omata, MD, PhD, from the Department of Gastroenterology, Graduate School of Medicine, University of Tokyo, Tokyo, Japan; Masamichi Kojiro, MD, PhD, from the Department of Pathology, Kurume University School of Medicine, Kurume, Japan; Yasuni Nakanuma, MD, PhD, from the Department of Human Pathology, Kanazawa University Graduate School of Medicine, Kanazawa, Japan; and Kenichi Takayasu, MD, PhD, from the Department of Diagnostic Radiology, National Cancer Center Hospital, Tokyo, Japan, for their contributions to this study.
Footnotes
Reprints: Masami Minagawa, MD, PhD, Department of Hepato-Biliary-Pancreatic Surgery, Department of Artificial Organ and Transplantation, Graduate School of Medicine, University of Tokyo, 7-3-1, Hongo, Bunkyo-ku, Tokyo, 113-8655, Japan. E-mail: minagawa-tky@umin.ac.jp.
REFERENCES
- 1.Arii S, Yamaoka Y, Futagawa S, et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224–1229. [DOI] [PubMed] [Google Scholar]
- 2.Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment: study of 850 patients. Cancer. 1985;56:918–928. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. The Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. [DOI] [PubMed] [Google Scholar]
- 6.Chevret S, Trinchet JC, Mathieu D, et al. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma: Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire. J Hepatol. 1999;31:133–141. [DOI] [PubMed] [Google Scholar]
- 7.Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760–1769. [DOI] [PubMed] [Google Scholar]
- 8.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38:207–215. [DOI] [PubMed] [Google Scholar]
- 9.Henderson J, Sherman M, Tavill A, et al. AHPBA/AJCC consensus conference on staging of hepatocellular carcinoma: consensus statement. HPB. 2003; 5:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liver Cancer Study Group of Japan. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 4th Japanese edition. Tokyo: Kanehara, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Liver Cancer Study Group of Japan. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 2nd English edition. Tokyo: Kanehara, 2003. [The 2000 4th Japanese edition corresponds to the 2003 2nd English edition.]
- 12.Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–1536. [DOI] [PubMed] [Google Scholar]
- 13.AJCC. AJCC Cancer Staging Manual, 6th ed. New York: Springer, 2002. [Google Scholar]
- 14.Makuuchi M, Belghiti J, Belli G, et al. IHPBA concordant classification of primary liver cancer: working group report. J Hepatobiliary Pancreat Surg. 2003;10:26–30. [DOI] [PubMed] [Google Scholar]
- 15.Ueno S, Tanabe G, Nuruki K, et al. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res. 2002;24:395–403. [DOI] [PubMed] [Google Scholar]
- 16.Poon RT, Fan ST. Evaluation of the new AJCC/UICC staging system for hepatocellular carcinoma after hepatic resection in Chinese patients. Surg Oncol Clin North Am. 2003;12:35–50. [DOI] [PubMed] [Google Scholar]
- 17.Anonymous. Primary liver cancers in Japan. Cancer. 1980;45:2663–2669. [DOI] [PubMed] [Google Scholar]
- 18.Anonymous. Primary liver cancer in Japan: the Liver Cancer Study Group of Japan. Cancer. 1984;54:1747–1755. [DOI] [PubMed] [Google Scholar]
- 19.Anonymous. Primary liver cancer in Japan, Sixth report: the Liver Cancer Study Group of Japan. Cancer. 1987;60:1400–1411. [DOI] [PubMed] [Google Scholar]
- 20.Anonymous. Primary liver cancer in Japan: clinicopathologic features and results of surgical treatment. Liver Cancer Study Group of Japan. Ann Surg. 1990;211:277–287. [PMC free article] [PubMed] [Google Scholar]
- 21.Anonymous. Predictive factors for long term prognosis after partial hepatectomy for patients with hepatocellular carcinoma in Japan: the Liver Cancer Study Group of Japan. Cancer. 1994;74:2772–2780. [DOI] [PubMed] [Google Scholar]
- 22.Ikai I, Arii S, Kojiro M, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101:796–802. [DOI] [PubMed] [Google Scholar]
- 23.Ikai I, Itai Y, Okita K, et al. Report of the 15th follow-up survey of primary liver cancer. Hepatol Res. 2004;28:21–29. [DOI] [PubMed] [Google Scholar]
- 24.A Japanese study group for alcoholic liver disease: a new diagnostic criteria of alcoholic liver disease. Takada T, Acta Hepatol Jpn. 1993;34:888–896. [Google Scholar]
- 25.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 26.Vauthey JN, Pawlik TM, Lauwers GY, et al. Critical evaluation of the different staging systems for hepatocellular carcinoma. Br J Surg. 2004;91:1072. [DOI] [PubMed] [Google Scholar]
- 27.Izumi R, Shimizu K, Ii T, et al. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology. 1994;106:720–727. [DOI] [PubMed] [Google Scholar]
- 28.Cance WG, Stewart AK, Menck HR. The National Cancer Data Base Report on treatment patterns for hepatocellular carcinomas: improved survival of surgically resected patients, 1985–1996. Cancer. 2000;88:912–920. [DOI] [PubMed] [Google Scholar]
- 29.Staudacher C, Chiappa A, Biella F, et al. Validation of the modified TNM-Izumi classification for hepatocellular carcinoma. Tumori. 2000;86:8–11. [DOI] [PubMed] [Google Scholar]
- 30.Chiappa A, Zbar AP, Podda M, et al. Prognostic value of the modified TNM (Izumi) classification of hepatocellular carcinoma in 53 cirrhotic patients undergoing resection. Hepatogastroenterology. 2001;48:229–234. [PubMed] [Google Scholar]
- 31.Vauthey JN, Klimstra D, Blumgart LH. A simplified staging system for hepatocellular carcinomas. Gastroenterology. 1995;108:617–618. [DOI] [PubMed] [Google Scholar]
- 32.Lui WY, Chiu ST, Chiu JH, et al. Evaluation of a simplified staging system for prognosis of hepatocellular carcinoma. J Formos Med Assoc. 1999;98:248–253. [PubMed] [Google Scholar]
- 33.Kanai T, Hirohashi S, Upton MP, et al. Pathology of small hepatocellular carcinoma: a proposal for a new gross classification. Cancer. 1987;60:810–819. [DOI] [PubMed] [Google Scholar]
- 34.Takayama T, Makuuchi M, Hirohashi S, et al. Early hepatocellular carcinoma as an entity with a high rate of surgical cure. Hepatology. 1998;28:1241–1246. [DOI] [PubMed] [Google Scholar]
- 35.Anonymous. Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology. 2000;31:840–845. [DOI] [PubMed] [Google Scholar]
- 36.Kudo M, Chung H, Haji S, et al. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology. 2004;40:1396–1405. [DOI] [PubMed] [Google Scholar]
- 37.Llovet JM, Bruix J, Fuster J, et al. Liver transplantation for small hepatocellular carcinoma: the tumor-node-metastasis classification does not have prognostic power. Hepatology. 1998;27:1572–1577. [DOI] [PubMed] [Google Scholar]
- 38.Peck-Radosavljevic M, Pidlich J, Bergmann M, et al. Preoperative TNM classification is a better prognostic indicator for recurrence of hepatocellular carcinoma after liver transplantation than albumin mRNA in peripheral blood: Liver Transplant Oncology Group. J Hepatol. 1998;28:497–503. [DOI] [PubMed] [Google Scholar]
- 39.Zavaglia C, De Carlis L, Alberti AB, et al. Predictors of long-term survival after liver transplantation for hepatocellular carcinoma. Am J Gastroenterol. 2005;100:2708–2716. [DOI] [PubMed] [Google Scholar]
- 40.Machi J, Bueno RS, Wong LL. Long-term follow-up outcome of patients undergoing radiofrequency ablation for unresectable hepatocellular carcinoma. World J Surg. 2005;29:1364–1373. [DOI] [PubMed] [Google Scholar]