Abstract
Background:
The principal role of sentinel lymph node (SLN) sampling and ultrastaging in colon cancer is enhanced staging accuracy. The utility of this technique for patients with colon cancer remains controversial.
Purpose:
This multicenter randomized trial was conducted to determine if focused assessment of the SLN with step sectioning and immunohistochemistry (IHC) enhances the ability to stage the regional nodal basin over conventional histopathology in patients with resectable colon cancer.
Patients and Methods:
Between August 2002 and April 2006 we randomly assigned 161 patients with stage I–III colon cancer to standard histopathologic evaluation or SLN mapping (ex vivo, subserosal, peritumoral, 1% isosulfan blue dye) and ultrastaging with pan-cytokeratin IHC in conjunction with standard histopathology. SLN-positive disease was defined as individual tumor cells or cell aggregates identified by hematoxylin and eosin (H&E) and/or IHC. Primary end point was the rate of nodal upstaging.
Results:
Significant nodal upstaging was identified with SLN ultrastaging (Control vs. SLN: 38.7% vs. 57.3%, P = 0.019). When SLNs with cell aggregates ≤0.2 mm in size were excluded, no statistically significant difference in node-positive rate was apparent between the control and SLN arms (38.7% vs. 39.0%, P = 0.97). However, a 10.7% (6/56) nodal upstaging was identified by evaluation of H&E stained step sections of SLNs among study arm patients who would have otherwise been staged node-negative (N0) by conventional pathologic assessment alone.
Conclusion:
SLN mapping, step sectioning, and immunohistochemistry (IHC) identifies small volume nodal disease and improves staging in patients with resectable colon cancer. A prospective trial is ongoing to determine the clinical significance of colon cancer micrometastasis in sentinel lymph nodes.
One hundred sixty-one patients with stage I–III colon cancer were assigned randomly to standard histopathologic evaluation or sentinel lymph node mapping and ultrastaging with pan-cytokeratin IHC in conjunction with standard histopathology. Targeted nodal assessment identified small volume nodal disease and improved staging in patients with resectable colon cancer.
Presence and extent of regional nodal metastasis predict outcome in patients with colon adenocarcinoma. Completeness of nodal resection and staging accuracy has significant implications in terms of diagnosis, treatment, and survival in patients with this disease.1 Up to 30% of patients with node-negative colon cancer staged by standard pathologic techniques ultimately suffer disease recurrence and tumor-related mortality following potentially curative primary resection.2 Variations in outcome among patients with node-negative early stage disease may reflect inadequate nodal resection and inaccuracies of pathologic staging.
Standard pathologic evaluation may overlook low volume nodal metastasis, thereby failing to identify nodes imperative to accurate staging. Inconsistencies in number of nodes harvested at time of pathologic processing impact significantly colon cancer staging accuracy. This nodal sampling error serves as the basis for guidelines establishing a 12 node minimum for adequate staging utilizing conventional techniques.3 Up to 78% of metastases are identified in subcentimeter nodes that may be overlooked during standard gross pathologic dissection of resected specimens.3–5 Microscopic examination of 1 or 2 hematoxylin and eosin-stained sections of a 5-mm node limits pathologic assessment to <1% of the entire node, making identification of small tumor cell aggregates challenging.
Nodal step-sectioning may improve staging accuracy; however, this technique cannot be applied to all harvested nodes, as processing time, human resource requirement, and cost would be prohibitive. Directed and detailed examination of a limited number of nodes at highest likelihood of metastases would be a practical way to enhance staging accuracy.
The recognition that there exists an orderly, sequential, and predictable dissemination of epithelial cancer cells from the site of primary disease, through regional lymphatic channels, to principal or “sentinel” first draining node(s) ushered in an entirely new means of patient-specific staging and surgical treatment into the realm of oncology. This enabled focused, detailed pathologic assessment of a few nodes most predictive of the status of the regional nodal basin without compromising diagnostic accuracy. Furthermore, this development was heralded and validated as a highly reliable means of increasing staging accuracy and limiting radical, potentially morbid operations to those sentinel node-positive patients with breast cancer and melanoma.
Regional lymphadenectomy with en bloc primary tumor resection remains the standard of care for resectable colon cancer. As there is minimal associated complexity or morbidity with lymphadenectomy for this indication, sentinel lymph node (SLN) mapping and biopsy for colon cancer does not provide added information that will alter the extent of operation. The principal advantage for SLN mapping in colon cancer is the identification of nodes that can undergo focused and detailed pathologic scrutiny critical to optimizing staging accuracy.
Although there appears to be a significant advantage, not only in terms of accuracy of staging but also identification of nodal metastasis earlier in the natural history of the disease, inconsistencies in surgical and pathologic techniques, and diagnostic criteria for SLN-positive disease have led some to question the utility of applying the sentinel node paradigm to colon cancer.6,7 Prospective randomized trials are lacking to address the three important issues related to sentinel node mapping and biopsy in colon cancer: (1) SLN upstaging; (2) biology of micrometastatic disease; and, (3) benefit of adjuvant therapy in patients with micrometastatic-only node positive disease.8 We have undertaken this randomized trial to determine whether step sectioning and cytokeratin immunohistochemistry of the SLN(s) more accurately stages lymph nodes and identifies nodal micrometastasis undetected by conventional histopathology in patients with colon cancer.
METHODS
This report complies with the reporting standards established by the revised Consolidated Standards of Reporting Trials consensus statement.9
Participants
An international, multicenter prospective randomized clinical trial was conducted by the United States Military Cancer Institute (USMCI) Clinical Trials Group. This study was approved by the Department of Clinical Investigation Human Use Committee, Walter Reed Army Medical Center and participating medical center Institutional Review Boards. This study was conducted from August 2002 to April 2006 at five academic medical centers located in the United States, Israel, and Serbia. During the study period 175 patients enrolled, provided informed consent, and were assigned randomly to undergo standard complete surgical resection of the tumor-bearing colon, with en bloc regional lymphadenectomy followed by either conventional histopathologic evaluation (using paraffin embedding, single section hematoxylin and eosin staining (H&E), and microscopy) or sentinel lymph node mapping, biopsy, and ultrastaging (step sections with pancytokeratin immunohistochemistry (IHC)) in conjunction with standard histopathologic evaluation. Patients were stratified according to clinical disease stage (stage I/II vs. stage III) and extent of resection (segmental or greater colectomy). Method of nodal staging was unblinded.
Eligible patients had biopsy-proven, primary, nonmetastatic colon carcinoma or colon tumors clinically consistent with cancer and subsequently confirmed by pathology, were older than 18 years, and capable of providing written informed consent. Patients with recurrent or metastatic colon carcinoma, those who received prior chemotherapy or radiation, and those without pathologically confirmed adenocarcinoma were excluded. Thirteen patients (7.4%) were excluded after randomization due to absence of invasive adenocarcinoma (n = 5), gastrointestinal stromal tumor (n = 1), distant metastatic disease (n = 2), surgical specimen fixation failure (n = 3), or failure to identify the SLN (n = 2; included in assessment of the SLN mapping and biopsy technique).
Interventions
Standard Histopathologic Evaluation
Subjects underwent standard surgical resection of the tumor-bearing colon, with en bloc regional lymphadenectomy. The entire formalin-fixed surgical specimen (colon and mesentery) underwent standard histopathologic evaluation and staging using conventional paraffin embedding, sectioning and H&E staining, and microscopy. The colonic mesentery was examined carefully for lymph nodes. Any firm tissue remaining after pressure on the mesenteric fat was excised, embedded in paraffin, sectioned at a thickness of 4 μm, stained with H&E, and evaluated microscopically. Nodes <3 mm in maximal dimension were embedded in their entirety. Nodes ≥3 mm were bisected along the longitudinal axis of the node. One section of each node was evaluated. Total number of nodes and those nodes with metastatic disease were recorded in accordance with standards of care.
Sentinel Lymph Node Mapping
Subjects underwent standard surgical resection. Immediately following removal of the colon and node-bearing mesentery, isosulfan blue dye (Lymphazurin 1%; Ben Venue Labs, Bedford, OH) was injected (ex vivo) subserosally (colon specimen left intact/unopened) at the proximal and distal margin of the tumor along the longitudinal axis of the specimen and at 90 degrees from these injection sites. The injection site was then gently massaged for 5 minutes. The amount of blue dye injected was 0.5 mL per centimeter of tumor diameter.
Sentinel nodes were defined as the first blue staining nodes to appear within 5–10 minutes of dye injection. All nodes that stained blue within that time period were dissected from the mesentery and submitted to pathology as separately labeled specimens. The specimen and separated SLNs were delivered in a fresh state to the Pathology Department within 30 minutes of lymphatic mapping. H&E staining and microscopy were performed identically for SLNs and non-SLNs as was done in the control arm of the study. The remaining resected colon and attached mesentery were fixed in formalin for standard histopathologic evaluation according to standard of care protocol. The ex vivo SLN mapping technique was used to facilitate standardized, uniform specimen processing and assessment, with improved technical quality control; however, aberrant lymphatic drainage could not be assessed.
Sentinel Lymph Node Pathologic Evaluation
After diagnosis of colon adenocarcinoma was confirmed, the SLNs were measured and bisected along the longitudinal axis of the node. Paraffin-embedded blue nodes (single face if node <3 mm and 2 faces in bivalved nodes ≥3 mm) underwent step sectioning at 40 μm intervals and at four levels, yielding sections approximately 4 μm thick. All four sections were stained with H&E. Two unstained slides were prepared at the second and fourth level of the block for IHC staining. Sentinel node ultrastaging was defined as step sectioning of the sentinel node(s) followed by pathologic evaluation of 4 H&E stained, and 2 IHC stained sections of each SLN.
IHC was performed on formalin-fixed and paraffin-embedded sections of the SLN using the avidin-biotin-peroxidase complex method. A commercially obtained pan-cytokeratin antibody cocktail was used in this study (Pan-keratin AE1/AE3, CAM 5.2, 35bH11; Ventana Medical Systems, Tucson, AZ). Endogenous peroxidase was suppressed by incubation with 1% hydrogen peroxide. Diaminobenzidine tetrahydrochloride (DAB; Biogenex, San Ramon, CA) was used as the chromogen. Formalin-fixed paraffin-embedded sections of tonsils and or skin were used as positive controls and a section from the SLN block incubated with negative control buffer. A total of 4 H&E and 2 cytokeratin immunostained sections were examined for each SLN block. A cytokeratin immunostain was considered positive if strongly positive individual cells or cell clusters were identified that demonstrated anatomic and cytologic features of colon carcinoma cells. The definition of positive sentinel node in this trial was a blue-staining node containing single cells or cell aggregates demonstrating morphologic features consistent with colon carcinoma apparent on evaluation of H&E and/or pan-cytokeratin IHC stained nodal sections.
The remaining formalin-fixed mesentery for study subjects randomized to the SLN arm was evaluated for non SLNs as described above for standard pathologic evaluation. Tumor histology, total number of lymph nodes, sentinel node mapping success, number of SLNs, number of non SLNs, number of SLNs positive by H&E, number of SLNs positive by IHC (categorized according to isolated tumor cells, cell clusters ≤0.2 mm, micrometastasis >0.2 and ≤2 mm, and macrometastasis >2 mm) and number of non-SLNs positive by H&E as per standard protocol were recorded. For SLNs containing positive staining individual cells or cell clusters, the nodal H&E sections were rereviewed to determine if the cells were clearly identifiable on those sections. Tumor staging was conducted in accordance with the American Joint Commission on Cancer (TNM) Staging (2002, 6th edition).
The protocol required that resected surgical specimens in this study be prepared with two different sets of instruments—one set for lymph node dissection, the other for primary tumor preparation. The nodes were washed with saline after harvesting. These measures reduced the likelihood of nodal epithelial cell contamination.
Clinical decisions regarding adjuvant chemotherapy were based on conventional pathologic nodal assessment and not influenced by findings of isolated cells or cell clusters in SLNs, as the prognostic importance of micrometastatic disease remains undefined. Subject participation in this study concluded with the surgical procedure and nodal staging. No follow-up was required for this clinical trial.
Quality Control
Surgeons participating in this trial were experienced surgical oncologists and colorectal surgeons. They received formal instruction and technical training in SLN mapping and the specimen handling used in this study. Prior to enrolling patients into the study each surgeon completed 6–12 learning cases and demonstrated proficiency with the SLN mapping technique. Six surgeons at 5 participating centers performed all SLN mapping procedures. Regional study directors monitored the technical aspects of this trial to ensure compliance with SLN mapping, specimen handling, and processing. Specimen procurement, handling, transport, processing, and analysis oversight were provided by senior regional study pathologists experienced with GI pathology and cytochemistry. Standard histopathologic assessment was conducted by the Department of Pathology at each participating center, supervised by a dedicated study pathologist in each institution, and reviewed by each senior regional study pathologist. Paraffin embedded nodal blocks were transported to regional study laboratories for diagnostic confirmation, specimen processing and pathologic assessment. All IHC analysis for the three participating centers in Serbia was conducted by a central laboratory headed by the regional study pathologist (SU-Serbia) and all SLN analyses in Israel and the United States were conducted by DP and CFA, respectively.
A single senior study pathologist (CFA) blinded to the nodal staging results conducted a centralized pathologic review of all SLN sections. Changes were made to 8 of the 87 cases (82 SLN group study subjects and 5 negative controls). The following changes in diagnosis were rendered (Fig. 1): (1) Isolated tumor cells (ITCs) instead of cell clusters (CC) in SLN (n = 1); (2) false-positive SLN instead of ITCs, due to knife tumor carry over effect (n = 1); (3) ITCs identified in SLNs by IHC, positive instead of negative SLN (n = 3); (4) number of positive SLNs increased, as ITCs found in SLNs other than SLN containing metastasis >2 mm in size (n = 2); (5) number of multiple IHC-positive SLNs decreased (from 7 to 6 SLNs), as one SLN contained CK positive mast cell (n = 1). All five negative controls for SLN IHC were confirmed to be indeed negative. All 5 patients with suspected colon adenocarcinoma presented with right colon tumors (4 symptomatic) and underwent segmental resection. Three tumors proved to be benign adenomas, one Crohn’s stricture and one was a localized GIST. Two, 4, 5, 6, and 8 SLNs were identified in these patients; mean number of total nodes evaluated pathologically in these 5 cases was 14 nodes. Although occasional false-positive staining was identified in mesothelial and endothelial cells in rare sections, mast cells or occasional histiocytes containing-anthracotic pigment/hemosiderin, no cytoplasmic or membranous staining was identified in morphologically malignant cells localized to a subcaspular sinus or intra nodal sinusoid (Fig. 1).
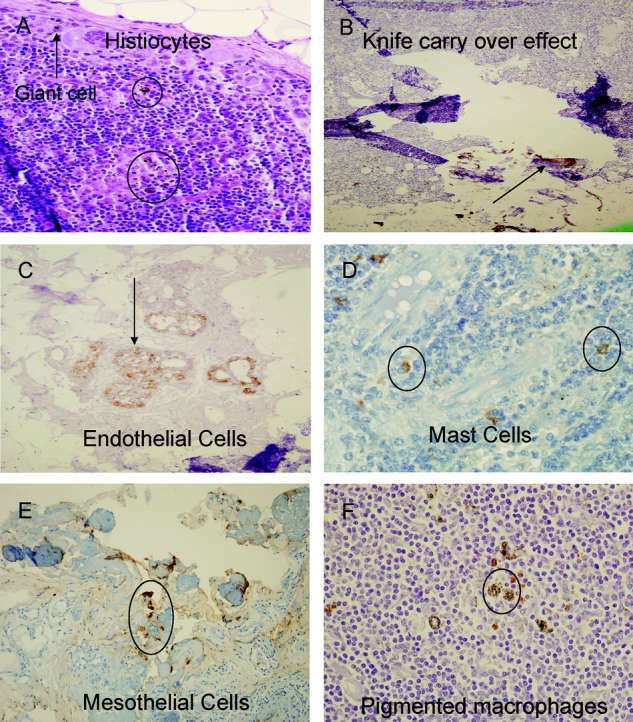
FIGURE 1. False-positive IHC staining in sentinel lymph nodes. A, H&E stained section of SLN in a patient that underwent biopsy of colon adenocarcinoma; typical postbiopsy changes of giant cells (arrow), and hemosiderin-laden histiocytes (circles). B, cytokeratin IHC section demonstrating extraneous matter and transferred tumor cells (arrow), knife carry-over effect. C, immunohistochemical endothelial cell staining (arrow). D, characteristic positive IHC staining of endogenous peroxidase-rich granular mast cells. E, positively staining mesothelium (circle) in omental fat fragment adherent to SLN on IHC. F, typical histologic appearance of nodal pigmented macrophages (circle).
Objectives
When this study was initiated, no randomized trial had been published to determine whether SLN mapping significantly increases staging accuracy for adenocarcinoma of the colon. The principal aim of this trial was to define the rate of upstaging of colon carcinoma lymph node metastasis with SLN mapping. The hypothesis to be tested was: H0: There is no difference in the rate of lymph node metastasis between conventional histopathologic and SLN ultrastaging in subjects with resected colon carcinoma versus H1: There will be a 25% difference in the rate of nodal metastasis between conventional histopathologic staging and SLN ultrastaging.
Outcomes
The primary outcome variable was the rate of nodal upstaging. Identification rate of the sentinel node, accuracy, sensitivity, and false-negative rate were calculated. Identification rate was calculated as the proportion of patients having SLNs identified with ex vivo peritumoral blue dye injection. Accuracy of SLN mapping and biopsy was defined as the proportion of patients with successful lymphatic mapping having SLN examination correctly reflect the tumor status of the nodal basin. Sensitivity of SLN mapping was defined as the proportion of patients with positive nodes found by routine H&E examination to have positive SLNs, and upstaging the proportion of patients with negative nodes found by conventional pathologic examination to have micrometastatic disease by focused examination of the SLN. False-negatives represented the proportion of patients with successful lymphatic mapping having tumor-positive non-SLNs but SLNs without apparent tumor cells. The false-negative rate was calculated as the proportion of false-negative over the sum of false-negative and true positive SLN cases. The following data were also collected/calculated: total nodes identified; number of SLNs identified per subject; total patients with positive and negative SLNs; total node-negative subjects per study group; total SLN+ and non-SLN− subjects as well as total SLN− and non-SLN+ subjects in SLN group; rate of SLN only micrometastasis; proportion of H&E−/IHC+, H&E+/IHC+ SLNs.
Sample Size
Sample size calculation was based on expected accrual of 50 subjects per year. The calculation was based on the assumption that 40% of the subjects in the standard pathology group would be node-positive compared with 65% of subjects in the SLN group − an absolute SLN upstaging rate of 25%. Controlling the probability of a Type I error at α = 0.05, a sample of 69 subjects per group would have 80% power to detect a 25% difference in proportion of subjects with node-positive status using a “two-tailed” test, for a total of 138 subjects. Accounting for dropouts/inability to identify SLN (∼20%), up to 172 subjects were planned to enroll in the study over a 4-year period.
Randomization
This trial was planned as a group sequential randomized study. Subjects were randomized to one of two arms: standard histopathologic evaluation or SLN mapping and ultrastaging with IHC in conjunction with standard histopathologic assessment. Randomization was balanced between the two treatment arms stratified by clinical stage and extent of resection. Randomization was performed at the Department of Clinical Investigation, Walter Reed Army Medical Center utilizing a stratified permuted block scheme and a separate randomization table for each participating study site with the aim of avoiding inequalities in treatment group assignment. The randomization sequence was concealed until the treatment group was assigned. The allocation sequence was generated by the study biostatistician (RH). Neither study participants nor those administering treatment were blinded to group assignment.
Statistical Methods
The categorical variables between groups were compared using Fisher exact test or χ2 test. Differences in observed sample means for single measurements were evaluated using analysis of covariance to adjust for potentially important clinical factors. To examine the influence of possible confounding variables on SLN identification and staging logistic regression analysis was used. To assess the independent predictive effect of a covariate for a nominal response (SLN positive rate) a logistic regression model was constructed and parameters estimated using maximum likelihood. Factors potentially significant (P < 0.05) on categorical contingency analysis were entered into the multivariate model. The Wald test statistic was computed for each effect in the model. Confidence limits and odds ratios were calculated for the maximum likelihood parameter estimates. Statistical analysis was performed using JMP and SAS software (JMP and SAS, Cary, NC). A P value <0.05 was considered significant.
RESULTS
Patient Characteristics
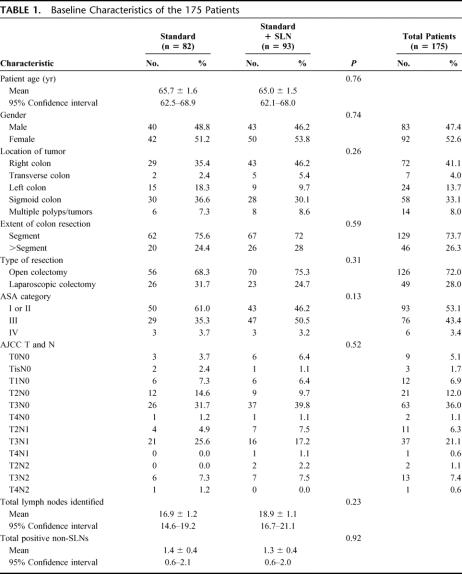
The study was initiated by the USMCI in August 2002. Enrollment ended in April 2006. One hundred seventy-five patients were randomly assigned to conventional histopathology or SLN mapping and ultrastaging with conventional histopathology. Eighty and eight-two patients in the control and intervention group completed the study protocol and were analyzed for nodal staging, respectively. Thirteen patients were excluded. The sentinel lymph node could not be localized in 2 patients and nodal fixation failure occurred in 3 cases. Two patients were found to have distant disease and 6 did not have colon adenocarcinoma, 5 of whom served as negative controls for IHC. Flow of participants through each stage of the trial is demonstrated in Figure 2. Statistical analysis included 162 patients. Baseline demographic and clinical characteristics of study participants are reported in Table 1 indicating no significant imbalance between the two study arms. In two-thirds of cases the primary tumor invaded through the muscularis propria into the subserosa or pericolic tissue (AJCC pT3) and involved regional nodes by standard pathologic assessment in one-third of patients (AJCC pN1 or N2, 57/162, 35.2%). Mean number of nodes analyzed per patient was 18.2 ± 0.9 (95% CI 16.5–19.9). A mean number of 2.7 ± 0.3 (95%CI 2.3–3.2) SLNs were identified in the 82 study arm patients.
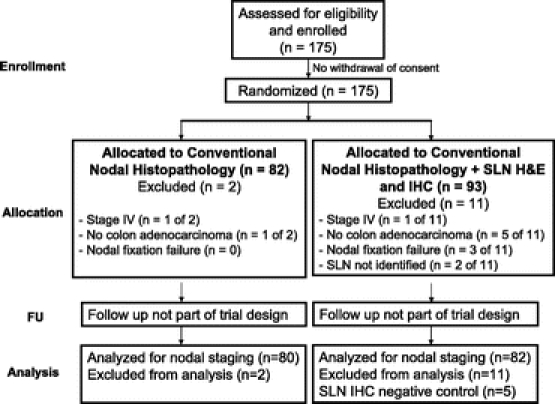
FIGURE 2. Flow of participants randomized to conventional nodal histopathology and sentinel lymph node mapping and targeted pathologic assessment for colon adenocarcinoma.
TABLE 1. Baseline Characteristics of the 175 Patients
Nodal Upstaging
Utilizing our predetermined definition of node-positive disease (individual tumor cells or cell aggregates identified by H&E and/or IHC), a significant nodal upstaging was identified with SLN ultrastaging (control vs. SLN: 38.7% vs. 57.3%, P = 0.019). When SLNs with cell aggregates ≤0.2 mm in size were excluded, no statistically significant difference in node-positive rate was apparent between the control and SLN arms (38.7% vs. 39.0%, P = 0.97). Similarly, no difference in proportion of node-positive patients staged by conventional nodal pathologic staging was detected between groups (control vs. SLN: 38.7% vs. 31.7%; P = 0.35). However, a 10.7% (6/56) nodal upstaging was identified by evaluation of H&E stained step sections of SLNs among study arm patients who would have otherwise been regarded as node negative by conventional pathologic assessment alone (Fig. 3).
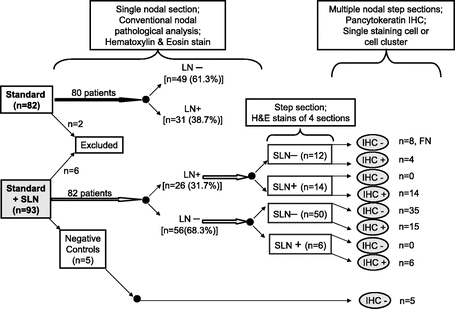
FIGURE 3. Distribution of nodal disease according to type. Nodal upstaging occurred in 10.7% (6/56) by evaluation of H&E stained step sections of SLNs alone in patients randomized to SLN mapping and targeted pathologic assessment. LN, lymph node; SLN, sentinel lymph node; H&E, hematoxylin & eosin; IHC, immunohistochemistry; FN, false-negative.
Sentinel Node Ultrastaging
SLNs were successfully identified in 82 of 84 patients (97.6%). A median of 2 SLNs were identified per patient (range: 1–15): 1 SLN in 27 (32.9%), 2 SLNs in 23 (28.0%), 3 SLNs in 11 (13.4%), 4 SLNs in 6 (7.3%), and 5 or more SLNs in 15 (18.3%) patients. Twenty-six patients (31.7%) were positive by conventional histopathology, and 47 (57.3%) were node positive by SLN ultrastaging (H&E and IHC) in the SLN arm. Accuracy and sensitivity of SLN mapping and biopsy was 90.2% (74/82) and 69.2% (18/26), respectively. No single clinical, pathologic, or surgical covariate emerged as an independent predictor of positive SLN.
There were 8 false-negative cases (9.8%), as shown in Table 2. Of multiple clinical and pathologic variables analyzed only one, number of SLNs identified, demonstrated a statistical correlation with the finding of false-negative SLN [for 1, 2, 3, and 4+ SLNs: TP vs. FN, 30.8% vs. 75.0%; 25.6% vs. 12.5%; 12.8% vs. 12.5%; and 30.8% vs. 0% (P = 0.049)]. Thus, 6 of 8 (75%) false-negative cases had only 1 SLN identified and no false-negative cases occurred when 4 or more SLNs were localized. Mean number of SLNs identified in false-negative (n = 7) and true positive (n = 39) SLN cases was 1.4 and 3.2 SLNs, respectively (P = 0.07). Distribution and volume of nodal disease is shown in Table 3 and Figure 4. Analysis of the pattern and distribution of metastasis in non-SLN and SLNs indicates that an exclusive site of metastasis in SLNs was found in 6 cases (10.7% of 56 non-SLN negative cases) by step section and H&E staining alone, and in 15 cases by more meticulous ultrastaging of the SLN incorporating cytokeratin IHC (26.8% of 56 non-SLN negative cases).
TABLE 2. Outcome: Sentinel Lymph Node Mapping and Targeted Pathological Assessment in Colon Cancer
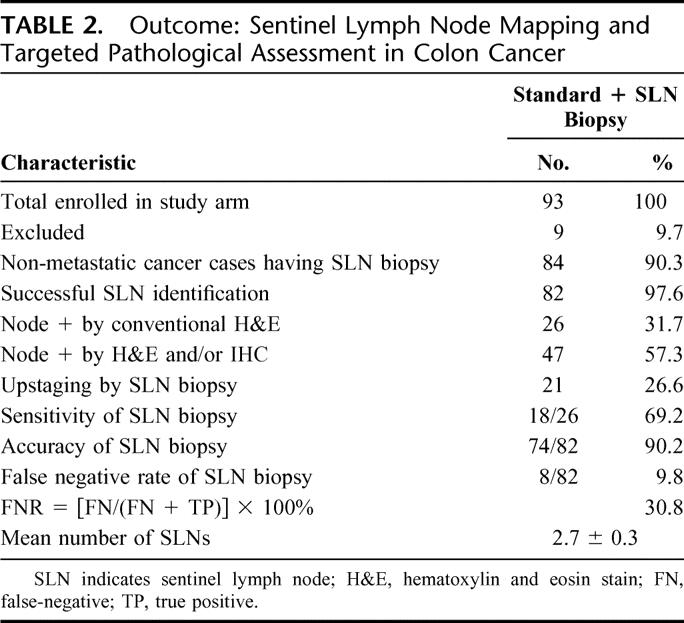
TABLE 3. Nodal Disease in 82 Patients Randomized to Sentinel Lymph Node Mapping and Targeted Pathological Assessment
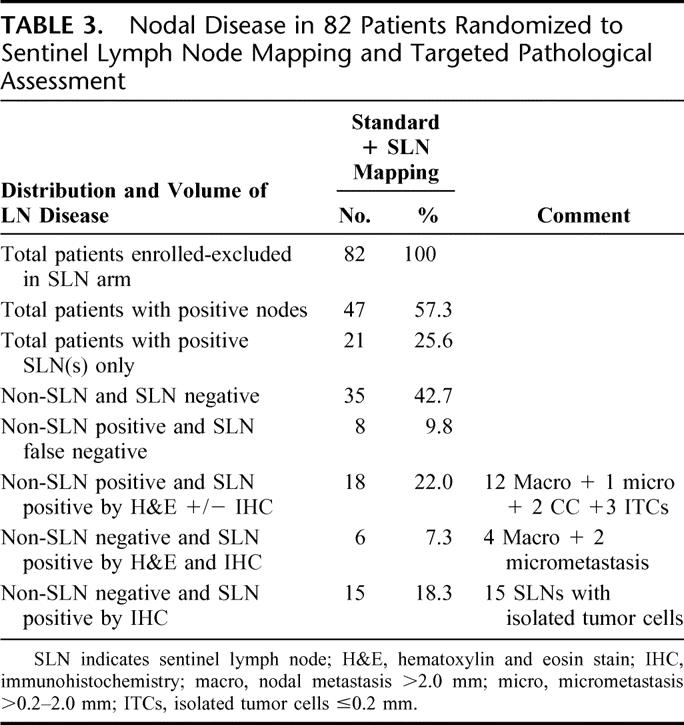
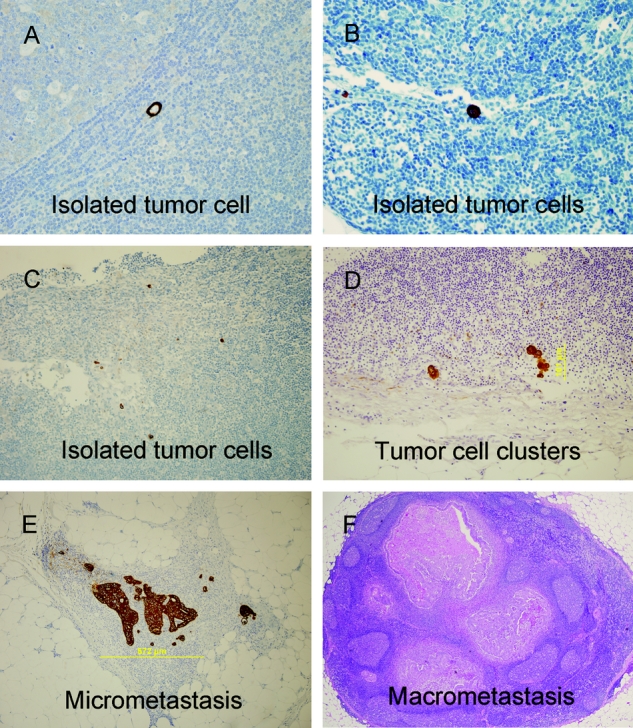
FIGURE 4. Volume of disease in sentinel nodes demonstrating cytoplasmic or membranous staining in morphologically malignant cells. A, isolated tumor cell. B and C, isolated tumor cells. D, tumor cell clusters, 0.10 mm in size. E, micrometastasis 0.57 mm in size. F, macrometastasis (>2 mm in size) on H&E stained section of SLN.
DISCUSSION
This multicenter randomized trial was conducted to determine if ex vivo SLN mapping followed by step section and cytokeratin IHC improves staging of resectable colon cancer over conventional histopathologic staging alone. Applying a predetermined definition of nodal positivity, a significant 19% nodal upstaging was identified with SLN mapping compared with the control arm. When nodes with isolated tumor cells or cell clusters ≤0.2 mm were excluded from the analysis, node-positive rate was identical in the two arms of the study. However, an 11% nodal upstaging (tumor deposits >0.2 mm) was demonstrated by pathologic assessment of H&E stained step sections of nodes within the SLN study arm, in patients who would have been deemed node negative by conventional pathologic assessment, and not offered adjuvant systemic chemotherapy.
Several large single institutional studies have demonstrated the technical feasibility and increased detection rate of tumor cell deposits in step sectioned nodes evaluated by H&E and IHC. Successful identification of the SLNs has been reported in the range of 96%–100% in large studies with the SLN accurately reflecting the tumor status of the nodal basin in 92%–96% of cases.10–13 The proportion of patients with negative nodes staged by conventional histopathology found to have tumor cells by focused examination of the sentinel nodes (upstaging) was 13%–31% in these single institutional series. A number of recent multi-institutional studies support the feasibility, accuracy and improved staging (13.4%–26.1% upstaging) with targeted nodal assessment in colon cancer indicating proportion of false-negative cases (3.6%–10.6%) comparable to those in other epithelial cancers.14–16 The current multicenter randomized collaborative study demonstrates comparable findings with SLN mapping and ultrastaging using H&E and IHC analysis of SLN step sections: successful identification, 98%; accuracy, 90%; upstaging, 19% (compared with the control arm); false-negatives, 10%. These favorable results have not been reproduced by other nonrandomized collaborative group trials pointing to the lack of uniformity in sensitivity and false-negative rates for lymphatic mapping in colon cancer. This inconsistency underscores a number of important technical issues that account for large discrepancies in published results, and that fuel considerable controversy over the value of SLN mapping in this disease.6,7
False-negative findings for colon SLN staging are attributable to a number of factors including extent of disease, mapping technique, timing and method of pathologic processing, number of SLNs evaluated, and method of ultrastaging.8 Local tumor invasion of adjacent pericolic nodes, nodes replaced by tumor, and extranodal disease extension are both indicators of tumor biology and predictors of false-negative SLN mapping. Viehl et al demonstrated that tumor volume is an important consideration in SLN mapping and that tumor-volume-adjusted blue dye dosing improves significantly SLN mapping success.17 The findings of the current study underscore the importance of number of identified blue nodes. It appears that false-negative SLN mapping for colon cancer is both a technical problem and pathologic reality, as false-negative findings could not be eliminated despite attempts to eliminate factors that could create significant variation in this trial, through rigorous surgical and pathologic quality control. Our results suggest that those false-negative cases where 1 or 2 blue nodes are identified likely represents technical failure (technical false-negatives); however, when multiple (>2 SLNs) blue nodes are discovered in false-negative cases, these likely represent pathologic failure or skip metastases (pathologic false-negatives). It is important to emphasize that, unlike the impact of false-negative sentinel nodes in breast cancer and melanoma staging, false-negative SLNs in colon cancer do not alter extent of operation or limit total number of nodes assessed by conventional histopathology.
Standardization of working definitions, training, mapping technique, and pathologic processing and review are critical to the success of SLN mapping for colon carcinoma. Although there are variations in SLN definition, the one applied in this study, blue nodes with the most direct drainage from the tumor to appear within 5–10 minutes of dye injection, is an accepted one applied consistently in multicenter trials.14,15 The ex vivo mapping technique was used, principally for the purpose of facilitating standardized, uniform specimen processing and assessment, recognizing that the principal limitation of the ex vivo technique is the inability to detect the small percentage (∼4%) of cases with aberrant lymphatic drainage.6,8,10,18–24 Volume of subserosal, peritumoral blue dye injected was determined by size of tumor (0.5 mL per 1.0 cm of tumor), as dye volume appears to be a significant predictor of successful SLN identification.17 Paramo et al plotted the learning curve for colon cancer SLN mapping and found that the curve flattened after the first 5 mapping procedures, with successful SLN identification rates >98%, thereafter.19 Surgical quality control was specifically addressed in the current trial. Six surgeons at 5 academic medical centers performed all SLN mapping procedures, after formal training and demonstrated proficiency with the technique on learning cases. There were 8 false-negative SLN cases in the study arm of the trial (9.8%), a rate that compares favorably with previous multicenter trial reports. A statistical correlation was found between number of SLNs identified and false-negative rate. No false-negative case occurred when ≥4 SLNs were localized suggesting a minimum threshold of 4 nodes for SLN mapping in colon cancer. In addition to monitoring of surgical technique, specimen procurement and handling, pathologic processing and analysis oversight was provided by senior regional study pathologists, and centralized blinded final pathologic review was conducted.
Hence, technical limitations contributing to false-negative results in SLN mapping for colon cancer can be overcome by attention to surgical technical detail, careful attention to standardized pathologic specimen processing and analysis, and collaborative and coordinated multidisciplinary efforts with institutional commitment to optimizing the staging colon cancer staging. A collaborative group trial conducted by the CALGB underscores how surgical “generalizability” to a larger, more technically heterogeneous group of surgeons and pathologists, concedes diagnostic accuracy.6 The CALGB 80001 trial was conducted by 25 surgeons at 13 member institutions on 72 enrolled study subjects undergoing SLN staging with step sections and H&E pathologic assessment alone.6 Seventy-two percent of surgeons performed <5 cases, accounting partly for the high (55%) false-negative rate. Dye volume was not adjusted for tumor size, as only 1 mL of isosulfan blue was used for all patients in the CALGB study, 65% of who had T3 or T4 colon cancers. Histopathologic staging was not “conventional” as 5 nodal sections were evaluated on subsets of SLN and non-SLNs, contributing further to false-negative staging and disputable inference: sentinel node sampling is ineffective for the detection of micrometastasis. Further evaluation of the same cohort of patients with IHC analysis of the SLNs, using the same definition of nodal positivity in the current study, reduced the false-negative results (12%) to a level comparable with that reported in this randomized trial.7 More stringent criteria (≥5 isolated tumor cells or cell clusters) yielded an intermediate, though unacceptably high false-negative rate of 32% despite an upstaging rate of 38% in the CALGB trial.7 These findings point to the fact that valid, agreed upon definitions of micrometastatic disease, consistent nodal mapping and ultrastaging techniques, surgical and pathologic quality assurance, and adequate statistical power will be imperative in future collaborative trials aimed at defining the biology of nodal micrometastasis.
The present study, though imperfect, addresses many of these fundamental issues, surgical-pathologic collaboration, standardization and quality, consistent SLN mapping and staging (antibodies, IHC techniques, and scoring), and centralized pathologic review. One limitation of the study was that IHC analysis was not performed on all non-SLNs in the SLN arm. The present analysis was not designed to validate the hypothesis that blue stained nodes are indeed sentinel nodes and significantly more likely to harbor colorectal cancer metastases, as this has previously been demonstrated conclusively.12 The current trial was powered to 80% likelihood that an incorrect null hypothesis would be rejected. Sample size estimate was based on the ability to detect a 25% upstaging between study arms; hence, at the current sample size and upstaging rate, the possibility of having rejected the null hypothesis—no difference in nodal metastasis between conventional staging and SLN ultrastaging—when it is indeed true (type I error) cannot be excluded. The predetermined definition of positive SLN used in this study did not appear to contribute to false-positive IHC results in patients without colon adenocarcinoma; however, this definition may lack sufficient rigor, and it points to a pivotal question, what volume of nodal micrometastatic disease is clinically meaningful? The lack of a definitive answer to this question leaves a precise definition of micrometastatic colon cancer elusive. More importantly, the biology of submicroscopic nodal metastasis and the impact on disease-specific outcomes has not been studied rigorously. The prognostic significance of so-called micrometastases remains controversial with ample, though imperfect, peer-reviewed literature supporting arguments in favor15,25–29 and against30–37 the hypothesis that single IHC positive cells or cell clusters are indicators of outcome in colon cancer. That number of nodes and not nodal volume per se (micro- vs. macro-metastases) influences prognosis in colon cancer, and that recent large uncontrolled analyses suggest increased recurrence-free survival in patients upstaged with enhanced sentinel node assessment and treated with adjuvant systemic chemotherapy, provide reasonable basis for large, controlled collaborative clinical trials.15,38 The principal aim of these future studies will be to define the clinically meaningful micrometastatic threshold, and the prognostic significance of micrometastasis; however, it will be a formidable undertaking to determine of node-negative patients staged conventionally, which develop disease recurrence, and those that harbor nodal micrometastasis are indeed identical.
A significant proportion of node-negative (∼25%) patients recur following resection of colon cancer with curative intent, in large part attributable to pathologic understaging. This number is similar to the ∼20% upstaging rate in a review of the literature with SLN mapping and ultrastaging.8 Sentinel node mapping and enhanced pathologic assessment directs pathologists to the few nodes most likely to harbor metastasis and makes ultrastaging with step sections, H&E staining, and IHC feasible and cost-effective. Although the fundamental principle that there exists an orderly and often predictable progression of epithelial cancer cells from primary tumor site to regional nodes that forms the basis of lymphatic mapping holds true for common malignancies such as melanoma, breast and colon cancer, important differences in SLN mapping for melanoma/breast cancer and colon cancer exist.
The principal advantage for SLN mapping and biopsy for melanoma and breast cancer is staging accuracy, and determining extent of regional lymph node dissection. As regional lymphadenectomy and en bloc tumor resection remains the standard of care for colon cancer, SLN mapping is not intended to alter the extent of operation. The probability that SLN mapping for colon cancer will detect nodal dissemination beyond the limits of intended lymphadenectomy and alter the extent of operation, and that these aberrant sentinel nodes will be the only positive evidence of disease spread is decidedly insignificant. The impact of SLN mapping failures in colon cancer, contrary to melanoma/breast cancer, is unlikely to result in under-treatment, as all relevant regional nodes are resected and evaluated at a minimum by conventional histopathology. Hence, SLN mapping and ultrastaging in colon cancer is purely supplementary regarding staging accuracy; it does not alter extent of operation or total number of nodes assessed by conventional means, and the impact of a false-negative result is less relevant when compared with that of melanoma/breast cancer. Important anatomic variations exist, however, in lymphatic drainage patterns in colon cancer relative to the integumentary system that may warrant a shift in how we approach lymphatic mapping for colon cancer. Sentinel lymph node mapping in melanoma and breast cancer yields one or a few nodes of prognostic importance rather than the entire regional nodal basin. The watershed distribution of the colon’s supporting lymphatic basin makes so-called sentinel node mapping, in fact first echelon node mapping, or targeted nodal assessment of first echelon nodes. The number of blue nodes retrieved appears to be important for targeted nodal assessment of H&E and cytokeratin IHC stained step sections, and the results of this trial, though preliminary, suggest a minimum of 4 nodes for this degree of pathologic scrutiny.
To our knowledge this is the first published randomized trial comparing sentinel nodal mapping and targeted pathologic assessment to conventional histopathology in colon cancer. Step sectioning and cytokeratin IHC of the blue nodes upstages significantly patients with resectable colon cancer in 19% of cases. However, the clinical utility of targeted nodal assessment was apparent in 11% of node-negative patients randomized to nodal mapping who were found to have nodal micrometastatic disease (>200 μm) and otherwise would not have received adjuvant systemic chemotherapy. The detection of micrometastasis is but one facet of a complex clinical picture and decision making process. Micrometastatic nodal disease should be included among the adverse prognostic features — tumor differentiation, obstruction, perforation, lymphovascular invasion and adherence to or invasion of local organs (T4)—that are considered when offering adjuvant chemotherapy to patients with high-risk Stage II colon cancer. Targeted nodal assessment in colon cancer increases staging accuracy over conventional pathologic techniques, and identifies a significant proportion of submicroscopic disease of unproven prognostic significance.
Currently there is no universally agreed upon criteria for the characteristics and volume of clinically relevant cytokeratin-positive colon cancer cell deposits in SLNs. Targeted nodal assessment in colon cancer may identify biologically important and prognostically relevant tumor metastasis; however, at the present time, the clinical significance of isolated cytokeratin positive tumor cells or cell clusters or micrometastasis remains undefined. Hence the clinical importance of sentinel node tumor deposits awaits the findings of prospective studies correlating volume of sentinel nodal disease with disease-free and disease-specific survival. The USMCI Clinical Trials Group has undertaken a large prospective trial to determine the biologic relevance of lymph node micrometastases in colon cancer.
ACKNOWLEDGMENTS
The authors thank the tireless efforts and support of our colleagues: Dr. Dragomir Damjanov, Dr. Zoran Milosevic, Clinic of Abdominal and Endocrine Surgery, Institute of Surgery, Clinical Center Novi Sad, Novi Sad, Serbia; Dr. Zoran Radovanovic, Department of Surgical Oncology, Institute of Oncology of Vojvodina, Sremska Kamenica, Serbia; Dr. Nebojsa Mitrovic, Dr. Damir Jasarovic, Department of Surgery, University Surgical Hospital “Dr Dragisa Misovic”, Belgrade, Serbia; Dr. Milan Korica, Dr. Aleksandar Gluhovic, Clinic of Abdominal and Endocrine Surgery, Institute of Surgery, Clinical Center Novi Sad, Novi Sad, Serbia; Tamar Hamburger, Department of Surgery, Hadassah-Hebrew University Medical Center Jerusalem, Israel; Earnestine Harvey, Department of Pathology, Walter Reed Army Medical Center, and the United States Military Cancer Institute, Washington, D.C.; Anne E. Dimke, Stephanie R. Moon, William P. Mahr, United States Military Cancer Institute, Washington, D.C.; Residents and attending pathologists, Department of Pathology, Walter Reed Army Medical Center.
Footnotes
The research for USMCI GI-01 was supported by grants from the United States Military Cancer Institute.
Alexander Stojadinovic and Aviram Nissan contributed equally to this study and will share first authorship.
The opinions or assertions contained herein are the private views of the authors (AS, CFA, CDS, JMN, TAB, GEP) and are not to be construed as official or reflecting the views of the Departments of the Army, or the Department of Defense.
Reprints: LTC Alexander Stojadinovic, MD, Gastrointestinal Cancer Program Leader, United States Military Cancer Institute, 6900 Georgia Avenue, Room 5C27B, N.W., Washington, D.C. 20307. E-mail: alexander.stojadinovic@na.amedd.army.mil.
REFERENCES
- 1.Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21:2912–2919. [DOI] [PubMed] [Google Scholar]
- 2.Cohen Am, Kelsen D, Saltz L, et al. Adjuvant therapy for colorectal cancer. Curr Probl Cancer. 1998;22:5–65. [Google Scholar]
- 3.Compton CC. Updated protocol for the examination of specimens from patients with carcinomas of the colon and rectum, excluding carcinoid tumors, lymphomas, sarcomas, and tumors of the vermiform appendix: a basis for checklists. Cancer Committee. Arch Pathol Lab Med. 2000;124:1016–1025. [DOI] [PubMed] [Google Scholar]
- 4.Herrera-Ornelas L, Justiniano J, Castillo N, et al. Metastases in small lymph nodes from colon cancer. Arch Surg. 1987;122:1253–1256. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Bigas MA, Maamoun S, Weber TK, et al. Clinical signficance of colorectal cancer: metastases in lymph nodes <5 mm in size. Ann Surg Oncol. 1996;3:124–130. [DOI] [PubMed] [Google Scholar]
- 6.Bertagnolli M, Miedema B, Redston M, et al. Sentinel node staging of respectable colon cancer: results of a multicenter study. Ann Surg. 2004;240:624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redston M, Compton CC, Miedema BW, et al. Analysis of micrometastatic disease in sentinel lymph nodes from resectable colon cancer: results of Cancer and Leukemia Group B Trial 80001. J Clin Oncol. 2006;24:878–883. [DOI] [PubMed] [Google Scholar]
- 8.Stojadinovic A, Allen PJ, Protic M, et al. Colon sentinel lymph node mapping: practical surgical applications. J Am Coll Surg. 2005;201:297–313. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Schulz KF, Altman DG; CONSORT. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. BMC Med Res Methodol. 2001;1:2. [DOI] [PMC free article] [PubMed]
- 10.Bilchik AJ, Nora D, Tollenaar RAEM, et al. Ultrastaging of early colon cancer using lymphatic mapping and molecular analysis. Eur J Cancer. 2002;38:977–985. [DOI] [PubMed] [Google Scholar]
- 11.Bilchik AJ, Nora DT, Sobin LH, et al. Effect of lymphatic mapping on the new tumor-node-metastasis classification for colorectal cancer. J Clin Oncol. 2003;21:668–672. [DOI] [PubMed] [Google Scholar]
- 12.Wong JH, Johnson DS, Namiki T, et al. Validation of ex vivo lymphatic mapping in hematoxylin-eosin node-negative carcinoma of the colon and rectum. Ann Surg Oncol. 2004;11:772–777. [DOI] [PubMed] [Google Scholar]
- 13.Saha S, Dan AG, Beutler T, et al. Sentinel lymph node mapping technique in colon cancer. Semin Oncol. 2004;31:374–381. [DOI] [PubMed] [Google Scholar]
- 14.Saha S, Monson KM, Bilchik A, et al. Comparative analysis of nodal upstaging between colon and rectal cancers by sentinel lymph node mapping: a prospective trial. Dis Colon Rectum. 2004;47:1767–1772. [DOI] [PubMed] [Google Scholar]
- 15.Saha S, Seghal R, Patel M, et al. A multicenter trial of sentinel lymph node mapping in colorectal cancer: prognostic implications for nodal staging and recurrence. Am J Surg. 2006;191:305–310. [DOI] [PubMed] [Google Scholar]
- 16.Bilchik A, DiNome M, Saha S, et al. Prospective multicenter trial of staging adequacy in colon cancer: preliminary results. Arch Surg. 2006;141:527–533. [DOI] [PubMed] [Google Scholar]
- 17.Viehl CT, Hamel CT, Marti WR, et al. Identification of sentinel lymph nodes in colon cancer depends on the amount of dye injected relative to tumor size. World J Surgery. 2003;27:1285–1290. [DOI] [PubMed] [Google Scholar]
- 18.Patten LC, Berger DH, Rodriguez-Bigas M, et al. A prospective evaluation of radiocolloid and immunohistochemical staining in colon carcinoma lymphatic mapping. Cancer. 2004;100:2104–2109. [DOI] [PubMed] [Google Scholar]
- 19.Paramo JC, Summerall J, Poppiti R, et al. Validation of sentinel node mapping in patients with colon cancer. Ann Surg Oncol. 2002;9:550–554. [DOI] [PubMed] [Google Scholar]
- 20.Bilchik AJ, Saha S, Wiese D, et al. Molecular staging of early colon cancer on the basis of sentinel node analysis: a multicenter phase II trial. J Clin Oncol. 2001;19:1128–1136. [DOI] [PubMed] [Google Scholar]
- 21.Feig BW, Curley S, Lucci A, et al. A caution regarding lymphatic mapping in patients with colon cancer. Am J Surg. 2001;182:707–712. [DOI] [PubMed] [Google Scholar]
- 22.Wood TF, Saha S, Morton DL, et al. Validation of lymphatic mapping in colorectal cancer: in vivo, ex vivo, and laparoscopic techniques. Ann Surg Oncol. 2001;8:150–157. [DOI] [PubMed] [Google Scholar]
- 23.Saha S, Wiese D, Badin J, et al. Technical details of sentinel lymph node mapping in colorectal cancer and its impact on staging. Ann Surg Oncol. 2000;7:120–124. [DOI] [PubMed] [Google Scholar]
- 24.Wiese DA, Saha S, Badin J, et al. Pathologic evaluation of sentinel lymph nodes in colorectal carcinoma. Arch Pathol Lab Med. 2000;124:1759–1763. [DOI] [PubMed] [Google Scholar]
- 25.Greenson JK, Isenhart CE, Rice R, et al. Identification of occult micrometastases in pericolic lymph nodes of Duke’s B colorectal cancer patients using monoclonal antibodies against cytokeratin and CC49. Correlation with long-term survival. Cancer. 1994;73:563–569. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi N, Ito I, Yanagisawa A, et al. Genetic diagnosis of lymph-node metastasis in colorectal cancer. Lancet. 1995;345:1257–1259. [DOI] [PubMed] [Google Scholar]
- 27.Broll R, Schauer V, Schimmelpenning H, et al. Prognostic relevance of occult tumor cells in lymph nodes of colorectal carcinomas: an immunohistochemical study. Dis Colon Rectum. 1997;40:1465–1471. [DOI] [PubMed] [Google Scholar]
- 28.Liefers GJ, Cleton-Jansen AM, van de Velde CJ, et al. Micrometastases and survival in stage II colorectal cancer. N Engl J Med. 1998;339:223–228. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg R, Hoos A, Mueller J, et al. Prognostic significance of cytokeratin-20 reverse transcriptase polymerase chain reaction in lymph nodes of node-negative colorectal cancer patients. J Clin Oncol. 2002;20:1049–1055. [DOI] [PubMed] [Google Scholar]
- 30.Palma RT, Waisberg J, Bromberg SH, et al. Micrometastasis in regional lymph nodes of extirpated colorectal carcinoma: immunohistochemical study using anti-cytokeratin antibodies AE1/AE3. Colorectal Dis. 2003;5:164–168. [DOI] [PubMed] [Google Scholar]
- 31.Cutait R, Alves VA, Lopes LC, et al. Restaging of colorectal cancer based on the identification of lymph node micrometastases through immunoperoxidase staining of CEA and cytokeratins. Dis Colon Rectum. 1991;34:917–920. [DOI] [PubMed] [Google Scholar]
- 32.Jeffers MD, O’Dowd GM, Mulcahy H, et al. The prognostic significance of immunohistochemically detected lymph node micrometastases in colorectal carcinoma. J Pathol. 1994;172:183–187. [DOI] [PubMed] [Google Scholar]
- 33.Adell G, Boeryd B, Franlund B, et al. Occurrence and prognostic importance of micrometastases in regional lymph nodes in Dukes’ B colorectal carcinoma: an immunohistochemical study. Eur J Surg. 1996;162:637–642. [PubMed] [Google Scholar]
- 34.Oberg A, Stenling R, Tavelin B, Lindmark G. Are lymph node micrometastases of any clinical significance in Dukes Stages A and B colorectal cancer? Dis Colon Rectum. 1998;41:1244–1249. [DOI] [PubMed] [Google Scholar]
- 35.Nakanishi Y, Ochiai A, Yamauchi Y, et al. Clinical implications of lymph node micrometastases in patients with colorectal cancers. A case control study. Oncology. 1999;57:276–280. [DOI] [PubMed] [Google Scholar]
- 36.Choi HJ, Choi YY, Hong SH. Incidence and prognostic implications of isolated tumor cells in lymph nodes from patients with Dukes B colorectal carcinoma. Dis Colon Rectum. 2002;45:750–755. [DOI] [PubMed] [Google Scholar]
- 37.Fisher ER, Colangelo L, Wieand S, et al. Lack of influence of cytokeratin-positive mini micrometastases in “Negative Node” patients with colorectal cancer: findings from the national surgical adjuvant breast and bowel projects protocols R-01 and C-01. Dis Colon Rectum. 2003;46:1021–1025. [DOI] [PubMed] [Google Scholar]
- 38.Wong JH, Steinemann S, Tom P, et al. Volume of lymphatic metastases does not independently influence prognosis in colorectal cancer. J Clin Oncol. 2002;20:1506–1511. [DOI] [PubMed] [Google Scholar]