Abstract
Objective:
To evaluate the role of transient receptor potential vanilloid 1 (TRPV1) in a rat hemorrhagic shock (HS) model using the TRPV1 antagonist, capsazepine (CPZ).
Summary Background Data:
TRPV1, distributed within the sensory nerve, plays a role in the regulation of cardiovascular functions. TRPV1 may be involved in the cardiovascular responses to HS.
Methods:
Male rats were anesthetized and HS was induced with the mean arterial pressure (MAP) at 30 mm Hg for 90 minutes. CPZ (5.0 μmol/kg) was administered at 30 minutes after the shock induction, and the 24-hour survival rates were assessed. The MAP, heart rate, and shed blood volume (SBV) were recorded throughout the experiment. Arterial blood gas analysis and the plasma catecholamines levels were measured before and after HS. Double-immunohistochemistry for Fos and tyrosine hydroxylase (TH) was performed in the rostral ventrolateral medulla (RVLM) of the brain.
Results:
CPZ significantly improved the 24-hour survival rates, which was accompanied by the increase in the MAP and the SBV, a decrease of the plasma catecholamines levels, and attenuation of the severe metabolic acidosis. Furthermore, CPZ reduced the percentage of double-labeled neurons for Fos and TH in the RVLM of the rat brain.
Conclusions:
TRPV1 may be involved in the regulation of the cardiovascular responses to HS, at least in part, by recruiting catecholaminergic neurons in the RVLM. CPZ appears to induce metabolic compensations, which may be potentially useful in HS.
TRPV1 may be involved in the regulation of the cardiovascular responses to hemorrhagic shock. Capsazepine appears to be potentially therapeutically useful for hemorrhage shock.
Transient receptor potential vanilloid 1 (TRPV1) is a ligand-gated, nonselective cation channel and widely distributed within the cardiovascular system that has principally been identified on capsaicin-sensitive sensory nerve (CSSN) endings.1,2 TRPV1, expressed by the neurons of the dorsal root ganglion constituting the origin of C and Aδ primary afferent fibers,3 has been shown to be activated by a variety of physical and chemical stimuli, including noxious heat, reduced pH, ischemia, and capsaicin,2,4,5 and involved in the modulation of cardiovascular functions, including changes in the blood flow and heart rate (HR).6,7 Capsazepine (CPZ) is a selective TRPV1 antagonist that has been shown to competitively inhibit the responses to capsaicin in mice and rats.8
Hemorrhagic shock (HS) is a broad-spectrum hemodynamic stimulus, eliciting a constellation of compensatory autonomic and humoral responses,9 which are initiated by afferent C- and Aδ-fibers.10 Cardiovascular receptors, such as the baroreceptors, are considered to be the chief players in mediating the reflex responses to acute hypovolemia, and their afferent sensory fibers converge in the central nervous system (CNS).11 In particular, physical stressors, like hemorrhage,12 appear to act mainly through primary afferent input to the brainstem catecholaminergic neurons.13 The catecholaminergic neuronal system in the medulla, including the rostral ventrolateral medulla (RVLM), plays a pivotal mediatory role for the compensatory neuroendocrine responses by relaying pressure and volume-related information,14 and it has been demonstrated that Fos expression in tyrosine hydroxylase (TH)-containing neurons in the ventrolateral medulla reflects the extent of the catecholaminergic neuronal activation in response to HS.15
In the present study, we examined the effects of CPZ on the survival rates in a rat HS model. To explore the mechanism underlying the effects of CPZ on the compensatory cardiovascular responses, we measured cardiovascular and metabolic parameters. In addition, we assessed the effects of CPZ on the expression of Fos in the catecholaminergic neurons of the RVLM to determine whether activation of TRPV1 in response to HS is associated with recruitment of catecholaminergic neurons in this brain region.
METHODS
Animals
A total of 80 Sprague-Dawley male rats (8–10 weeks old; Clea Japan, Inc., Tokyo, Japan) were housed in a controlled environment with a 12-hour light/dark cycle. The rats were allowed access to standard laboratory food and water ad libitum. All the animal experiments were performed in accordance with the guidelines of the Institute for Experimental Animals of Shiga University of Medical Science, after obtaining approval from the Institutional Review Board.
Hemorrhagic Shock Model
The rats were anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg). HS was induced at our laboratory as described previously.16 Briefly, the left femoral artery was cannulated with a heparinized (10 units/mL) polyethylene tube, which was connected to a three-way in-line stopcock for blood withdrawal and blood pressure monitoring. Blood was withdrawn into a syringe until the mean arterial pressure (MAP) decreased to 30 mm Hg, and it was maintained at 30 mm Hg for 90 minutes by withdrawing/reinfusing blood, as necessary. The animals were then observed for an additional 30 minutes. Each rat was administered an intravenous injection of vehicle (saline containing 10% Tween 80/10% ethanol; control group) or capsazepine (CPZ: dissolved in vehicle; Sigma Chemical Co., St Louis, MO; CPZ group) 30 minutes after the shock induction. The remaining rats (sham group) were anesthetized and treated as unchallenged controls: their left femoral arteries were cannulated, but no blood was withdrawn.
Survival Rate
To determine the 24-hour survival rates after HS, the 50 rats were administered vehicle (1 mL/kg; n = 10) or CPZ (0.005, 0.05, 0.5, 5.0 μmol/kg, n = 10, respectively) intravenously 30 minutes after the shock induction. At the end of the experimental period (at time 120 minutes), the animals were returned to their individual cages and allowed access to food and water, and the 24-hour survival rates were determined. For the subsequent experiments, we used 5.0 μmol/kg of CPZ to assess the cardiovascular and metabolic parameters, and the changes in the distribution of Fos expression in the RVLM of the rat brain.
Evaluation of ABG and Plasma Levels of Catecholamines
Blood samples were collected before the shock induction (at time 0 minutes) and at the end of the experimental period for measurement of arterial blood gas (ABG; Rapid laboratory 348, Bayer Medical). All the blood samples in each group (sham, control, and CPZ: n = 10, respectively) were collected in heparinized tubes and measured immediately after the withdrawal. We also measured the plasma levels of catecholamines, that is, epinephrine and norepinephrine, at the same 2 time-points. Samples were collected into ice-cold polypropylene tubes containing EDTA and centrifuged immediately (1500g for 5 minutes at 4°C). The separated plasma specimens were kept frozen at −80°C until the assay. The plasma levels of catecholamines were then measured using a HPLC system equipped with an immobilized enzyme reactor and an electrochemical detector (HLC-8030, Tosoh). An analog recording system (BIO COLOR GRAPH 2G82, NEC San-ei Co.) was used for continuous monitoring of the MAP and HR throughout the experimental period. The volume of blood withdrawn to maintain the MAP at 30 mm Hg was recorded as the shed blood volume (SBV). The SBV was measured separately during the first 30 minutes (before treatment) and between 30 and 90 minutes of the start of blood withdrawal (after treatment).
Immunohistochemistry for Fos and TH
At the end of the experimental period, 7 rats from each of the groups (sham, control, and CPZ) were deeply anesthetized by an overdose of pentobarbital (100 mg/kg) and perfused transcardially with normal saline, followed by 4% paraformaldehyde and 0.2% picric acid in 0.1 mol/L phosphate buffer (PBS; pH 7.4). The brain was removed and postfixed and stored overnight at 4°C submerged in 20% sucrose for 4 days, and cut into 20-μm sections using a cryostat.
For Fos and TH double-label immunohistochemistry, the brainstem tissue sections from each of the rat groups were processed as described previously.17 Briefly, the sections were washed in 0.3% Triton X-100 in PBS, followed by incubation with primary rabbit Fos antiserum (Ab-2; Oncogene Research Products, Darmstadt, Germany; 1:5,000 dilution in PBS) for 48 hours at 4°C. Then, after washing in PBS, the sections were incubated in biotinylated goat anti-rabbit IgG (Vector Laboratories Inc.; 1:1,000) followed by incubation with avidin-biotin complex (Vector Elite ABC Kit; Vector Laboratories Inc., Burlingame, CA; 1:2000 in PBST). A combination of 0.04% diaminobenzidine tetrahydrochloride (Dojindo Laboratories), 0.01% nickel ammonium sulfate (Nacalai Tesque, Inc., Kyoto, Japan), and 0.01% hydrogen peroxide dissolved in 0.05 mol/L Tris/HCl buffer was used for the chromogen reaction. Following the Fos staining, the sections were incubated with mouse monoclonal anti-TH antibody (Chemicon International; 1:2000 in PBS) as the secondary antibody for 48 hours at 4°C.
Quantification of Fos/TH Neurons
The cell counts (Fos-expressing and TH-containing cells) were conducted under a 100× objective by an observer blinded to the treatment groups, using a computer-associated image analysis system, the CCD camera VB-7000 (Keyence, Osaka, Japan), and the results were expressed as the percentage of double-labeled neurons relative to the total number of TH-containing neurons in the RVLM of the rat brain.
Statistical Analysis
All the data were expressed as means ± SEM. Statistical significance was determined by analysis of variance (ANOVA) with repeated measures, followed by Fisher's post hoc test. Differences between the 2 groups at various time points were evaluated by ANOVA. The unpaired t test was used for other comparisons between the 2 groups. Fisher exact test was used to examine the differences in the survival rates between the animal groups. The cell count data from the double-label immunohistochemical study was also analyzed by ANOVA, and differences between groups were analyzed by Fisher's protected least significant difference. Statistical significance was assumed at P < 0.05.
RESULTS
Administration of CPZ increased the 24-hour survival rates in a dose-dependent manner (0.005 μmol/kg; 20%, 0.05 μmol/kg; 30%, 0.5 μmol/kg; 40% in each group), and 5.0 μmol/kg dose significantly improved to 80% as compared with that in the control group (10%, P < 0.05).
To explore the mechanisms by which CPZ improved the survival rates, we measured systemic hemodynamic parameters (HR, MAP, and SBV). There were no significant differences in the baseline (at time 0 minutes), HR (355.20± 11.71/353.67 ± 13.54 beats/min) or MAP (88.7 ± 2.39/90.2± 3.46 mm Hg) between the CPZ and control groups, and CPZ had no effect on HR and MAP in the sham group (data not shown). At time 120 minutes, the MAP (43.1± 3.46/28.3± 0.80 mm Hg) was significantly higher in the CPZ group as compared with that in the control group, although the difference in the HR (328.67± 14.59/297.33± 9.06 beats/min) was not statistically significant (Fig. 1). Before the treatment (administration of CPZ/vehicle), there was no significant difference in the SBV between the CPZ and control groups (2.96± 0.13/3.00± 0.08 mL/100 g body weight). However, after the treatment in the respective groups, a significant difference of the SBV was noted (1.37± 0.07/1.01± 0.14 mL/100 g body weight) (Fig. 2).
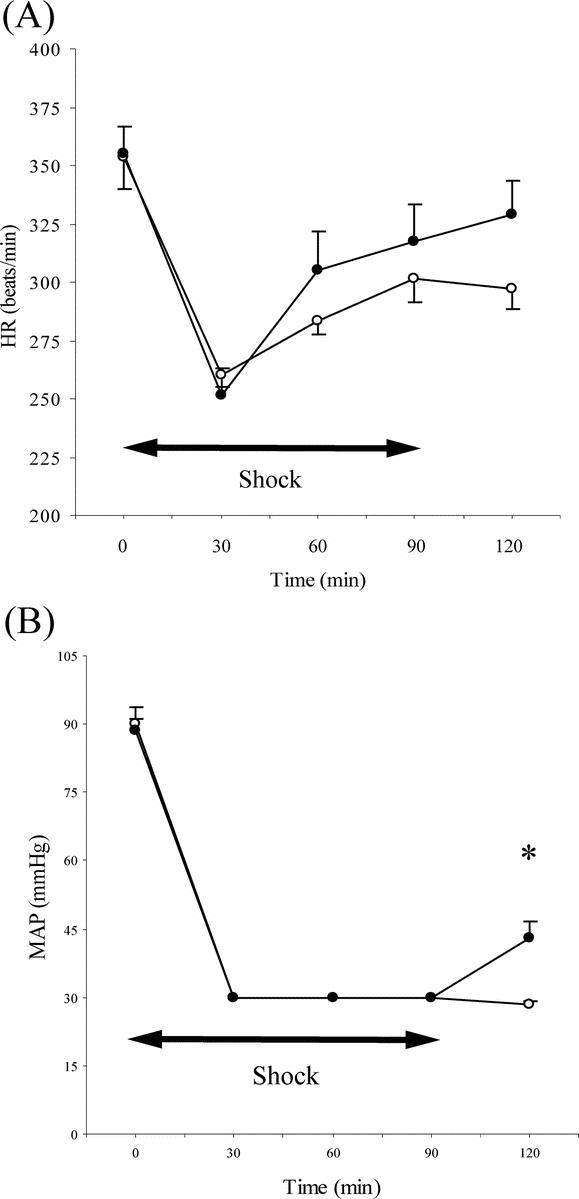
FIGURE 1. Heart rate (HR; A) and mean arterial pressure (MAP; B) during the experiment in the rats of the control and the CPZ groups (n = 10 in each group). Clear circles, control group (hemorrhagic shock + vehicle); filled circles, CPZ group (hemorrhagic shock + CPZ). *P < 0.01 for CPZ group versus control group.
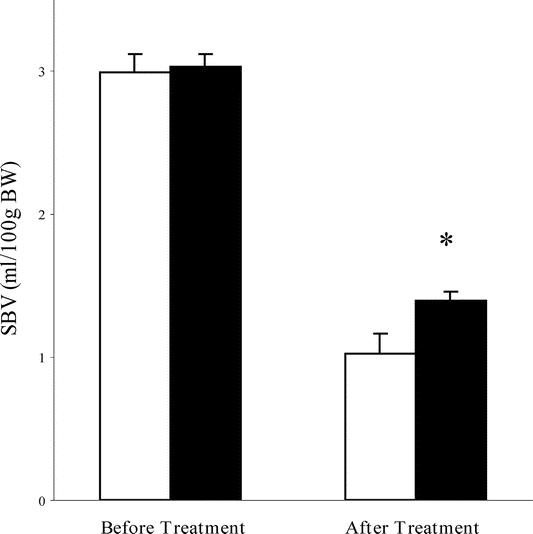
FIGURE 2. Cumulative shed blood volume (SBV) before and after treatment with CPZ/vehicle (n = 10 in each group). Clear column, control group (hemorrhagic shock + vehicle); filled column, CPZ group (hemorrhagic shock + CPZ). *P < 0.05 for CPZ group versus control group.
The metabolic acidosis with HS was further characterized by conducting ABG analysis, as shown in Table 1. There were no significant differences in ABG or acid-base parameters (at time 0 minutes) between the 2 groups. At time 120 minutes, severe metabolic acidosis, including the pH, base excess (BE), bicarbonate (HCO3), developed in the control group, whereas the severity was significantly attenuated in the CPZ group. In the control group, a moderate increase of the arterial pO2 was observed at time 120 minutes, with a significant decrease in the arterial pCO2. A similar increase of the arterial pO2 was also observed in the CPZ group; however, no decrease of the arterial pCO2 was observed noted in this group.
TABLE 1. Arterial Blood Gas and Acid-Base Parameters, and Plasma Levels of Catecholamines Before and at the End of the Experimental Period
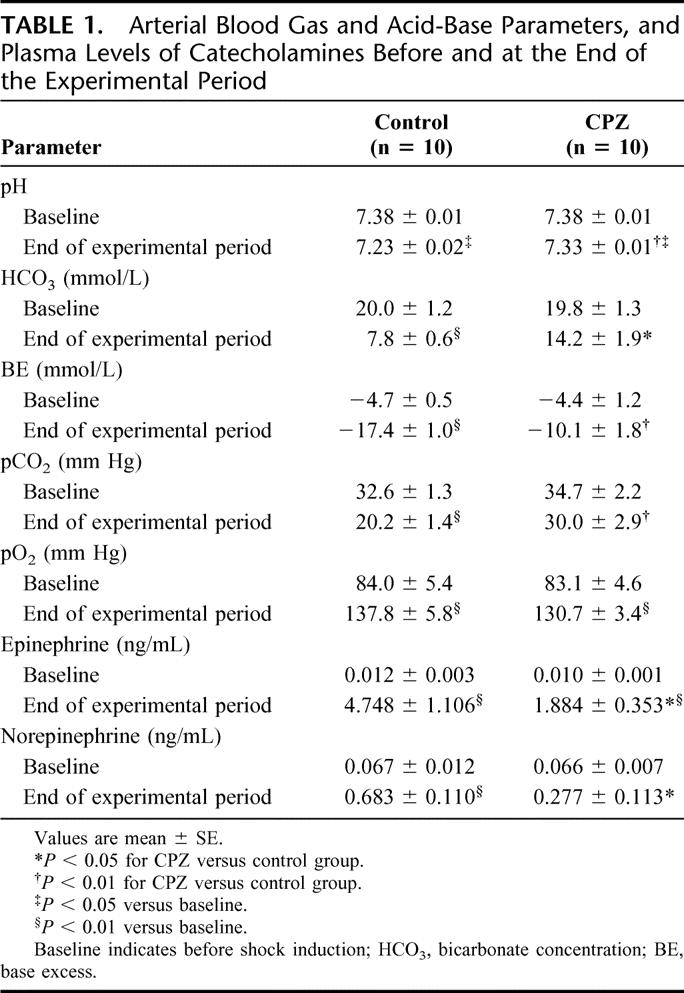
To explore the involvement of catecholamines, we measured the plasma levels of epinephrine and norepinephrine. The plasma levels of catecholamines significantly increased at time 120 minutes in the control group. On the other hand, while the plasma catecholamines levels were also significantly increased as compared with the baseline levels in the CPZ group, the levels were significantly lower than those in the control group (Table 1). CPZ administration had no effect on plasma catecholamines levels in the sham group (data not shown).
Finally, we assessed the double-immunohistochemistry for Fos and TH in the RVLM catecholaminergic neurons after the induction of HS (Fig. 3). The percentage of double-labeled neurons (Fos + TH) in the control group was significantly higher (61.5% ± 2.0%) as compared with that in the sham group (37.3% ± 3.0%). On the other hand, CPZ, at the dose at which it significantly attenuated the adverse cardiovascular responses and induced metabolic compensations, significantly decreased the percentage of double-labeled neurons in the RVLM in the rat model of HS (43.8% ± 4.7%) (Fig. 3D).
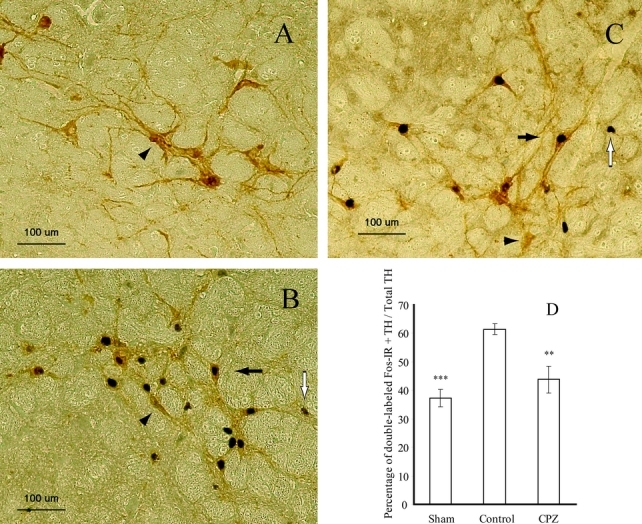
FIGURE 3. Representative photomicrographs of double-labeled neurons for Fos and TH in the rostral ventrolateral medulla (RVLM) in the sham (A), control (B), and CPZ (C) groups. Black arrows, Fos/TH double-labeled neurons; white arrows, Fos-immunoreactive neurons; arrowheads, TH neurons. Effects of CPZ on Fos expression in the TH neurons in the RVLM (D). The results are expressed in terms of the percentage of double-labeled neurons for Fos and TH in the RVLM. **P < 0.01 for CPZ group versus control group. ***P < 0.001 for sham group versus control group; n= 7 in each group.
DISCUSSION
In the present study, we found that the administration of CPZ to rats with HS was associated with a dose-dependent improvement in the 24-hour survival rates, and 5.0 μmol/kg of CPZ attenuated the development of severe metabolic acidosis, and reduced plasma levels of catecholamines and Fos expression in the TH-containing catecholaminergic neurons in the RVLM. These findings suggest that catecholaminergic neurons in the brainstem, known to be critical regulators of cardiovascular responses, may be activated by TRPV1 stimulation induced by HS, which may, at least in part, be associated with the lethal injury in this HS model.
Cardiovascular responses to HS are regulated by both central and peripheral mechanisms, which include neurohormonal mechanisms, such as the renin-angiotensin system, arginine-vasopressin, and sympathetic nervous systems.18 Recently, it has been reported that activation of peripheral cannabinoid 1 receptors may contribute to the pathogenesis of HS, and that this effect may be mediated by the anandamide (AEA).19 AEA has been shown to be an endogenous ligand for TRPV1.6 Thus, it is likely that AEA may be involved in the hemodynamic responses to HS through TRPV1 activation.
In the present study, we used CPZ as a widely used competitive antagonist of TRPV1, and this agent has also previously been used in rat ischemia reperfusion and endotoxic shock models.20 Iodo-resiniferatoxin, (I-RTX) has recently been shown to be a high-affinity antagonist of TRPV1.21 However, in some subsequent studies, it has also been shown to have agonistic activity in some doses22 and have limited utility for behavioral study.23 Accordingly, we used CPZ as the TRPV1 antagonist despite its only modest affinity for TRPV1. In addition, CPZ may also have nonspecific actions in addition to its effects on the TRPV1, such as on ion-channels.24 Further studies must be conducted to investigate the specificity of TRPV1 ligands to determine the physiological roles of TRPV1.
We observed a decrease of the arterial pH and pCO2, serum HCO3, and BE, and an increase in the arterial pO2 at time 120 minutes in the control group, which is consistent with a previous report.25 And the arterial pO2 increased moderately as compared with the baseline values in the control group, reflective of the hyperventilatory response to compensate for the metabolic acidosis developing during HS, which is also corroborated by the significant decrease in the arterial pCO2 as compared with that at the baseline (Table 1). On the other hand, no such evidence of severe metabolic acidosis with hypocapnia was observed in the CPZ group. In addition, the SBV after treatment was significantly higher in the CPZ group as compared with that in the control group (Fig. 2), which appears to increase the circulating intravascular volume in HS. In this context, CPZ may be therapeutically useful for the prevention of circulatory disturbance and metabolic acidosis, and the consequent complications of HS.
The plasma catecholamines levels in the CPZ group at time 120 minutes were significantly lower as compared with those in the control group, which is consistent with a previous report.26 It has been shown that signals from the CSSN during HS can evoke a massive discharge of epinephrine from the adrenal medulla.27 A recent study has demonstrated that neonatal capsaicin treatment (capsaicin-denervated rats) almost completely abolished TRPV1 expression in the CSSN.28 According to our preliminary data, capsaicin-denervated rats did not show any significant increase, and indeed, had slightly lower levels of the plasma catecholamines as compared with those in the CPZ group at time 120 minutes. In addition, CPZ administration also had no effect on the plasma catecholamine levels in capsaicin-denervated rats with HS (data not shown). These results suggest that the massive release of plasma catecholamines during HS may, in part, be mediated by the TRPV1 expressed on the CSSN.
There are 2 sets of cardiovascular sensors, namely, the arterial baroreceptors and receptors in the cardiopulmonary region, whose inputs to the CNS are thought to play important roles responses to acute blood loss.18,29 Most afferent fibers from the cardiac receptors are unmyelinated C fibers, those are selectively destroyed by neonatal capsaicin treatment, and the destructed afferent fibers caused a significant reduction of reflex increase in the serum epinephrine levels following coronary occlusion.30 We speculate that HS-induced activation of TRPV1 expressed on the CSSN may play pivotal roles in relaying pressure and volume-related information to the CNS. The RVLM is considered to be a major efferent output center, including the regulation of sympathetic activity and cardiovascular responses. The present study was conducted to determine whether TRPV1 might be involved in sympathetic neural activation in the RVLM during HS. The functional neuroanatomic study of Fos expression showed that catecholaminergic neurons in the RVLM were indeed activated following HS, and that CPZ administration significantly blunted this neuronal activation in the RVLM.
A dramatic increase in the plasma epinephrine levels during hypotension has been reported in both humans31 and animals.9 In rats, Pittet et al32 previously reported that the development of HS was associated with a significant increase in the plasma epinephrine levels over a period of 2 hours, which is consistent with our results. However, species differences in the sympathoadrenal mechanisms involved in the cardiovascular responses to prolonged HS have been reported.18 Further studies are necessary to determine the effects of CPZ, specifically in humans before this agent can be applied in the clinical setting. In this study, we used rats anesthetized with pentobarbital, and the effect of the drug lasted for more than 2 hours in both the control and the CPZ groups. Anesthetics are known to weaken sympathoadrenal responses and affect also the cardiovascular responses to HS.33,34 Although our results with respect to the cardiovascular parameters and of the neuroanatomic study by Fos immunohistochemistry were correlated well with each other, further studies using conscious rat models are needed to exclude the effects of anesthetic agents on the responses to HS.
The mechanisms underlying the protective effects of CPZ during HS are still not clearly understood. The present experiment focused mainly on the CNS mechanisms, although it may not depend solely on central sympathetic nerve regulation. A recent study has demonstrated that TRPV1 activation induces the release of neurotransmitters, which may also mediate the cardiovascular responses.35 Possible involvement of these TRPV1-induced neurotransmitters in the cardiovascular responses in HS needs to be clarified to investigate the integrated cardiovascular responses to HS. Nonetheless, our results do provide important evidence to suggest that activation of TRPV1 may be involved in the regulation of cardiovascular responses to potentially fatal HS, at least in part, by recruiting catecholaminergic neurons in the RVLM.
Although the present study used an HS model without resuscitation, CPZ administration during HS improved the survival rates. In clinical practice, the preoperative approach to hypotension in patients with trauma has included prompt intravenous infusion of isotonic fluids. On the other hand, a previous study has expressed concern that intravenous infusion of fluids before the hemorrhage is surgically controlled may be detrimental in the clinical setting.36 The mechanisms underlying the detrimental effects of fluid administration in humans are difficult to ascertain. Further studies are needed to evaluate the effect of CPZ in HS using a rat model with resuscitation. Nonetheless, our results suggest that CPZ may be a useful tool for the treatment of patients with uncontrolled HS in such situations as prehospital care.
CONCLUSION
The present study demonstrates that the TRPV1 antagonist, CPZ, improves the survival rates in animal models of HS. CPZ attenuates both the increase in the plasma catecholamines levels associated with activation of the catecholaminergic neurons in the brainstem and the severe metabolic acidosis in HS. These findings suggest that activation of TRPV1 may be involved in the regulation of the cardiovascular responses to HS, at least in part, by engaging catecholaminergic neurons in the RVLM. Thus, CPZ appears to offer a useful tool in the treatment of HS.
Footnotes
Reprints: Hiroshi Yamamoto, MD, PhD, Department of Surgery, Shiga University of Medical Science, Seta-Tsukinowa-cho, Otsu, Shiga, Japan, 520-2192. E-mail: yhiroshi@belle.shiga-med.ac.jp.
REFERENCES
- 1.Zahner MR, Li DP, Chen SR, et al. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol. 2003;551:515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. [DOI] [PubMed] [Google Scholar]
- 3.Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tominaga M, Caterina MJ, Malmberg AB, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. [DOI] [PubMed] [Google Scholar]
- 5.Pan HL, Chen SR. Sensing tissue ischemia: another new function for capsaicin receptors? Circulation. 2004;110:1826–1831. [DOI] [PubMed] [Google Scholar]
- 6.Malinowska B, Kwolek G, Gothert M. Anandamide and methanandamide induce both vanilloid VR1- and cannabinoid CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:562–569. [DOI] [PubMed] [Google Scholar]
- 7.Ralevic V, Kendall DA, Randall MD, et al. Vanilloid receptors on capsaicin-sensitive sensory nerves mediate relaxation to methanandamide in the rat isolated mesenteric arterial bed and small mesenteric arteries. Br J Pharmacol. 2000;130:1483–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos AR, Calixto JB. Ruthenium red and capsazepine antinociceptive effect in formalin and capsaicin models of pain in mice. Neurosci Lett. 1997;235:73–76. [DOI] [PubMed] [Google Scholar]
- 9.Darlington DN, Shinsako J, Dallman MF. Responses of ACTH, epinephrine, norepinephrine, and cardiovascular system to hemorrhage. Am J Physiol. 1986;251:612–618. [DOI] [PubMed] [Google Scholar]
- 10.Lembeck F. A network of defense. In: Henry JL, Couture R, Cuello AC, eds. Substance P and Neurokinins. Berlin: Springer, 1987:380–387. [Google Scholar]
- 11.Evans RG, Ventura S, Dampney RA, et al. Neural mechanisms in the cardiovascular responses to acute central hypovolaemia. Clin Exp Pharmacol Physiol. 2001;28:479–487. [DOI] [PubMed] [Google Scholar]
- 12.Chan RK, Brown ER, Ericsson A, et al. A comparison of two immediate-early genes, c-fos and NGFI-B, as markers for functional activation in stress-related neuroendocrine circuitry. J Neurosci. 1993;13:5126–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawchenko PE, Swanson LW. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science. 1981;214:685–687. [DOI] [PubMed] [Google Scholar]
- 14.Plotsky PM, Cunningham ET Jr, Widmaier EP. Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocr Rev. 1989;10:437–458. [DOI] [PubMed] [Google Scholar]
- 15.Chan RK, Sawchenko PE. Spatially and temporally differentiated patterns of c-fos expression in brainstem catecholaminergic cell groups induced by cardiovascular challenges in the rat. J Comp Neurol. 1994;348:433–460. [DOI] [PubMed] [Google Scholar]
- 16.Mori T, Yamamoto H, Tabata T, et al. A free radical scavenger, edaravone (MCI-186), diminishes intestinal neutrophil lipid peroxidation and bacterial translocation in a rat hemorrhagic shock model. Crit Care Med. 2005;33:1064–1069. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto H, Lee CE, Marcus JN, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;10:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schadt JC, Ludbrook J. Hemodynamic and neurohumoral responses to acute hypovolemia in conscious mammals. Am J Physiol. 1991;260:305–318. [DOI] [PubMed] [Google Scholar]
- 19.Wagner JA, Varga K, Ellis EF, et al. Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature. 1997;390:518–521. [DOI] [PubMed] [Google Scholar]
- 20.Orliac ML, Peroni R, Celuch SM, et al. Potentiation of anandamide effects in mesenteric beds isolated from endotoxemic rats. J Pharmacol Exp Ther. 2003;304:179–184. [DOI] [PubMed] [Google Scholar]
- 21.Wahl P, Foged C, Tullin S, et al. Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist. Mol Pharmacol. 2001;59:9–15. [DOI] [PubMed] [Google Scholar]
- 22.Charrua A, Avelino A, Cruz C, et al. Iodoresiniferatoxin (IRTX) applied topically to the rat bladder acts as a potent TRPV1 agonist. Soc Neurosci Annu Meeting Abstr. 2004;743:7. [Google Scholar]
- 23.Seabrook GR, Sutton KG, Jarolimek W, et al. Functional properties of the high-affinity TRPV1 (VR1) vanilloid receptor antagonist (4-hydroxyl-5-iodo-3-methoxyphenylacetate ester) iodo-resiniferatoxin. J Pharmacol Exp Ther. 2002;303:1052–1060. [DOI] [PubMed] [Google Scholar]
- 24.Docherty RJ, Yeats JC, Piper AS. Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurons in culture. Br J Pharmacol. 1997;121:1461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tagliavini S, Bertolini E, Bazzani C, et al. Influence of TRH on regional blood flow and metabolic acidosis in a model of volume-controlled hemorrhagic shock in rats. Neuropeptides. 1991;20:233–238. [DOI] [PubMed] [Google Scholar]
- 26.Shackford SR, Norton CH, Ziegler MG, et al. The effect of hemorrhage and resuscitation on serum levels of immunoreactive atrial natriuretic factor. Ann Surg. 1988;207:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donnerer J. Reflex activation of the adrenal medulla during hypoglycemia and circulatory dysregulations is regulated by capsaicin-sensitive afferents. Naunyn Schmiedebergs Arch Pharmacol. 1988;338:282–286. [DOI] [PubMed] [Google Scholar]
- 28.Guo A, Vulchanova L, Wang J, et al. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. [DOI] [PubMed] [Google Scholar]
- 29.Little RA, Marshall HW, Kirkman E. Attenuation of the acute cardiovascular responses to haemorrhage by tissue injury in the conscious rat. Q J Exp Physiol. 1989;74:825–833. [DOI] [PubMed] [Google Scholar]
- 30.Godin-Ribuot D, Ribuot C, Lamontagne D, et al. Reflex adrenal medullary secretion during coronary occlusion mediated by cardiac receptors with afferent vagal fibres in the rat. Pflugers Arch. 1997;434:159–165. [DOI] [PubMed] [Google Scholar]
- 31.Sander-Jensen K, Mehlsen J, Stadeager C, et al. Increase in vagal activity during hypotensive lower-body negative pressure in humans. Am J Physiol. 1988;255:149–156. [DOI] [PubMed] [Google Scholar]
- 32.Pittet JF, Brenner TJ, Modelska K, et al. Alveolar liquid clearance is increased by endogenous catecholamines in hemorrhagic shock in rats. J Appl Physiol. 1996;81:830–837. [DOI] [PubMed] [Google Scholar]
- 33.Adamicza A, Tarnoky K, Nagy A, et al. The effect of anaesthesia on the haemodynamic and sympathoadrenal responses of the dog in experimental haemorrhagic shock. Acta Physiol Hung. 1985;65:239–254. [PubMed] [Google Scholar]
- 34.Van der Meer C, Cramer WC, Versluys-Broers JA, et al. The possible role of catecholamines in the protective effect of general anesthetics against kidney lesions in rats subjected to hemorrhagic hypotension. Arch Int Pharmacodyn Ther. 1980;247:145–154. [PubMed] [Google Scholar]
- 35.Wang L, Wang DH. TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation. 2005;112:3617–3623. [DOI] [PubMed] [Google Scholar]
- 36.Crawford ES, Hess KR, Cohen ES, et al. Ruptured aneurysm of the descending thoracic and thoracoabdominal aorta: analysis according to size and treatment. Ann Surg. 1991;213:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]


