Abstract
Objective:
The aim of our study was to detect bile acids and total bilirubin in saliva of gastrectomized patients, to confirm objectively presence of biliary laryngopharyngeal reflux and its relationship with laryngeal mucosa damage.
Summary Background Data:
Recently, it has been hypothesized that biliary-reflux may reach the upper aerodigestive tract and enhance development of laryngeal malignancies; nevertheless, the presence of duodenogastric contents in this region has never been revealed.
Methods:
We carried out a prospective observational case-control study on 52 patients (cases) previously submitted to gastric surgery, mainly to subtotal Billroth II resection, and on 51 healthy volunteers (controls). Patients were submitted to clinical interview, esophagogastroduodenal endoscopy, endoscopic laryngeal evaluation, and saliva collection. In all saliva samples, bile acids, total bilirubin, and pepsinogen II were assayed.
Results:
In cases, group bile acids levels were recorded in 17 of 52 (32.6%) patients, while in 35 of 52 (67.4%) they were undetectable. All controls were negative to bile acids. In positive cases to bile acids, we found a significant (P < 0.05) correlation between bile acids, total bilirubin, and pepsinogen II values and a significant (P < 0.05) higher prevalence of symptoms and findings of laryngeal damage and of previous laryngeal neoplastic lesions.
Conclusions:
We found detectable levels of bile acids and total bilirubin in saliva of patients submitted to previous gastric surgery, prospecting an intriguing diagnostic role of this dosage in the study of biliary laryngopharyngeal reflux. We finally revealed a high incidence of laryngeal disorders in patients with positive bile acids in saliva.
Recently, the clinical association between gastrectomy and laryngeal malignancies has been demonstrated, suggesting that biliary reflux may, in some cases, reach the upper aerodigestive tract and promote carcinogenesis. Nevertheless, the presence of duodenal contents in this region has never been directly revealed.
The term duodenogastroesophageal reflux (DGER) indicates the retrograde progression of duodenal content across the stomach into the esophagus.1 Etiopathogenically, DGER can be categorized as primary, due to an altered pyloric motility, or secondary if correlated to an impairment of pyloric anatomic integrity, following gastric surgery.2
The clinical importance of the damaging action of DGER on the gastroesophageal mucosa, and its correlation to inflammatory, preneoplastic, or frankly neoplastic patterns, has been well documented in the literature.3,4 Several studies have shown that bile acids are detectable in the esophagus of patients with DGER by means of prolonged ambulatory aspiration techniques or using fiberoptic probes measuring bilirubin (Bilitec 2000 monitoring). Ambulatory aspiration studies found a significant correlation between the total bilirubin and bile acids levels of aspirated samples and the fiberoptic reading of bilirubin concentrations.5,6 This evidence suggests that they are good tracer for DGER. It has been confirmed, moreover, that patients with complicated DGER (esophagitis, Barrett's esophagus) had greater amounts of duodenal reflux than patients with uncomplicated DGER; and on the basis of experimental findings, some authors have hypothesized that bile acids may play a role in the Barrett's-metaplasia-dysplasia carcinoma sequence in the esophagus.7,8
Recently, it has been hypothesized that biliary reflux may reach the upper aerodigestive tract,9 and it may enhance the development of laryngeal malignancies.10–13 A retrospective case-control study14 confirmed a significant association between gastric surgery and laryngeal cancer risk with an odd ratio of 4.3 (95% confidence interval [CI], 2.4–7.9) and with an increased risk 20 years after surgery.
Even though the clinical association between laryngeal cancer and the chronic exposure to secondary DGER has been demonstrated, the presence of duodenogastric contents in the upper aerodigestive tract has never been objectively revealed in these patients. Furthermore, we are unaware of any literature indicating the presence of duodenal contents in saliva of patients with bile reflux. For these reasons, we tried to detect bile acids and bilirubin in the saliva of patients submitted to previous gastrectomy and hypothesized that their presence could be related to symptoms and findings of laryngeal damage. Indeed, patients submitted to gastric surgery may represent an epidemiologic model for studying the effects of duodenal reflux on pharynx and larynx. To rule out the endogenous production of bile acids in the oral cavity mucosa, we tested pepsinogen II as a marker of gastric reflux.15
MATERIALS AND METHODS
Subjects and Specimen Collection
The research design was a prospective observational case-control study. Subjects were recruited from the clinical practice of the Gastroenterology Department of the Catholic University of the Sacred Heart “A. Gemelli” Hospital.
Patients previously submitted to gastric surgery were enrolled after an esophagogastroduodenal endoscopy and sent to the Department of Otorhinolaryngology. In the first instance, patients were submitted to clinical interview and to a laryngeal evaluation by a fiberoptic flexible endoscope. In a separate second consultation, saliva was collected. Laryngeal reflux symptoms and endoscopic findings were classified according to Reflux Symptom Index (RSI)16 and Reflux Findings Score (RFS).17
Between January 2005 and July 2005, 52 consecutive cases were enrolled. The cases-group was made up by 32 men and 20 females, with a median age of 62.8 ± 14.5 years (range, 25–89 years). Five patients of 52 (9.6%) had a smoking history and 8 (15.3%) revealed to be alcohol consumers. In addition, 51 healthy volunteers (control group) took part to the study. They were selected randomly and matched basing on sex and age, as well as smoking and alcohol habits. None of the volunteers had symptoms or history of reflux disease or previous surgery of the upper gastrointestinal tract. All patients gave their informed consent to participate to the study.
Saliva was collected in the morning at 8 o'clock using the Salivette system.18 Patients were asked to keep the Salivette in the mouth for 15 minutes. The specimens were centrifuged immediately at 4000 rpm for 5 minutes obtaining an 80% of saliva recovery; then they were divided in aliquots and frozen at 80°C until the assays. No difference in the volume of saliva collected between cases and controls was found.
Regardless of collection technique, all subjects (overnight fasting for fluid or food) were asked to refrain smoking in the morning of the test. They denied the use of cream or lotions or inhalers containing steroids immediately prior to saliva collection, and activities that could cause oral bleeding, including brushing or flossing teeth. We never stimulated salivation.
Methods
A total of 103 samples of saliva were collected. The volume of the saliva in each container was recorded, the pH of saliva was measured by potentiometric method using a pH glass electrode (Radiometer ABL 700), and all samples were assayed for bile acids, total bilirubin, and pepsinogen II.
Samples were measured by commercially available enzymatic colorimetric assay (Kit bile acids, Olympus) for determination of total bile acids in human serum and applied on an automated analyser (Olympus AU400). To increase the method sensitivity at low concentrations, the calibration curve was obtained by successive dilutions of the standard Randox (5.0 μmol/L) at the following standard concentrations: 0,6–1,2-2,5–5,0 μmol/L. A commercial control of 4.3 μmol/L (Sentinel), was diluted in physiologic saline solution to a final concentration of 0.3 μmol/L, to verify the interassay reproducibility (CV = <5%).
The total bilirubin concentration was measured on the same analyzer (Olympus AU400) by means of a currently used colorimetric method for total serum bilirubin (Total Bilirubin Kit and control, Olympus).
The pepsinogen II levels were obtained by an Enzyme Linked Immuno-Sorbent Assay (EDITM Human Pepsinogen II ELISA Kit), which has a sensitivity of 0.1 ng/mL. Samples, standards, and controls have been assayed in triplicate.
Statistical Analysis
Statistic analysis of the data was performed by SSPS computer software. Continual variables have been expressed as mean ± SD and comparison between groups were performed by means of Mann-Whitney U test and T test. Comparison of categorical variables was performed by means of Pearson's χ2 or Fisher exact test tests. Linear correlation has been evaluated by Spearman's nonparametric methods. A P value <0.05 was considered significant.
RESULTS
Study Population
The 52 patients submitted to previous gastric surgery (cases), recruited for the study, had a mean interval time after surgery of 16.96 years (range, 1–50 years). Looking at particular surgical procedures, 5 of 52 had Billroth I resection (subtotal excision of the stomach with gastroduodenal anastomosis), 42 Billroth II (subtotal excision of the stomach with gastrojejunal anastomosis and duodenal closure), 4 selected vagotomy plus antrectomy, and 1 had total gastrectomy with Roux reconstruction. The gastric surgery was due to gastric cancer in 15 of 52 patients, lymphoma in 2 cases, and peptic ulcer in 35 cases.
Analytical Results
In the cases a positive saliva bile acids test was recorded in 17 of 52 (32.6%) patients, with a mean value of 1.0 μmol/L (SD ±0.4 μmol/L) and a range of 0.5 to 5.0 μmol/L. The distribution of the positive values of bile acids is represented in Figure 1. In 35 of 52 (67.4%) patients, we did not find any bile acid in saliva.
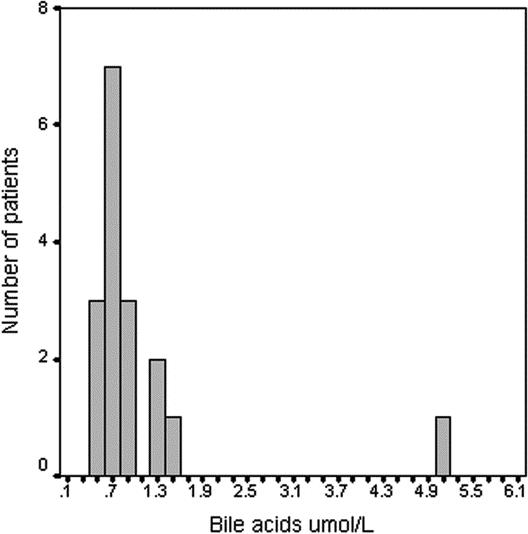
FIGURE 1. Distribution of positive values of bile acids assays in cases with positive tests.
In 13 positive cases to bile acids, we observed a significant (P < 0.05) correlation between bile acids and total bilirubin (Fig. 2). The mean total bilirubin value in saliva was 0.17 mg/dL (SD, 0.05 mg/dL).
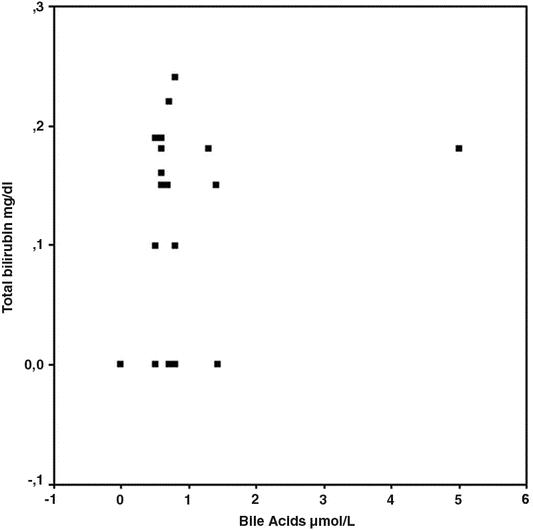
FIGURE 2. Scatter plot about correlation between total bilirubin and bile acids values (P < 0.05).
In all controls, bile acids and total bilirubin were not detectable.
In saliva samples of cases, the mean pH value was 5.9 ± 0.7 with a range of 5.0 to 7.7. We did not find significant differences in pH mean values between cases and controls (5.9 vs. 6.2) and between positive cases to bile acids and negative ones (5.4 vs. 6.0).
Pepsinogen II levels were measurable in all positive bile acid samples, with a mean value of 1.3 ng/mL (SD, ±0.4 ng/mL; range, 0.8–2.0 ng/mL), suggesting the presence of components of gastric juice in the saliva. The distribution of the positive value is presented in Figure 3. Only in 1 case, submitted to total gastrectomy, did we observe positive bile acids test with negative value of pepsinogen II. All patients, in whom no bile acids could be found in saliva, resulted to be negative at the pepsinogen II assay. The pepsinogen II and bile acid levels were significantly correlated as shown in Figure 4 (P < 0.05).
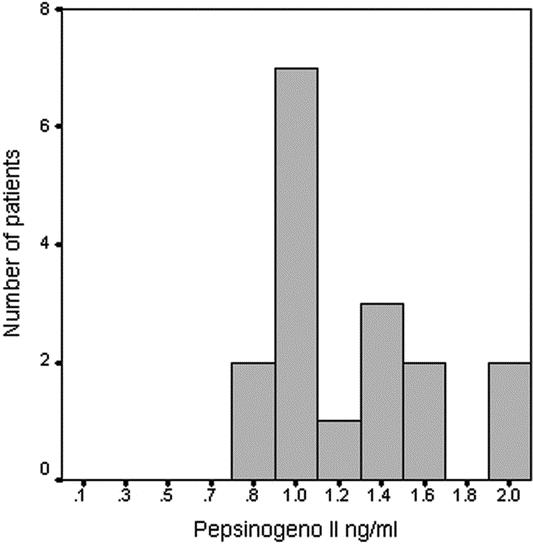
FIGURE 3. Distribution of positive values of pepsinogen II in cases with positive tests.
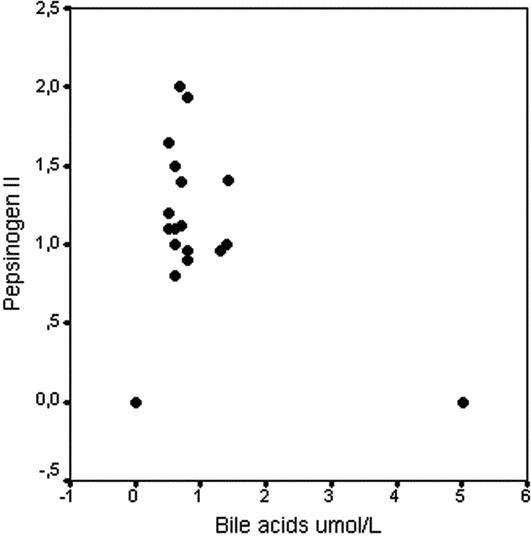
FIGURE 4. Scatter plot about correlation between bile acids and pepsinogen II levels (P < 0.05).
Endoscopic Gastric Findings
During gastric endoscopy, an “open cardias” was revealed in a significantly higher number of positive cases to bile acids if compared with negative ones (88.2% vs. 48.2%; P < 0.05). The cardias was suspected to be hypotonic if it remained open when the operator invited patients to perform a profound inspiration. Manometric evaluation revealed that 77% of patients with open cardias had an incompetent lower esophageal sphincter.
Furthermore, a significant (P < 0.05) difference between positive cases to bile acids and negative ones could be observed in the incidence of gastritis, 47.1% versus 25.7% respectively, and of hiatal hernia (64.7% vs. 25.7%). Finally, no difference could be found concerning esophagitis (29.8% vs. 22.8%), bile or bile-stained mucosa in the stomach (76% vs. 60%) and bile in the esophagus (5.8% vs. 2.8%) (Fig. 5).
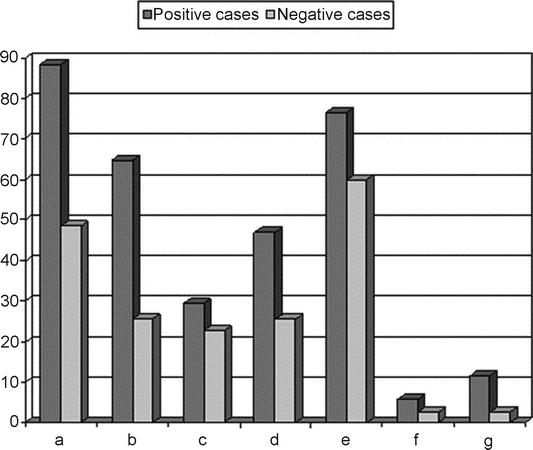
FIGURE 5. Endoscopic esophagogastroduodenal findings in positive cases to bile acids and negative ones: a, hypotonic cardias; b, hiatal hernia; c, esophagitis; d, gastritis; e, bile or bile-stained mucosa in the stomach; f, bile or bile-stained mucosa in the esophagus; g, intestinal metaplasia.
Endoscopic and Clinical Laryngeal Findings
Laryngeal reflux symptoms were classified according to RSI (range, 1–46). Figure 6 shows the incidence of symptoms in positive cases to bile acids respect to negative ones. We found a significantly (P < 0.05) higher mean value of RSI in the positive cases (13.4 ± 6.7) when compared with the negative ones (4.2 ± 6.2).
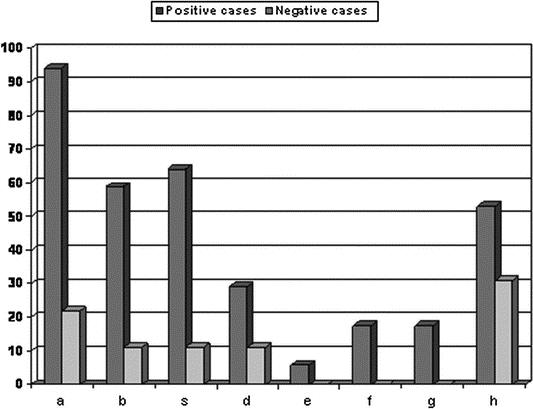
FIGURE 6. Reflux symptoms incidence (Reflux Symptoms Index) in positive cases to bile acids and negative ones: a, hoarseness or a problem with your voice; b, clearing your throat; c, sensations of something sticking in your throat or a lump in your throat; d, troublesome or annoying cough; e, breathing difficulties or choking episodes; f, coughing after you ate or after lying down; g, difficulty swallowing food, liquids, or pills; h, heartburn, chest pain, indigestion, or stomach, acid coming up.
Endoscopic laryngeal findings were classified according to the RFS (range, 1–26). The incidence of each finding in positive cases and negative ones to bile acids is reported in Figure 7. We found a significant (P < 0.05) higher mean value of RFS in positive cases (8.6 ± 5.1) when compared with negative ones (2.8 ± 5.0).
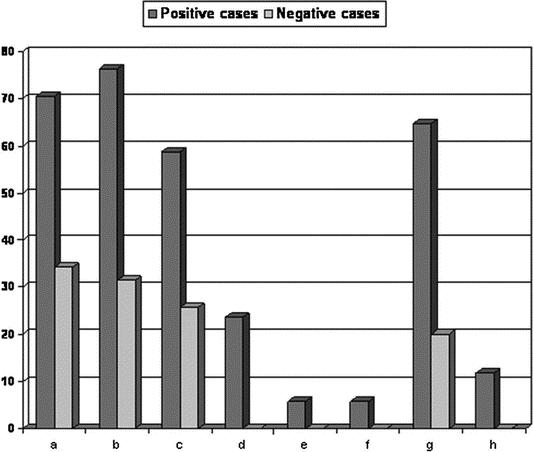
FIGURE 7. Endoscopically revealed reflux findings, (Reflux Findings Score) in positive to bile acids and negative ones: a, posterior commissure hypertrophy; b, diffuse laryngeal edema; c, vocal fold edema; d, thick endolaryngeal mucus; e, ventricular obliteration; f, pseudosulcus, infraglottic edema; g, erythema/hyperemia; h, granuloma/granulation.
A previous history of laryngeal surgery was identified in 7 of 52 (13.5%) of the cases, due to precancerous lesions in 3 cases (2 positive and 1 negative to bile acids) and to squamous cell carcinoma in 4 patients (3 positive and 1 negative to bile acids). Nobody in the controls had a previous history of laryngeal disorders. In patients with previous laryngeal precancerous lesions, the mean interval time between gastric resection and diagnosis of laryngeal lesions was 18.3 ± 15.8 years; whereas in patients with previous laryngeal cancer, it was 34.2 ± 14.9 years. The incidence of laryngeal precancerous or cancer lesions was higher in positive cases to bile acids if compared with negative ones, respectively, 5 of 17 (29.4%) versus 2 of 35 patients (5.7%) (P < 0.05). The logistic regression model revealed a significant odds ratio of 1.92 (95% CI, 1.17–40.2). We did not find a correlation between the different incidence of positive saliva bile acids and interval time after surgery.
DISCUSSION
It is generally recognized that gastroesophageal reflux disease (GERD) can be related to disorders of the upper aerodigestive tract in a distinct condition referred as laryngopharyngeal reflux (LPR). However, recently there is increasing evidence that reflux of bile compounds could be related to several laryngeal disorders and that bile acids may contribute to inflammation and carcinogenesis in the pharynx or larynx as well as in the esophagus.10,11 Sasaki et al9 indeed demonstrated that bile acids appear active in both acid and nonacid environment and that they can be topically injurious to intact laryngeal mucosa.
New diagnostic techniques as bilimetry and multichannel intraluminal impedance are actually routinely used to identify also non acid LPR, but they are at the moment invasive, time-consuming, and not easily available procedures in clinical practice. However, nobody tried to detect directly duodenal contents in the pharynx and in the oral cavity of patients with secondary bile reflux. As secondary DGER has been documented in the majority of patients who underwent gastric surgery,19 we studied, in this population, the bile acids presence in saliva and its possible harmful effect on larynx. We found saliva bile acids in 32.6% patients previously submitted to gastric surgery with a significant (P < 0.05) correlation with total bilirubin values. These data agree with the incidence of DGER (5%–35%) after gastric surgery reported in the literature.20 Nevertheless, basing on low concentrations of bile acids investigated in the oral cavity of these patients, the circadian rhythm of their secretion and that we made a single sample in a day, our results might underestimate the total bile reflux. These reasons could explain why we didn't find any bile acids in saliva of 2 gastrectomized patients with laryngeal precancerous.
The likely mechanisms explaining the presence of bile acids or bilirubin in saliva may include: 1) endogenous production in the oral mucosa, 2) duodenogastroesophageal reflux, and 3) transudate from serum. Because in all controls they were not detectable in saliva, we can rule out the hypothesis of an endogenous production and of a transudation from serum. Furthermore, we found a greater incidence of hypotonic cardias in patients with positive bile acids test (Fig. 5). These results suggest that DGER may be the actual mechanism of the bile salts and bilirubin presence in the saliva. To confirm this hypothesis, we decided to test pepsinogen in the saliva as a marker of gastric juice reflux. Pepsinogen I and II are precursor molecules for pepsin. Pepsinogen I is synthesized and secreted into the gastric lumen, by chief (pepsin) cells and mucous neck cells of the gastric corpus, whereas pepsinogen II is secreted into the gastric lumen by the pyloric glands of the gastric antrum also by the chief and neck cells of the gastric corpus; thus we tested the second one.15 We found pepsinogen II exclusively in patients who were positive to bile acids and/or total bilirubin, with a significant correlation (P < 0.05). Only 1 patient positive to bile acids/bilirubin tests was negative to pepsinogen II; indeed, he was the only patient submitted to total gastrectomy and obviously he couldn't produce pepsinogen.
In several studies,12,13 it has been hypothesized that biliary reflux after gastric resection may enhance the development of laryngeal malignancies. Recently, we showed that bile reflux after gastrectomy may represent, as already demonstrated for acid reflux,21–23 a further risk factor or cofactor for the onset of laryngeal carcinogenesis with a 10-fold increased risk 20 years after gastric resection.14 Finally, some authors tried to confirm experimentally the damaging potential on the mucosa of the upper aerodigestive tract of noxious refluxate originating from duodenal secretion.24,25
Even though many reasons suggested a possible harmful action of intestinal contents (bile acids, trypsin, etc.) after gastric resection on the multistratified epithelium of the upper aerodigestive tract, and despite experimental models having been elaborated, few clinical data show direct effects of biliary reflux on the larynx. In our study, we found a significantly higher RSI and RFS score in gastrectomized patients positive to bile acids than in negative ones, confirming our previous clinical report. Furthermore, we found not only a significant higher incidence (P < 0.05) of precancerous or frankly neoplastic laryngeal lesions in cases than in controls, but also a greater incidence of laryngeal leukoplakia or cancer, in gastrectomized patients with a positive bile acid test, than in patients with a negative test. We revealed, moreover, that the mean interval time between gastric resection and diagnosis of laryngeal leukoplakia or cancer was 18.3 and 34.2 years, respectively. Logistic regression model reveals that chronic bile reflux exposure almost doubled (1.92) the risk of pre or frankly neoplastic laryngeal lesion (95% CI, 1.17–40.2). These data suggest that bile acids or some other duodenal contents may play a role in the precancerous-carcinoma sequence in the larynx. Therefore, we confirmed objectively our previous data that indirectly showed a bile reflux involvement in laryngeal carcinogenesis.14
Noxious effects of specific bile acids, their form (conjugated or deconjugated), or concentrations required for damage in the upper aerodigestive tract are only now beginning to be understood. There are marked differences in the bile acid behavior depending on the pH of the solutions in which they reside.26 In this regard, the literature confirms an important mucosal injury by conjugated bile salts at acid pH (1.2–1.5), whereas unconjugated salts significantly increase mucosal permeability and injury at pH 7 or greater.27 In our patients, we found a mean value of saliva pH of 5.9 ± 0.7 (range, 5.0–7.7). For this reason, although we measured total bile acids and not their different forms, we may suppose that in our gastrectomized patients, the prevalent and harmful role on laryngeal tissue has been played by unconjugated bile acids in respect of conjugated one.
Considering that literature data28 have shown a high concentration of N-nitrous compounds conjugated with bile acids in remnant stomach after partial gastric surgery (particularly after BII resection), we suggested that these toxic substances could represent one of the multifactorial mechanisms of laryngeal tumorigenesis.
CONCLUSION
In this study, we found that levels of bile acids and total bilirubin are detectable specifically only in saliva of patients submitted to gastric surgery with secondary reflux. In this manner, not only did we confirm directly the exposure of the upper aerodigestive tract to the duodenal contents, but we also revealed that bile acids and total bilirubin positive patients have a significantly higher incidence of laryngeal findings and symptoms and a higher risk of preneoplastic lesions or frankly malignancies, if compared with negative patients. Therefore, we suggest that bile acids and total bilirubin assays in saliva may be considered simple, specifics, noninvasive, and not expensive markers of proximal reflux of duodenal contents. Finally, taking into account our previous reports and these new observations, biliary laryngopharyngeal reflux after gastric resection could be included among risk factors of inflammatory, precancerous, and neoplastic laryngeal lesions. Clinical studies correlating chronic bile exposure and laryngeal damage are required, and more sensitive diagnostic techniques able to reveal even slight concentrations of duodenal contents in upper aerodigestive tract could be of great clinical value.
Footnotes
Reprints: Eugenio De Corso, MD, PhD, Università Cattolica del Sacro Cuore, Istituto di Otorinolaringoiatria, Policlinico “A. Gemelli,” Largo A. Gemelli n. 1, 00168, Roma, Italia. E-mail: eugenio.decorso@tin.it.
REFERENCES
- 1.Vaezi MF, Richter JE. Importance of duodenogastroesophageal reflux in the medical outpatient practice. Hepatogastroenterology. 1999;46:40–47. [PubMed] [Google Scholar]
- 2.Vaezi MF, Richter JE. Duodenogastroesophageal reflux and methods to monitor nonacidic reflux. Am J Med. 2001;3:111(suppl 8A):160–168. [DOI] [PubMed]
- 3.Sharma P, Sampliner R. GERD, DGER, or both in Barrett's esophagus? Am J Gastroenterol. 1997;92:903–904. [PubMed] [Google Scholar]
- 4.Vaezi MF, Richter JE. Synergism of acid and duodenogastroesophageal reflux in complicated Barrett's esophagus. Surgery. 1995;117:699–704. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell MT, Byrne PJ, Brazil N, et al. An ambulatory bile reflux monitoring system: an in vitro appraisal. Physiol Meas. 1994;15:57–65. [DOI] [PubMed] [Google Scholar]
- 6.Stipa F, Stein HJ, Feussner H, et al. Assessment of non-acidic esophageal reflux aspiration test and fiberoptic bilirubin monitoring. Dis Oesophagus. 1997;10:24–28. [DOI] [PubMed] [Google Scholar]
- 7.Iftikhar SY, Ledingham S, Evans DF, et al. Alkaline gastro-oesophageal reflux: dual probe pH monitoring. Gut. 1995;37:465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iftikhar SY, Ledingham S, Steele RJ, et al. Bile reflux in columnar-lined Barrett's oesophagus. Ann R Coll Surg Engl. 1993;75:411–416. [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki CT, Marotta J, Hundal J, et al. Bile-induced laryngitis: is there a basis in evidence? Ann Otol Rhinol Laryngol. 2005;114:192–197. [DOI] [PubMed] [Google Scholar]
- 10.Cianci R, Fedeli G, Cammarota G, et al. Is the alkaline reflux a risk factor for laryngeal lesions? Am J Gastroenterol. 2000;95:2398. [DOI] [PubMed] [Google Scholar]
- 11.Galli J, Cammarota G, Calo’ L, et al. The role of acid and alkaline reflux in laryngeal squamous cell carcinoma. Laryngoscope. 2002;112:1861–1865. [DOI] [PubMed] [Google Scholar]
- 12.Cianci R, Galli J, Agostino S, et al. Gastric surgery as a long-term risk factor for malignant lesions of the larynx. Arch Surg. 2003;138:751–754. [DOI] [PubMed] [Google Scholar]
- 13.Galli J, Calo’ L, Cadoni G, et al. Bile reflux as possible risk factor in laryngo-pharyngeal inflammatory and neoplastic lesions. Acta Otorhinolaryngol Ital. 2003;23:377–382. [PubMed] [Google Scholar]
- 14.Cammarota G, Galli J, Cianci R, et al. Association of laryngeal cancer with previous gastric resection. Ann Surg. 2004;240:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potluri S, Friedenberg F, Parkman HP, et al. Comparison of a salivary/sputum pepsin assay with 24-hour esophageal pH monitoring for detection of gastric reflux into the proximal esophagus, oropharynx, and lung. Dig Dis Sci. 2003;48:1813–1817. [DOI] [PubMed] [Google Scholar]
- 16.Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice. 2002;16:274–277. [DOI] [PubMed] [Google Scholar]
- 17.Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS). Laryngoscope. 2001;111:1313–1317. [DOI] [PubMed] [Google Scholar]
- 18.Lamey PJ, Nolan A. The recovery of human saliva using the Salivette system. Eur J Clin Chem Clin Biochem. 1994;32:727–728. [PubMed] [Google Scholar]
- 19.Sears RJ, Champion GL, Richter JE. Characteristics of distal partial gastrectomy patients with esophageal symptoms of duodenogastric reflux. Am J Gastroenterol. 1995;90:211–215. [PubMed] [Google Scholar]
- 20.Zhang C, Liu ZK, Yu PW. Effects of bile reflux and intragastric microflora changes on lesions of remnant gastric mucosa after gastric operation. World J Gastroenterol. 2004;15:1537–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen MYM, Ott DJ, Casolo BJ, et al. Correlation of laryngeal and pharyngeal carcinomas and 24-hour pH monitoring of the esophagus and pharynx. Otolaryngol Head Neck Surg. 1998;119:462. [PubMed] [Google Scholar]
- 22.Copper MP, Smit CF, Stanojcic LD, et al. High incidence of laryngopharyngeal reflux in patients with head and neck cancer. Laryngoscope. 2000;110:1007–1011. [DOI] [PubMed] [Google Scholar]
- 23.Freije JE, Beatty TW, Campbell BH, et al. Carcinoma of the larynx in patient with gastroesophageal reflux. Am J Otolaryngol. 1996;17:386–390. [DOI] [PubMed] [Google Scholar]
- 24.Sung MW, Roh JL, Park BJ, et al. Bile acid induces cyclo-oxygenase-2 expression in cultured human pharyngeal cells: a possible mechanism of carcinogenesis in the upper aerodigestive tract by laryngopharyngeal reflux. Laryngoscope. 2003;113:1059–1063. [DOI] [PubMed] [Google Scholar]
- 25.Adhami T, Gholdblum JL. The role of gastric and duodenal agents in laryngeal injury: am experimental canine model. Am J Gastroenterol. 2004;99:2098–2106. [DOI] [PubMed] [Google Scholar]
- 26.Kauer WK, Peters JH, DeMeester TR, et al. Mixed reflux of gastric and duodenal juices is more harmful to the oesophagus than gastric juice alone: the need for surgical therapy re-emphasized. Ann Surg. 1995;222:525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harmon JW, Jhonson LF, Mayodonovitch CL. Effects of acid and bile salts on the rabbit oesophageal mucosa. Dig Dis Sci. 1981;19:875–881. [DOI] [PubMed] [Google Scholar]
- 28.Galli J, Cammarota G, De Corso E, et al. Biliary laryngopharyngeal reflux: a new pathological entity. Curr Opin Otolaryngol Head Neck Surg. 2006;14:128–132. [DOI] [PubMed] [Google Scholar]


