Abstract
Objective:
A prospective randomized controlled trial (RCT) of multimodal perioperative management protocol in patients undergoing elective colorectal resection for cancer.
Aims:
This study evaluates the use of a multimodal package in colorectal cancer surgery in the context of an RCT.
Methods:
Patients for elective resection for colorectal cancer were offered trial entry. Participants were stratified by sex and requirement for a total mesorectal excision and centrally randomized. Multimodal patients received intravenous fluid restriction, unrestricted oral intake with prokinetic agents, early ambulation, and fixed regimen epidural analgesia. Control patients received intravenous fluids to prevent oliguria, restricted oral intake until return of bowel motility, and weaning regimen epidural analgesia. Adherence to both regimens was reinforced using a daily checklist and protocol guidance sheets. Discharge decision was made using preagreed criteria. The primary endpoint was postoperative stay, and achievement of independence milestones. Secondary endpoints were postoperative complications, readmission rates, and mortality. Analysis was by intention to treat.
Results:
Seventy patients were recruited. Approximately one fourth underwent TME. Median ages were similar (69.3 vs. 73.0 years). The median stay was significantly reduced in the multimodal group (5 vs. 7 days; P < 0.001, Mann-Whitney U test). Patients in the control arm were 2.5 times as likely to require a postoperative stay of more than 5 days. Patients in the multimodal group had less cardiorespiratory and anastomotic complications but more readmissions. There were 2 deaths, both controls.
Conclusions:
This RCT provides level 1b evidence that a multimodal management protocol can significantly reduce postoperative stay following colorectal cancer surgery. Morbidity and mortality are not increased.
The use of a multimodal recovery protocol significantly improved outcomes in patients undergoing elective colorectal cancer resection. This randomized controlled trial demonstrates a reduction in hospital stay, earlier recovery of body functions, and faster return of independent status. Postoperative complications are not increased.
Multimodal or “fast track” programs have been widely reported to accelerate patient recovery and shorten hospital stays. In cohort and matched control studies, Kehlet and others have shown that median postoperative stays of 2 to 3 days following colonic surgery may be consistently achieved without an appreciable increase in complications.1–4 This is done using a combination of preoperative patient information, thoracic epidurals, avoidance of fluid overload, prokinetics, early diet and ambulation, and preemptive analgesia. MacFie’s group in the United Kingdom have taken the investigation further with a recent randomized controlled trial (RCT) involving patients with both colonic as well as rectal surgery.5 This study, however, is weakened by the omission of bowel preparation in the intervention group and the stipulated use of a vertical incision in the control group. Use of mechanical bowel preparation may worsen outcomes in the control group,6 and patients who had vertical incisions have more postoperative pain and slower recovery of respiratory function.7 Both factors are therefore potential confounders and are standardized in this study. Fazio’s group in the United States reported mean length of postoperative stays of 4.7 days, with a protocol that omits thoracic epidurals in a cohort comprising predominantly younger patients with inflammatory bowel disease.8 An additional RCT by the same group, however, has not shown a reduction in the initial hospital stay.9
In the present study, the authors have conducted a protocol-driven RCT comparing multimodal therapy against standard practice. This study is focused on patients typically seen in mainstream colorectal cancer practice. Efforts are made to minimize potential confounders.
METHODS
Recruitment
All elective patients presenting with colorectal cancer between May 2003 and October 2004 were eligible for inclusion into the trial. No upper age limits were set, and both colonic and rectal surgery were included. Patients were excluded if they were unable to mobilize independently over 100 m at preoperative assessment, had contraindications to thoracic epidurals, or had preexisting clinical depression. Patients were also excluded if they were having palliation only, or undergoing a joint operation involving another surgical specialty.
Statistical Considerations and Randomization
The study was powered (α = 0.05, β = 0.80) to detect a 30% difference in proportion of patients regaining preoperative independence (ie, 50% in multimodal arm vs. 20% in control arm by postoperative day 3); 45 patients per arm were required.
All eligible patients were given written information detailing both arms of the study. Patients who consented to participate were stratified by sex, and whether a total mesorectal excision (TME) was planned. Randomization was done centrally by the Research and Development Unit (RDU), using a random number generator. Details required for stratification were communicated to the RDU and an individualized allocation provided over the telephone. The RDU were not otherwise involved in the running of the study. The results are analyzed on an intention-to-treat basis.
Bowel Preparation and Intravenous Fluid Restriction
All patients were admitted the morning prior to surgery for standard bowel preparation with sodium dihydrogen phosphate dihydrate (Fleet Phospho-soda EC DeWitt & Co.), given on admission and 12 hours later. Both groups were allowed oral fluids up to 3 hours before surgery. In addition, control arm patients received 125 mL/h of intravenous fluid starting from 2200 hours on the night of admission. Multimodal arm patients received no supplementary intravenous fluids until 3 hours before surgery but were encouraged to make up the loss through oral rehydration.
In the control arm, the anesthetist was free to follow the normal intraoperative fluid practice. In the multimodal arm, intraoperative fluids were restricted to 1500 mL unless bleeding in excess of 500 mL occurred.
Postoperatively, patients in the control arm were allowed 30 mL/h of oral fluids. This was increased stepwise (30 mL/h to 60 mL/h to free oral fluids) every 12 hours unless there was nausea. Sufficient supplementary intravenous fluids were given to maintain a urine output of at least 0.5 mL/kg per hour and a systolic blood pressure of >90 mm Hg. Patients in the multimodal arm were allowed free oral fluids immediately after the operation. Intravenous fluids were discontinued when the patient was able to tolerate 200 mL of water over 30 minutes. Urine output was not used to guide intravenous fluid use in the multimodal arm, although persistent anuria triggered an assessment by a senior clinician.
Diet
Nasogastric tubes were inserted in all patients. In patients in the multimodal arm, nasogastric tubes were removed in the recovery room. In control patients, nasogastric tubes were removed the following morning unless there was >200 mL of free drainage overnight.
In the control arm, diet was commenced only on signs of returning bowel motility (audible bowel sounds, flatulence, and/or defecation). In the multimodal arm, diet was allowed immediately after the operation. Patients in the multimodal arm received regular domperidone, magnesium hydroxide 8%, and liquid protein/calorie supplements from admission.
Thoracic Epidurals and Pain Relief
A thoracic epidural was attempted in the anesthetic room in all patients, and a bupivacaine 0.167% and diamorphine infusion used. Patient controlled analgesia with morphine and cyclizine was used if a thoracic epidural was not possible. In the control arm, the epidural infusion rate was titrated against pain and narcotization, and removed when the rate was ≤1 mL/h. In the multimodal arm, the infusion rate was not adjusted unless there were features of narcotization, and epidurals were discontinued 48 hours postoperatively. Oral paracetamol (1 g every 6 hours) and ibuprofen (400 mg every 6 hours) were given from the immediate postoperative period in the multimodal arm but were given as required in the control arm.
Mobilization
In the control arm, patients were sat out and assisted to mobilize on the first postoperative day, but not normally aggressively mobilized until discontinuation of the thoracic epidural. Urinary catheters were removed following epidural catheter removal.
In the multimodal arm, mobilization was encouraged from the night of the operation. Patients were encouraged to meet predefined mobility targets over the postoperative days. Urinary catheters were removed 24 hours postoperatively following colonic resection and at 72 hours after TME.
Discharge Criteria
Patients were eligible for discharge when they were self-caring (able to dress, shower, and groom themselves), were tolerating oral fluid and diet, had stoma or bowel function, were mobilizing independently (ie, able to ambulate to toilet/bath and 100 m unassisted), and were comfortable on oral analgesia. Assessment of these parameters was made by ward nurses using preset definitions. Patients who met discharge criteria but were not confident of independent care were discharged to a nonmedically supervised community hospital for convalescence.
Patients were readmitted at the request of the primary care physician or if the patient made direct contact with the hospital describing deteriorating health at home.
Protocol Integrity
Both arms were protocol driven, with checklists for patients, nursing staff, and surgical staff to help compliance. Teaching sessions and dummy runs were held before trial commencement to clarify potential points of confusion and reduce protocol violations.
Patients were admitted to one of two nursing areas depending on the results of randomization. Although the interventions are protocol driven, a geographically separate location was considered desirable to minimize protocol contamination.
Follow-up
Arrangements were made to review all patients at 10 to 14 days postoperatively.
Ethics Approval
Ethics committee approval was gained from the West Somerset Ethics Committee (LREC 1.95).
RESULTS
In the study period, 160 patients presented for possible colorectal excision for cancer; 53 were excluded (27 had metastatic disease, 13 were unfit for surgery or opted not to have surgery, 1 had clinical depression, 4 underwent joint surgery with another surgical specialty, and in 8, informed consent was not possible). Of the remaining 107 patients, 26 patients declined to take part. Eighty-one patients were therefore recruited and randomized; 11 were subsequently withdrawn. Seven patients were withdrawn because metastatic disease was discovered post randomization (6 preoperatively, 1 intraoperatively). One patient deteriorated rapidly and was unfit for surgery on admission. Three patients withdrew consent prior to admission and did not undergo either of the protocols. One patient withdrew consent during her admission, but consent was gained for continued collection of data and the results are included under intention-to-treat principles. Of the 70 patients, 35 were in the control arm and 35 were in the multimodal arm (Fig. 1).
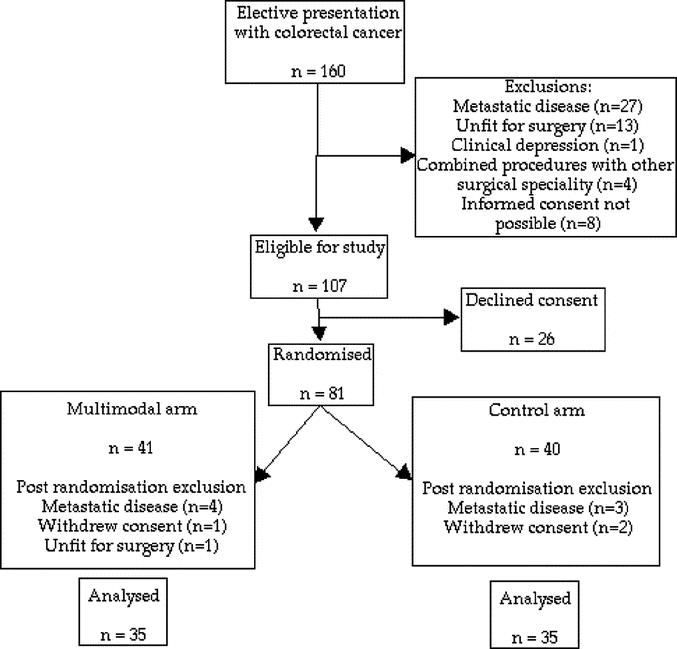
FIGURE 1. CONSORT diagram of recruitment.
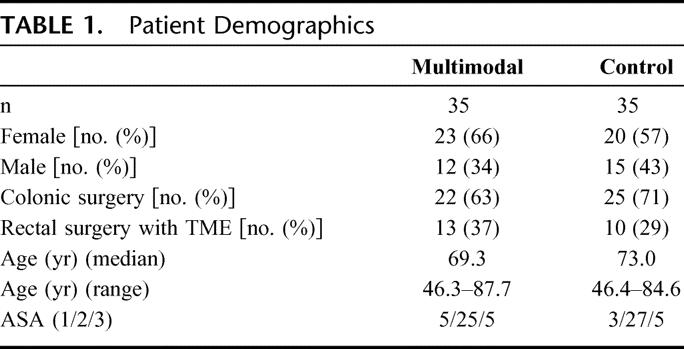
As a result of stratification, the study arms were similar in terms of sex distribution and requirement for a TME. There were slightly more men in the control arm and slightly more rectal excisions in the multimodal arm. Age distributions and American Society of Anesthesiologists grades were similar (Table 1).
TABLE 1. Patient Demographics
Thoracic epidurals were successfully inserted in 34 of 35 control patients and 32 of 35 multimodal patients. Three epidurals in the control group and one in the multimodal group were ineffective and removed in the early postoperative stage. Overall, 31 of 35 (86%) control patients and 31 of 35 (86%) multimodal patients received effective epidurals. Median times of epidural use were 2 days in the multimodal arm and 3 days in the control arm.
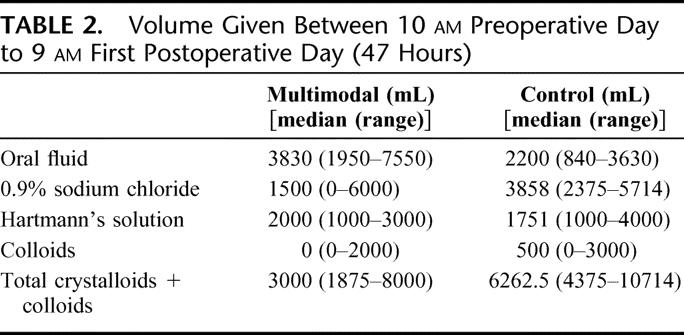
The use of intravenous fluid was successfully restricted by the protocol. Over the first 47 hours of admission, the multimodal group received less than half the fluid given to the control group. This deficit was partly balanced by an increased oral fluid intake (Table 2). Blood transfusions were not different between groups.
TABLE 2. Volume Given Between 10 am Preoperative Day to 9 am First Postoperative Day (47 Hours)
Although the choice of incision was left to the surgeon, there were no marked differences in the incision used. Seventeen of 35 (49%) patients in each arm had a midline incision, and the remainder had a transverse incision.
Multimodal patients had shorter median times to tolerance of solid diet, faster independent mobility, earlier passage of stool per rectum or per stoma, and were self-caring sooner (Table 3).
TABLE 3. Achievement of Discharge Criteria
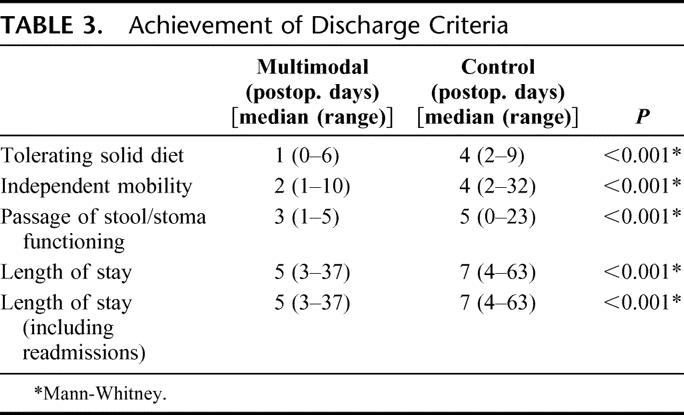
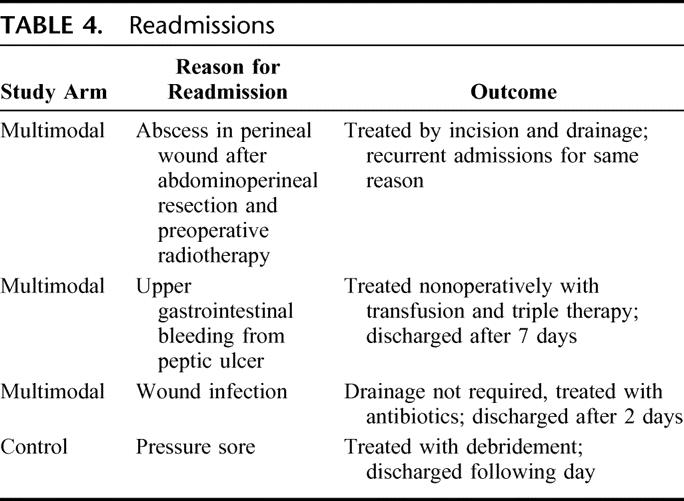
Median postoperative stay of patients in the multimodal arm is 5 days as compared with 7 in controls. This difference is significant (P < 0.001, Mann-Whitney U test). If the additional inpatient days from readmissions are factored into the analysis, the results are still statistically significant (Table 3). Most patients were discharged home, with only 2 patients in the multimodal arm requiring community hospital convalescence against 7 in the control arm. There were 3 of 35 (9%) readmissions in the multimodal arm and 1 of 35 (3%) in the control arm (Table 4).
TABLE 4. Readmissions
In multimodal patients, median stay following TMEs was 5.5 days (range, 4–37 days), while that following colonic surgery was 4 days (range, 3–13 days). In control patients, median stay following TMEs was 8.5 days (range, 4–63 days), while that following colonic surgery was 7 days (range, 5–35 days).
Two deaths occurred, both in controls. The first patient was an 83-year-old woman who underwent a sigmoid colectomy and a right hemicolectomy. She deteriorated suddenly on the sixth postoperative day after a slow recovery and suffered a fatal pulmonary embolus. The other patient was a 73-year-old woman who underwent a right hemicolectomy. Three days postoperatively, she suffered a myocardial infarction and, despite resuscitation and intensive care support, died 2 days later.
Four anastomotic leaks occurred: 3 (8.6%) in control patients and 1 (2.9%) in multimodal patients. Despite early feeding, nausea and vomiting were similar in multimodal patients, with 4 control patients requiring reinsertion of a nasogastric tube, as opposed to 3 multimodal patients.
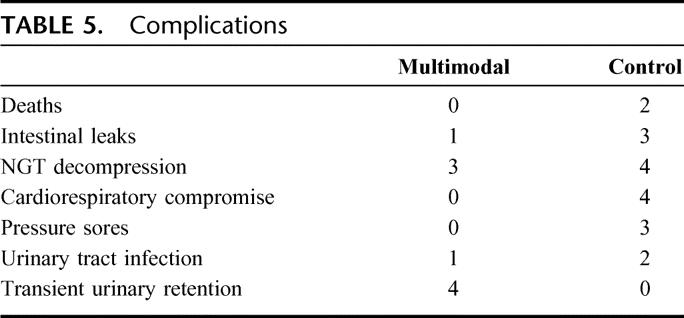
There were 4 cases of cardiorespiratory insufficiencies, with 2 cases requiring brief intensive care admissions. All of these were in control patients. Four patients in the multimodal arm required temporary recatheterization following urinary retention. The rates of urinary tract infection were not dissimilar. Pressure sores were also seen in control patients, with one requiring readmission for debridement (Table 5).
TABLE 5. Complications
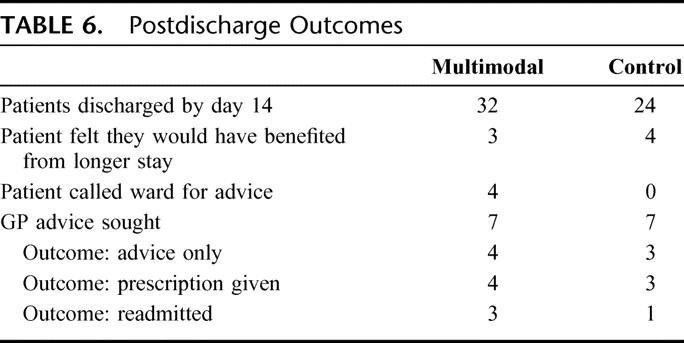
Twenty-four patients in the control arm and 32 patients in the multimodal arm were home by day 14. Of these patients, 7 patients felt they would have benefited from additional days of inpatient stay. None of these patients required readmission. There was no increase in the primary care physician workload between the two arms. Seven patients in each arm contacted their primary care physician, but most required either advice and/or a prescription only (Table 6).
TABLE 6. Postdischarge Outcomes
DISCUSSION
This RCT demonstrates improved recovery without increased morbidity using a protocol similar to Kehlet’s multimodal recovery program. Unlike previous studies, this study’s design standardizes preoperative information, bowel preparation, type of surgical incision, and the use of thoracic epidurals in both trial arms, thereby minimizing confounders.
A recent Cochrane review of clinical trials comparing no mechanical bowel preparation versus mechanical bowel preparation suggests poorer outcomes in patients receiving bowel preparation.6 Newer multimodal protocols should avoid the use of bowel preparation, at least for nonrectal surgery. In this study, the influence of bowel preparation on study findings has been reduced by its implementation in all patients.
Transverse incisions, which affect fewer dermatomes, have been reported to cause less postoperative pain and allow faster recovery of respiratory function.7 Although vertical incisions remain the most popular incision in open surgery, transverse incisions were used in a large proportion of patients in this study. Vertical incisions were used when previous vertical scars were present or where the surgeon felt that greater access was required.
In this study, the principal differences between the two arms concern early diet and ambulation, intravenous fluid restriction, the pattern of thoracic epidural use, and the early removal of urinary catheters.
Early enteral feeding is predicated on radiologic and electrophysiologic studies that indicate a return of small bowel function in 4 to 8 hours postincision, right colon function by 24 hours, and left colon function by 72 hours.10 Feasibility, safety, and effectiveness of early (within 24 hours of operation) feeding have been found in several RCTs.9–12 Patients given early enteral feeding had earlier resolution of ileus, shorter hospital stays, and fewer complications. In other studies, discharge criteria have not included defecation or stoma function; such a policy might reduce hospital stay further.5,13
Avoidance of intravenous fluid overloading is an important element in many protocols. Current practices in fluid regimens have been based on 40-year-old concepts proposed by Shires postulating a decrease in functional extracellular fluid volume postoperatively, requiring fluid replacement. Despite further research, this decrease has not been convincingly demonstrated.14,15 It is common practice for large volumes of fluid to be given to patient perioperatively, well in excess of actual losses. Most of this fluid is given in the immediate perioperative period, and a mean of 3 to 4.5 L of intravenous fluid has been reported.16
In healthy volunteers, fluid infusion causes a significant and persistent deterioration of pulmonary function,17 and a fluid retention of up to 48 hours.18–20 These results have been replicated in clinical studies of surgical patients. Standard fluid regimens with 0.9% saline as compared with restricted fluid regimens delayed return of gastric motility following elective colonic surgery and caused persistent water retention.21 Brandstrup et al, in a recent large RCT of patients undergoing elective colorectal resections, reported significantly increased complications when using a liberal perioperative fluid regimen compared with a restricted volume regimen designed to maintain the patient’s body weight constant.22 The reduced incidence of cardiopulmonary and anastomotic leaks reported by Brandstrup et al22 parallel those observed in this study. However, no meaningful statistical analysis can be performed on the data from the present study as it was not powered at its outset to detect such differences. Although the liberal fluid regimen of Brandstrup et al22 has been criticized for delivering too much fluid, the results of their study and those of the present study suggest that the pattern of perioperative intravenous fluid administration has a major influence upon cardiorespiratory and anastomotic complications.
Recently, RCTs of perioperative fluid optimization using the esophageal Doppler to direct intraoperative colloid infusion suggest that further improvements in outcome can be achieved.23 However, mean length of stay are longer in these studies than in multimodal series. The benefits of Doppler optimization in those managed in a multimodal protocol have not been studied. In the present study, the type of intravenous fluid was not controlled in either arm. The potential metabolic and hemodynamic adverse effects of 0.9% saline is increasingly being recognized,24,25 and the use of more physiologically friendly fluids needs further investigation.
The use of thoracic epidurals in abdominal surgery is widespread in the United Kingdom.26 This has been on the basis on multiple papers and reviews indicating superior relief of postoperative pain, with a favorable adverse effects profile.27–31 Research continues to find the optimum infusion (constituents, concentration, and total volume), and the optimum timing and duration of infusion. Thoracic epidurals are used in colonic surgery primarily for postoperative pain relief but may also be used to reduce the amount of general anesthetic used (allowing faster recovery) and to allow earlier return of function (ambulation, enteral alimentation, deep breathing). In this study, both arms had thoracic epidural analgesia, but the multimodal arm had a fixed infusion rate (to reduce the risk of breakthrough pain on early mobilizing) and a planned removal time of 48 hours (to allow unencumbered mobilization).
This study did not include laparoscopic surgery as at the time no data were available to establish the superiority of laparoscopic over open surgery in patients managed using a multimodal perioperative management protocol.32 While potential benefits may be established by later studies, it is essential that such studies include robust control of perioperative and postoperative management to ensure that altered surgical and patient expectations do not influence the management.
There appears to be synergism between modalities. Individually, the intervention modalities appear to improve outcome, but the degree of improvement is not usually marked. In combination, however, the improvements to the rate of recovery appear to be strong and very robust. In addition, no one intervention appears to be critical to the process. The occasional protocol violations, which are difficult to guard against in a complex multipronged trial of this nature, do not appear to be detrimental to the outcome.
CONCLUSION
This study provides level 1b evidence that a multimodal recovery program can significantly shorten hospital stay in patients undergoing elective colorectal resection. There appears to be faster return of function, without a concomitant increase in complications.
Footnotes
Reprints: Ian A. Eyre-Brook, MD, FRCS, Department of Surgery, Musgrove Park Hospital, Taunton and Somerset NHS Trust, Taunton, United Kingdom. E-mail: ck.tenosce@gmail.com.
REFERENCES
- 1.Basse L, Hjort Jakobsen D, Billesbolle P, et al. A clinical pathway to accelerate recovery after colonic resection. Ann Surg. 2000;232:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basse L, Thorbol JE, Lossl K, et al. Colonic surgery with accelerated rehabilitation or conventional care. Dis Colon Rectum. 2004;47:271–277; discussion 277–278. [DOI] [PubMed]
- 3.Hjort Jakobsen D, Sonne E, Basse L, et al. Convalescence after colonic resection with fast-track versus conventional care. Scand J Surg. 2004;93:24–28. [DOI] [PubMed] [Google Scholar]
- 4.Kehlet H, Mogensen T. Hospital stay of 2 days after open sigmoidectomy with a multimodal rehabilitation programme. Br J Surg. 1999;86:227–230. [DOI] [PubMed] [Google Scholar]
- 5.Gatt M, Anderson AD, Reddy BS, et al. Randomized clinical trial of multimodal optimization of surgical care in patients undergoing major colonic resection. Br J Surg. 2005;92:1354–1362. [DOI] [PubMed] [Google Scholar]
- 6.Guenaga KF, Matos D, Castro AA, et al. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev. 2005;1:CD001544. [DOI] [PubMed] [Google Scholar]
- 7.Lindgren PG, Nordgren SR, Oresland T, et al. Midline or transverse abdominal incision for right-sided colon cancer: a randomized trial. Colorectal Dis. 2001;3:46–50. [DOI] [PubMed] [Google Scholar]
- 8.Delaney CP, Fazio VW, Senagore AJ, et al. ‘Fast track’ postoperative management protocol for patients with high co-morbidity undergoing complex abdominal and pelvic colorectal surgery. Br J Surg. 2001;88:1533–1538. [DOI] [PubMed] [Google Scholar]
- 9.Delaney CP, Zutshi M, Senagore AJ, et al. Prospective, randomized, controlled trial between a pathway of controlled rehabilitation with early ambulation and diet and traditional postoperative care after laparotomy and intestinal resection. Dis Colon Rectum. 2003;46:851–859. [DOI] [PubMed] [Google Scholar]
- 10.Stewart BT, Woods RJ, Collopy BT, et al. Early feeding after elective open colorectal resections: a prospective randomized trial. Aust NZ J Surg. 1998;68:125–128. [DOI] [PubMed] [Google Scholar]
- 11.Ortiz H, Armendariz P, Yarnoz C. Is early postoperative feeding feasible in elective colon and rectal surgery? Int J Colorectal Dis. 1996;11:119–121. [DOI] [PubMed] [Google Scholar]
- 12.Reissman P, Teoh TA, Cohen SM, et al. Is early oral feeding safe after elective colorectal surgery? A prospective randomized trial. Ann Surg. 1995;222:73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson AD, McNaught CE, MacFie J, et al. Randomized clinical trial of multimodal optimization and standard perioperative surgical care. Br J Surg. 2003;90:1497–1504. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen OM, Engell HC. Changes in extracellular sodium content after elective abdominal vascular surgery. Acta Chir Scand. 1986;152:587–591. [PubMed] [Google Scholar]
- 15.Virtue RW, LeVine DS, Aikawa JK. Fluid shifts during the surgical period: RISA and S35 determinations following glucose, saline or lactate infusion. Ann Surg. 1966;163:523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tambyraja AL, Sengupta F, MacGregor AB, et al. Patterns and clinical outcomes associated with routine intravenous sodium and fluid administration after colorectal resection. World J Surg. 2004;28:1046–1051; discussion 1051–1052. [DOI] [PubMed]
- 17.Holte K, Jensen P, Kehlet H. Physiologic effects of intravenous fluid administration in healthy volunteers. Anesth Analg. 2003;96:1504–1509. [DOI] [PubMed] [Google Scholar]
- 18.Holte K, Sharrock NE, Kehlet H. Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth. 2002;89:622–632. [DOI] [PubMed] [Google Scholar]
- 19.Drummer C, Heer M, Baisch F, et al. Diuresis and natriuresis following isotonic saline infusion in healthy young volunteers before, during, and after HDT. Acta Physiol Scand Suppl. 1992;604:101–111. [PubMed] [Google Scholar]
- 20.Drummer C, Gerzer R, Heer M, et al. Effects of an acute saline infusion on fluid and electrolyte metabolism in humans. Am J Physiol. 1992;262:F744–F754. [DOI] [PubMed] [Google Scholar]
- 21.Lobo DN, Bostock KA, Neal KR, et al. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet. 2002;359:1812–1818. [DOI] [PubMed] [Google Scholar]
- 22.Brandstrup B, Tonnesen H, Beier-Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakeling HG, McFall MR, Jenkins CS, et al. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth. 2005;95:634–642. [DOI] [PubMed] [Google Scholar]
- 24.Lobo DN, Stanga Z, Simpson JA, et al. Dilution and redistribution effects of rapid 2-litre infusions of 0. 9% (w/v) saline and 5% (w/v) dextrose on haematological parameters and serum biochemistry in normal subjects: a double-blind crossover study. Clin Sci (Lond). 2001;101:173–179. [PubMed] [Google Scholar]
- 25.Reid F, Lobo DN, Williams RN, et al. (Ab)normal saline and physiological Hartmann’s solution: a randomized double-blind crossover study. Clin Sci (Lond). 2003;104:17–24. [DOI] [PubMed] [Google Scholar]
- 26.O’Higgins F, Tuckey JP. Thoracic epidural anaesthesia and analgesia: United Kingdom practice. Acta Anaesthesiol Scand. 2000;44:1087–1092. [DOI] [PubMed] [Google Scholar]
- 27.Fotiadis RJ, Badvie S, Weston MD, et al. Epidural analgesia in gastrointestinal surgery. Br J Surg. 2004;91:828–841. [DOI] [PubMed] [Google Scholar]
- 28.Flisberg P, Rudin A, Linner R, et al. Pain relief and safety after major surgery: a prospective study of epidural and intravenous analgesia in 2696 patients. Acta Anaesthesiol Scand. 2003;47:457–465. [DOI] [PubMed] [Google Scholar]
- 29.Liu SS, Carpenter RL, Mackey DC, et al. Effects of perioperative analgesic technique on rate of recovery after colon surgery. Anesthesiology. 1995;83:757–765. [DOI] [PubMed] [Google Scholar]
- 30.Carli F, Mayo N, Klubien K, et al. Epidural analgesia enhances functional exercise capacity and health-related quality of life after colonic surgery: results of a randomized trial. Anesthesiology. 2002;97:540–549. [DOI] [PubMed] [Google Scholar]
- 31.Block BM, Liu SS, Rowlingson AJ, et al. Efficacy of postoperative epidural analgesia: a meta-analysis. JAMA. 2003;290:2455–2463. [DOI] [PubMed] [Google Scholar]
- 32.Basse L, Jakobsen DH, Bardram L, et al. Functional recovery after open versus laparoscopic colonic resection: a randomized, blinded study. Ann Surg. 2005;241:416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]


