Abstract
Objective:
We report patient outcomes from esophageal resection with respect to morbidity and cancer survival comparing open thoracotomy and laparotomy (Open), with a thoracoscopic/laparotomy approach (Thoracoscopic-Assisted) and a total thoracoscopic/laparoscopic approach (Total MIE).
Methods:
From a prospective database of all patients managed with cancer of the esophagus or esophagogastric junction, patients who had a resection using one of three techniques were analyzed to assess postoperative variables, adequacy of cancer clearance, and survival.
Results:
The number of patients for each procedure was as follows: Open, 114; Thoracoscopic-Assisted, 309; and Total MIE, 23. The groups were comparable with respect to preoperative variables. The differences in the postoperative variables were: less median blood loss in the Thoracoscopic-Assisted (400 mL) and Total MIE (300 mL) groups versus Open (600 mL); longer time for Total MIE (330 minutes) versus Thoracoscopic-Assisted (285 minutes) and Open (300 minutes); longer median time in hospital for Open (14 days) versus Thoracoscopic-Assisted (13 days), Total MIE (11 days) and less stricture formation in the Open (6.1%) versus Thoracoscopic-Assisted (21.6%), Total MIE (36%). There were no differences in lymph node retrieval for each of the approaches. Open had more stage III patients (65.8%) versus Thoracoscopic-Assisted (34.4%), Total MIE (52.1%). There was no difference in survival when the groups were compared stage for stage for overall median or 3-year survival.
Conclusion:
Minimally invasive techniques to resect the esophagus in patients with cancer were confirmed to be safe and comparable to an open approach with respect to postoperative recovery and cancer survival.
We compared the outcomes from esophageal resection for cancer where the approaches were: an open thoracotomy and laparotomy or with minimally invasive techniques used in the thorax and abdomen. There was no major difference in morbidity, mortality, and clearance of the tumor. Cancer survival was not different for each of the approaches.
Esophageal resection for cancer remains the gold standard, not only in providing the optimal chance for cure but also the best palliation for dysphagia. Because of the substantial morbidity from the open surgical approach to the chest, there have been attempts to use approaches that avoid a thoracotomy. However, to date there has been no clear evidence that the avoidance of thoracotomy using a transhiatal approach to resect the esophagus improves outcomes either in relation to morbidity1,2 or survival with the disease.3 The latter randomized trial showed a trend to a benefit for the transthoracic approach thought to be due to the ability to perform a more complete lymph node dissection.3 The extent to which the lymph nodes should be dissected remains contentious.4 Promoters of the open approaches to esophageal resection strongly support a radical approach to a mediastinal lymphadenectomy, whereas the advocates of the transhiatal approach hold the view that a more extensive lymphadenectomy does not influence survival.5
With improved experience and skills for performing laparoscopic and thoracoscopic surgery, there have been a number of reports where these approaches have been used in association with the thoracic dissection of the esophagus6–8 or gastric mobilization,9,10 or for both.11,12 These reports have confirmed that these approaches are possible, safe and have reasonable outcomes when compared with the literature. Conceptually, a minimally invasive approach to esophageal resection (MIE) does appear to offer the potential for a more radical approach to mediastinal resection, under vision, when compared with transhiatal esophagectomy. Recent reviews of the role of MIE have maintained that the benefits from this approach are controversial because the operations are more complex than those required for other malignancies. There are concerns relating to the adequacy of tumor and lymph node clearance, and most series reported to date have not shown an apparent reduction in morbidity or mortality.4 Wu and Posner identified issues such as the optimal approach, cost effectiveness, advantages over open techniques and the role of MIE in combined modality therapy and call for more comparative studies to determine the worth of MIE.13 There has been very little written about the oncological impact and the impact on prognosis from the resected cancer using MIE.
Our unit has been performing thoracoscopic mobilization for esophageal cancer since 1993. The results from our first 162 cases have been reported previously.13 We concluded that the procedure was safe with acceptable outcomes. We subsequently embarked upon a pilot study of a consecutive series of patients having Total MIE using thoracoscopic esophageal mobilization and laparoscopic gastric mobilization and a small right upper quadrant incision to create the gastric tube. We felt that there was little benefit over the thoracoscopic and laparotomy approach, so this operation was discontinued. We have recently reported this series with short- and medium-term follow-up data questioning whether there is a significant benefit from Total MIE.14 In both of these series of patients, the tumors were in the intrathoracic esophagus or localized in the esophagogastric junction (EGJ) allowing resection and a gastric pull-up to the neck. Prior to using MIE techniques, we had used an open approach via laparotomy and thoracotomy to resect these cancers. Concurrent with those series of patients in which MIE techniques were used, we have used the open approach for cancers located at the EGJ (Siewert Type II and III) as well as the lower esophagus, where a substantive resection of the upper stomach was required, to allow appropriate tumor clearance and necessitating an intrathoracic anastomosis.
In this report, we wish to compare the outcomes from two approaches to MIE with open esophageal resection in a contemporary series of patients from a single unit. Aside from the amount of esophagus and stomach resected, the dissection within the abdomen and the chest was similar allowing assessment of the potential benefits or otherwise for the MIE approach over open surgery. We report the peri-operative outcomes as well as longer-term outcomes in relation to the cancers that were treated.
METHODS
A prospective computer database (Microsoft Access, Washington, DC, 2001) of all patients presenting with cancer of the esophagus or esophagogastric junction has been maintained since 1987. Patient details are compiled at the time of presentation, following investigation, following treatment and after follow-up visits. From January 1993, when we began MIE techniques, to December 2004, there have been 1048 patients recorded. Up until 1993, esophageal resections were performed using either an open transthoracic resection (Open) or a transhiatal blunt dissection with the abdominal component being performed via a laparotomy. We commenced performing the esophageal mobilization thoracoscopically for cancers confined to the esophagus (not into the gastric cardia) in 1993 and have continued to use this approach. The Open thoracic approach has been confined to patients with cancers that cross into the gastric cardia where a more aggressive gastric resection is required. For the period between December 1998 and October 2000, a consecutive series of the patients had a thoracoscopic esophageal mobilization and had a laparoscopic mobilization of the stomach, which was used for the reconstruction. After this period, this approach was not continued because the early results did not appear to be superior to the thoracoscopic/laparotomy group. We compare the short- and long-term outcomes of those patients who had an open transthoracic resection (Open) and a thoracoscopic and laparotomy approach (Thoracoscopic-Assisted) from 1993 to December 2004 as well as those patients who had a thoracoscopic and laparoscopic approach (Total MIE) from December 1998 to October 2000.
Patients routinely had an epidural cannula for postoperative analgesia unless there were technical problems with respect to needle insertion. The surgical approaches using MIE have been previously described.13,14 In brief, for the thoracoscopic mobilization, the patient is placed in the prone position following insertion of a double lumen tube to allow one-lung anesthesia. The surgeon inserts a cannula into the right chest and the right lung is deflated. Following division of the azygous vein, the esophagus was mobilized, including the periesophageal tissue in the lower mediastinum. The subcarinal package of nodal tissue was removed unless the patients had high-grade dysplasia/in situ disease or they were elderly and/or had significant comorbidities. For patients with squamous cell carcinoma of the lower or mid esophagus, the lower mediastinal nodes and the subcarinal nodes were removed as described. The superior mediastinum and neck were not dissected in any patient.
In the Total MIE group, the stomach was also mobilized completely on the right gastric and right gastroepiploic arcades using ultrasonic shears (Johnson & Johnson, Endosurgery). The nodal tissue at the base of the left gastric pedicle was dissected with the stomach and the left gastric pedicle divided flush with the celiac axis using a vascular stapler (Johnson & Johnson, Endosurgery). A small incision (approximately 5 cm) was made in the right upper quadrant to deliver the stomach and the esophagus (which had been divided in the neck). Division of the lesser curve of the stomach creates the gastric tube. The tube is replaced into the abdomen and delivered to the neck. As with the thoracoscopic group, the anastomosis is performed between the esophageal remnant with interrupted sutures.
In the patients who had a laparotomy (Open, Thoracoscopic-Assisted), the stomach was mobilized on the right gastric and right gastroepiploic arcades. The nodal tissue around the left gastric pedicle was dissected and the pedicle ligated flush with the celiac axis. In patients having an Open thoracic approach, the lesser curve was divided in the abdomen to ensure an adequate resection of the tumor and the nodal tissue. The group that had a thoracoscopic approach had the stomach and esophagus (divided in the neck) delivered into the wound and the lesser curve was divided and a gastric tube fashioned. The tube was then taken to the neck via the posterior mediastinum and an anastomosis was performed in the neck. All patents had a pyloromyotomy or pyloroplasty and a feeding jejunostomy.
The patients who had an Open thoracic approach had a posterolateral thoracotomy via the 5th or 6th intercostal space. The azygos vein was routinely divided and the lower mediastinum dissected along with the subcarinal nodal tissue as previously described. The stomach was pulled into the chest and an anastomosis performed between esophagus and stomach, end to side with either interrupted sutures or a circular stapling device above the level of the azygous vein. From 1998, following the resection, the operating surgeon routinely dissected the specimen placing the nodes into three groups: subcarinal (when dissected), mediastinal, and left gastric nodes/pedicle. The abdominal lymph node stations dissected and collected in the latter group typically involved the paracardial nodes (right and left), left gastric nodes, lesser curve nodes, suprapancreatic nodes, and celiac nodes. The groups were analyzed separately histologically.
Data collected and entered into the database included patient demographics, comorbidity; tumor site, and morphology. Operative information included blood loss and duration of surgery. The complications were documented fully, including all unexpected events whether major or minor. A significant respiratory infection was defined as clinical suspicion of a respiratory infection, usually associated with a fever, with or without radiologic or microbiologic confirmation, for which active intervention was initiated ranging from the use of intravenous antibiotics, to the return to ICU with or without ventilation.
With respect to pathology, patients with invasive cancer were analyzed to assess the size of tumor, number of nodes dissected in each group, margins of resection, and the postoperative stage of the tumors. Patients were staged using the AJCC staging system.15
The differences between the groups were assessed using Statistical Package for Social Science (SPSS, Chicago, IL). Continuous data were compared by the Mann-Whitney U test and the ordinal data by χ2 test. Survival was analyzed by the Kaplan-Meier method. Survival comparison was made through the log-rank test. P < 0.05 was considered significant. The study complied with the Australian National Health and Medical Research Council's guidelines on research involving human subjects.
RESULTS
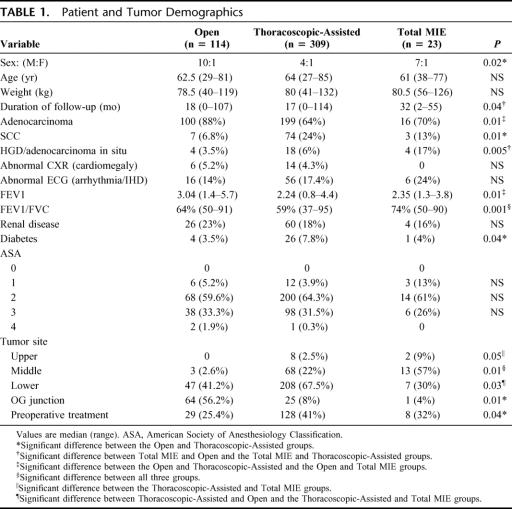
Of the 483 patients where operation was commenced, resection did not proceed in 25 patients because of advanced disease, a further 10 patients (3%) were converted from Thoracoscopic-Assisted to a transhiatal esophagectomy and 2 patients (8%) were converted from Total MIE to a Thoracoscopic-Assisted; 446 patients remained for this comparative study after excluding these patients. This comprised of 114 patients who had an Open, 309 Thoracoscopic-Assisted, and 23 Total MIE. Table 1 shows the patient and tumor demographics and the associated preoperative comorbidities in these patients. There was a male predominance because of the high incidence of adenocarcinoma. There was no difference in age and weight between the groups. Patients who had an Open were more likely to have their tumor at the EGJ and the lower esophagus; thus, there were more patients with adenocarcinoma. The Thoracoscopic-Assisted group had more patients with compromised respiratory function and diabetes when compared with the other groups. There were more patients who had had chemo-radiotherapy or chemotherapy as part of protocols for phase II and III trials in the Thoracoscopic-Assisted group.
TABLE 1. Patient and Tumor Demographics
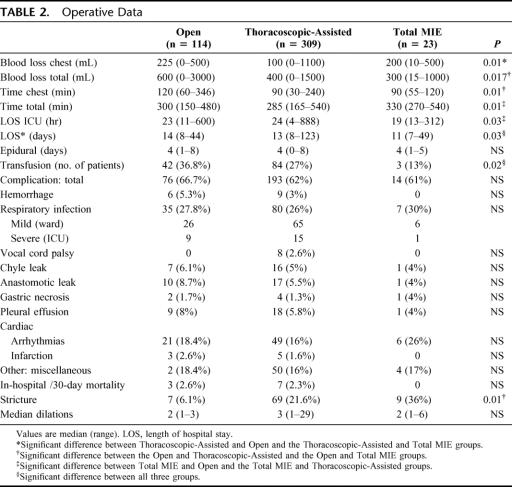
Operative data and postoperative morbidity are shown in Table 2. The total time for the procedures was not significantly different for Open and Thoracoscopic-Assisted groups; however, operation in the Total MIE group took significantly longer than with the other two approaches. The thoracic component of the Open took longer than both the MIE groups. The Thoracoscopic-Assisted group lost less blood and had fewer requirements for transfusion during the admission than the Open group. The Total MIE group spent less time in the intensive care unit compared with the other two approaches The MIE groups spent less median time in hospital when compared with Open: 3 days for Total MIE and 1 day for Thoracoscopic-Assisted.
TABLE 2. Operative Data
The overall complication rate was not different for each group. This number includes a large number of minor complications, which did not have a substantive impact on overall recovery. The incidence of respiratory infections was not different for all groups nor was the need to return to the intensive care unit with respiratory compromise. Because of the cervical anastomosis, the two MIE approaches added the risk of vocal cord palsy, which occurred in 2.6% of the Thoracoscopic-Assisted group, half recovering within 6 weeks.
The in-hospital operative mortality was 2.6% for the Open approach, 2.2% for the Thoracoscopic-Assisted group, and there were no deaths in the Total MIE group. We have had no operative mortality for any approach in the last 5 years. In the Thoracoscopic-Assisted group, the cause of death was respiratory related in 5 patients, myocardial infarction in 1 case, and a postoperative bleed with subsequent death in 1 patient. In the Open group, the cause of death was respiratory complications in 1 patient, gastric conduit necrosis with subsequent multisystem failure in 1 patient, and a single patient had adult respiratory distress syndrome following significant blood loss intraoperatively. This patient had a Barrett's cancer, above a recurrent hiatus hernia, adherent to the descending aorta.
There was a higher stricture rate for the patients who had a cervical anastomosis following both types of MIE. In those patients who were alive at 12 months, there was a significantly higher incidence of patients complaining of regurgitation and required acid suppressing medication in those who had a Thoracoscopic-Assisted compared with an Open. The numbers in the Total MIE were too small for appropriate analysis.
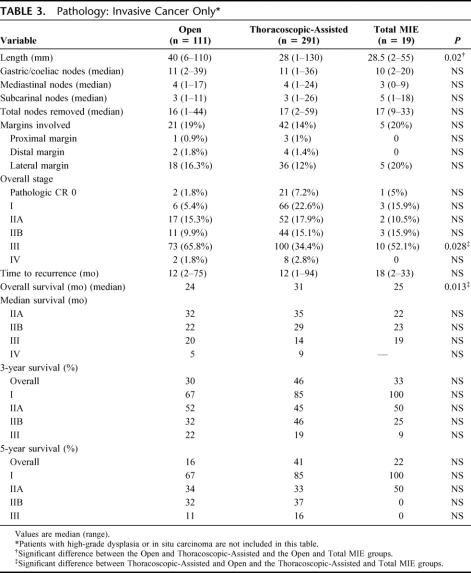
With respect to the pathology of the resected specimens, Table 3 shows the results for 421 patients who had invasive cancer. The Open group had longer tumors and, along with Total MIE group, had more patients who were stage III with lymph node metastasis. The Thoracoscopic-Assisted group had more patients with stage 0, who had had a pathologic complete response, after preoperative chemo-radiation. There was no significant difference in margin involvement between the three approaches. With respect to lateral margin involvement, when the comparison is made between Open and Thoracoscopic-Assisted for patients who had surgery alone (no preoperative therapy), the Open incidence was 15% versus 8% in the Thoracoscopic-Assisted group.
TABLE 3. Pathology: Invasive Cancer Only
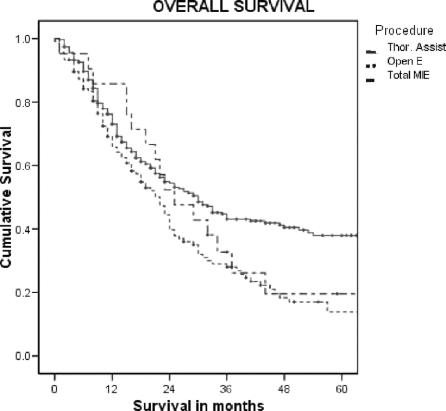
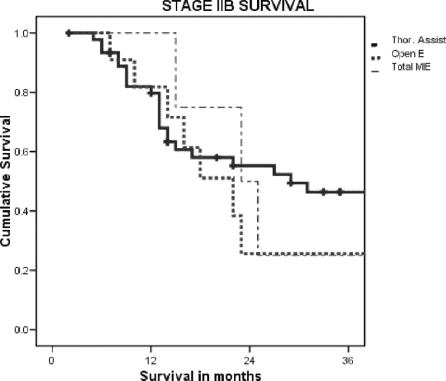
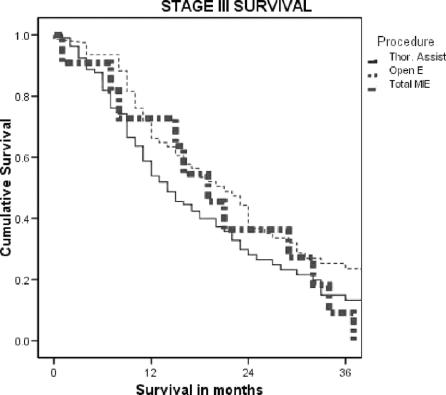
The nodal harvest for each of the defined regions dissected was not different when the approaches were compared. There was no difference in the time to recurrence between the three groups for patients with invasive cancer (in situ disease was excluded). The overall survival was worse in the Open and Total MIE groups. However, when examined stage for stage, there was no difference in survival between the groups where there were enough events to gain a median survival. The results for the overall survival, stages IIB and III, for each of the groups are shown in Figure 1.
FIGURE 1. Overall survival for the three approaches.
DISCUSSION
The difference in the demographics between the three approaches to esophageal resection, assessed by the authors, related primarily to the selection of patients to an Open esophagectomy if it was apparent, preoperatively, that a more aggressive gastric resection may be required because of the site of the cancer at the EGJ with suspected or definite invasion into the gastric cardia. The presence of a higher incidence of mid-esophageal lesions in the Total MIE group relates to the small number of patients. This was a consecutive series of patients that would have otherwise had a thoracoscopic/laparotomy approach. When comparing the outcomes from the three approaches to esophageal resection for cancer, two using MIE techniques and one using a totally Open technique, we have shown equivalence in most short-term postoperative outcomes with some advantages for avoiding the Open thoracotomy, such as less blood loss and earlier discharge. However the magnitude of these improvements was not large. The three approaches had a similar morbidity profile with no outstanding benefits shown in any of the areas where problems occurred. We did not show any detrimental effects with respect to the clearance of the tumor either locally or with respect to the lymph node clearance; and when compared stage for stage, there was no difference in the survival of the patients with invasive cancer. All procedures were undertaken in a single unit with a high volume of patients with esophageal cancer, with 3 surgeons and trainees performing the surgery. If we were to consider MIE experimental and the Open approach the traditional gold standard, the outcomes from these approaches in our unit bear comparison with other centers to assess whether the comparison and conclusions we make are valid.
The reported morbidity in this series was high at 61% to 65%. We have been inclusive of all events that would be considered outside normal postoperative recovery. Many of these events did not cause the patient a major problem. Others have reported similar figures where they appear to have been inclusive of all problems.16 The operative mortality for this group of patients is comparable to that seen from contemporary studies from major centers undertaking esophageal resection.17–21
It was hoped that we would see a reduction in respiratory complications because of less restriction to movement and breathing when the thoracotomy or laparotomy or both were avoided. In assessing the literature, it is surprising to find a number of reports where the respiratory outcomes are an integral part of the report, but the authors have not provided adequate definitions to compliment the figures provided. In comparisons of the transthoracic approach and transhiatal esophagectomy, the incidence of respiratory complications has been reported to be between 18% to 26%,1,2 being similar for both approaches. A meta-analysis of the randomized trials comparing these approaches22 and a recent randomized trial3 suggested there was a higher pulmonary complication rate for the Open approach. One large study of a three-field dissection for esophageal cancer with a 14% recurrent nerve palsy rate reports a postoperative pneumonia rate of 5%23 without defining what this means. However, contemporary studies with definitions, similar to ours, show similar outcomes from pneumonia in patients having an Open esophagectomy.16,23–25 Others report fewer respiratory complications,19,21 one because of a more aggressive approach to their patients with the use of temporary minitracheostomy.21 Although it was not clear what was defined as a significant respiratory problem, the largest study of Total MIE, with 222 patients, reports a 7.7% incidence of pneumonia,10 much less than what we saw. The Total MIE approach we used still had a small right upper quadrant incision. Whether this had an influence on the respiratory function cannot be assessed. Our Thoracoscopic-Assisted group had worse respiratory function preoperatively than the Open esophagectomy group. This suggests there was some selection bias toward the minimally invasive approach for the more compromised patients. However, in a comparative study such as this, it is difficult to draw any conclusions from this observation. Thus, overall our respiratory outcomes for all approaches appear acceptable, but there was no improvement using MIE over an Open esophagectomy.
The technical problems such as anastomotic leak, chyle leak, and postoperative stricture formation are no worse for each approach and are similar to those reported in contemporary series of Open esophagectomy.16,18,19 Clearly, there were more strictures in the patients with the cervical anastomosis, and we found these patients to be more problematic with some patients requiring many episodes of dilatation before they stabilize. These patients are managed by two of the authors (D.C.G. and B.M.S.) who have a policy of regular, progressive endoscopic dilatation. We have shown a larger rate of recurrent nerve palsy for the Thoracoscopic-Assisted approach compared with Open esophagectomy. We have not had any cases over the last 6 years since we stopped using instrument retraction in the neck and maintained the dissection at the tracheoesophageal groove closely on the esophagus.
The lymph node harvest in our series was from a limited dissection of the mediastinum and upper abdomen. Whether this should have been more aggressive remains a source of continued discussion and debate. The median number of nodes we report for all approaches is similar to some reports for Open resection20 but less than reported for other series of patients who had a formal two-field lymph node3 or three-field dissection.24,25 The MIE dissection we have done does provide the ability to increase the nodal dissection, under vision, more than could be done using the transhiatal approach. A randomized controlled trial of Open versus transhiatal esophagectomy showed a trend to improved survival for the Open approach attributed to the nodal dissection.3 In that trial, the transhiatal group had a mean of 16 nodes, whereas the Open group had a mean of 31 nodes. We did not attempt to dissect the nodes in the superior mediastinum. MIE techniques for this approach have been reported from Japan26,27 with nodal counts similar to that achieved at Open surgery.27 Groups who do dissect this area and the neck have much higher rates of vocal cord palsy.
Our patients that had an Open esophagectomy had more cancers at the EGJ and had less use of neoadjuvant chemoradiotherapy and more patients with stage III disease; consequently, the patients had a poorer overall survival. However, where there were enough events to allow a comparison, when compared stage for stage, there was no difference in survival between the different approaches. The numbers in the Total MIE group are not large enough to allow reasonable comparison with the literature. The Open esophagectomy and Thoracoscopic-Assisted approaches in our series, when compared with contemporary series, have resulted, stage for stage, in comparable 3-year23 and 5-year17,18 survivals. Groups performing a more extensive dissection at Open surgery have reported better overall survival and outcomes stage for stage at 3 years.20,25 However, this latter difference may relate to stage migration as 30% of patients in one study moved to stage IV25 because of the more extensive dissection.
In a systematic review of randomized controlled trials of video-assisted thoracic surgery, there was evidence for a benefit when the operation was performed for pneumothorax and minor resections, with patients having less postoperative pain and a shorter length of hospital stay. However, there was no advantage for patients having a lobectomy29 where a mediastinal dissection would have been necessary. Other attempts at avoiding a thoracotomy such as transhiatal esophagectomy have not shown outstanding differences in outcomes, although reviews have suggested a reduction to a minor degree in some postoperative variables.2 We think that the mediastinal dissection is the source of the high morbidity, and the approach to this is not a major factor. As stated in a recent review, “the surgical trauma of the mediastinal dissection is independent of the incision size.”30 The patient takes time to recover from this dissection, taking away some of the possible advantages that may occur by not having a large incision on the abdomen or the chest or both.
CONCLUSION
By comparison with the Open approach to esophageal resection, we have confirmed our previous reports, which stated that MIE for esophageal cancer was a safe option in our hands.6,14 Importantly, we have now shown that the pathologic and oncological outcomes using MIE are not compromised when compared with the Open approach. We found the total endoscopic esophagectomy to be more technically demanding with no real advantage to the patient over the Thoracoscopic-Assisted approach aside from discharge 2 days earlier. Thus, we have not continued to use this technique since that period of assessment. The Thoracoscopic-Assisted approach had the advantage of less blood loss with a more efficient approach into the thoracic cavity by avoiding a thoracotomy, although there was a higher anastomotic stricture rate. We continue to use this approach for appropriate esophageal cancers and the Open approach when a more significant gastric resection is required for cancer clearance. We have not provided any evidence that there should be widespread adoption of MIE techniques for esophageal resection. MIE will not allow patients, who are compromised by comorbidities, the possibility of a surgical resection where they may otherwise have had to seek alternative management. By reducing the operative trauma with MIE, we do not replace those important elements of good patient selection, appropriate staging, and medical assessment and management in centers that have an ongoing experience of a significant volume of esophageal surgery.
ACKNOWLEDGMENTS
The authors acknowledge the assistance and support from the Mater Private Hospital, Brisbane Australia.
FIGURE 2. Stage IIB survival for the three approaches.
FIGURE 3. Stage III survival for the three approaches.
Footnotes
Reprints: Bernard Smithers, MD, Princess Alexandra Hospital, Ipswich Road, Woolloongabba, Queensland, 4102, Australia. E-mail: M.Smithers@mailbox.uq.edu.au.
REFERENCES
- 1.Rindani R, Martin C, Cox M. Transhiatal versus Ivor-Lewis oesophagectomy: is there a difference? Aust NZ J Surg. 1999;69:187–194. [DOI] [PubMed] [Google Scholar]
- 2.Rente J, Bull D, Harpole D, et al. Transthoracic versus transhiatal esophagectomy: a prospective study of 945 patients. J Thorac Cardiovasc Surg. 2003;125:1114–1120. [DOI] [PubMed] [Google Scholar]
- 3.Hulscher J, Van Sandick J, De Boer A, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–1669. [DOI] [PubMed] [Google Scholar]
- 4.Stein H, Siewert J. Improved prognosis of resected esophageal cancer. World J Surg. 2004;28:520–525. [DOI] [PubMed] [Google Scholar]
- 5.Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg. 1999;230:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smithers BM, Gotley DC, McEwan D, et al. Thoracoscopic mobilization of the esophagus: a 6-year experience. Surg Endosc. 2001;15:176–182. [DOI] [PubMed] [Google Scholar]
- 7.Law S, Fok M, Chu K, et al. Thoracoscopic esophagectomy for esophageal cancer. Surgery. 1997;122:8–14. [DOI] [PubMed] [Google Scholar]
- 8.Akaishi T, Kaneda I, Higuchi N. Thoracoscopic en bloc total esophagectomy with radical mediastinal lymphadenectomy. J Thorac Cardiovasc Surg. 1996;112:1534–1541. [DOI] [PubMed] [Google Scholar]
- 9.Jagot P, Sauvanet A, Berthoux L, et al. Laparoscopic mobilization of the stomach for oesophageal replacement. Br J Surg. 1996;83:540–542. [DOI] [PubMed] [Google Scholar]
- 10.Bonavina L, Bona D, Binyom P, et al. Laparoscopy assisted surgical approach to esophageal carcinoma. J Surg Res. 2004;117:52–57. [DOI] [PubMed] [Google Scholar]
- 11.Luketich J, Alvelo-Rivera M, Buenaventura P, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg. 2003;238:486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen N, Roberts P, Follette D, et al. Thoracoscopic and laparoscopic esophagectomy for benign and malignant disease: lesson learned from 46 consecutive procedures. J Am Coll Surg. 2003;197:902–913. [DOI] [PubMed] [Google Scholar]
- 13.Wu P, Posner M. The role of surgery in the management of oesophageal cancer. Lancet Oncol. 2003;4:481–488. [DOI] [PubMed] [Google Scholar]
- 14.Leibman Smithers BM, Gotley D, et al. Minimally invasive esophagectomy: short and long term outcomes. Surg Endosc. 2006;20:428–433. [DOI] [PubMed] [Google Scholar]
- 15.American Joint Committee on Cancer. TNM Classification of Malignant Tumors, 6th ed. New York: Springer-Verlag, 2002. [Google Scholar]
- 16.Atkins B, Shah A, Hutcheson K, et al. Reducing hospital morbidity and mortality following esophagectomy. Ann Thorac Surg. 2004;78:1170–1176. [DOI] [PubMed] [Google Scholar]
- 17.Visbal A, Allen M, Miller D, et al. Ivor Lewis esophagogastrectomy for esophageal cancer. Ann Thorac Surg. 2001;71:1803–1808. [DOI] [PubMed] [Google Scholar]
- 18.Karl R, Schreiber R, Boulware D, et al. Factors affecting morbidity, mortality and survival in patients undergoing Ivor Lewis esophagogastrectomy. Ann Surg. 2000;231:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin S, Shaw I, Dresner S. Early complications after Ivor Lewis subtotal esophagectomy with two-field lymphadenectomy: risk factors and management. J Am Coll Surg. 2002;194:285–297. [DOI] [PubMed] [Google Scholar]
- 20.Mariette C, Taillier G, Van Seuningen I, et al. Factors affecting postoperative course and survival after en bloc resection for esophageal carcinoma. Ann Thorac Surg. 2004;78:1177–1183. [DOI] [PubMed] [Google Scholar]
- 21.Law S, Wong KH, Kwog KF, et al. Predictive factors for postoperative pulmonary complications and mortality after esophagectomy for cancer. Ann Surg. 2004;240:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hulscher J, Tijssen J, Obertop H, et al. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg. 2001;72:306–313. [DOI] [PubMed] [Google Scholar]
- 23.Swanson S, Batirel H, Bueno R, et al. Transthoracic esophagectomy with radical mediastinal and abdominal lymph node dissection and cervical esophagogastrostomy for esophageal cancer. Ann Thorac Surg. 2001;72:1918–1925. [DOI] [PubMed] [Google Scholar]
- 24.Tachibana M, Kinugasa S, Yoshimura H, et al. Clinical outcomes of extended esophagectomy with three-field lymph node dissection for esophageal squamous cell carcinoma. Am J Surg. 2005;189:98–109. [DOI] [PubMed] [Google Scholar]
- 25.Altorki N, Kent M, Ferrara C, et al. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg. 2002;236:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawahara K, Maekawa T, Okabayashi T, et al. Video-assisted thoracoscopic esophagectomy for esophageal cancer. Surg Endosc. 1999;13:218–223. [DOI] [PubMed] [Google Scholar]
- 27.Osugi H, Takemura M, Higashino M, et al. A comparison of video-assisted thoracoscopic oesophagectomy with radical lymph node dissection for squamous cell cancer of the oesophagus with open operation. Br J Surg. 2003;90:108–113. [DOI] [PubMed] [Google Scholar]
- 28.Deleted in proof.
- 29.Sedrakyan A, van der Meulen J, Lewsey J, et al. Video assisted thoracic surgery for the treatment of pneumothorax and lung resections: systemic review of randomised clinical trials. BMJ. 2004;229:1008–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Law S, Wong J. Current management of esophageal cancer. J Gastrointest Surg. 2005;9:291–310. [DOI] [PubMed] [Google Scholar]