Abstract
Background:
Sentinel lymph node biopsy can be associated with delays in operating room schedule and with significant pain during the preoperative 99mTc colloid injection. To avoid these problems, we developed a novel radiolabeled blue dye that can be injected intraoperatively.
Methods:
We performed a phase I/II trial (IND#70627) of sterile pyrogen-free 125I-methylene blue to identify sentinel nodes in patients with breast cancer. Twelve women were studied. Two women each were given peritumoral or circumareolar injections of 100, 200, 300, 400, 500, or 1000 μCi of 125I methylene blue.
Results:
Sentinel nodes were detected in 11 of 12 patients, with a low-dose 200 μCi patient being the single exception. The number of sentinel nodes detected per patient ranged from 0 to 3 (mean = 1.66 nodes/case). Radioactivity at the tumor injection site [counts per second (cps) averaged over 10 seconds] ranged from 3346 to 47,300 cps and was highly dose-dependent (r = 0.90, P = 0.0002). In contrast, the in vivo node counts ranged from 0 to 1228 cps, while ex vivo counts ranged from 0 to 1516 cps. The in vivo nodal counts were dose-dependent (r = 0.67, and P = 0.0231). Radiation was carefully monitored inside the operating room and in pathology. Even with the 1-mCi dose, the radioactive blue dye produced significantly lower personnel exposure than historically seen with 99mTc.
Conclusions:
This method eliminates the painful preoperative injections of 99mTc colloid, is performed by the surgeon in the operating room, is associated with lower radiation exposures for personnel, and avoids the delays caused by nonoperating room personnel. These observations warrant a more extensive trial of this method using the 1000-μCi dose of 125I methylene blue dye for sentinel lymph node biopsies.
Sentinel lymph node biopsy can be associated with delays in operating room schedule and with significant pain during the preoperative 99mTc colloid injection. 125I methylene blue, a novel radiolabeled blue dye that can be injected intraoperatively, was created to obviate these problems. Intraoperative injection of 125I methylene blue allowed for effective detection of the axillary sentinel node in 11 of 12 female patients with stage I/II breast cancer in a phase I/II study. These observations warrant a more extensive trial of this method using the 1000-μCi dose of 125I methylene blue dye for sentinel lymph node biopsies.
Sentinel lymph node biopsy was initially developed as a technique for the detection of regional lymph node metastasis in patients with melanoma by Morton et al.1 The evolution of the procedure as a means to detect the presence of axillary lymph node metastasis in patients with breast cancer developed shortly thereafter. In less than 10 years following the initial reports utilizing this technique, dozens of studies have appeared in the scientific literature validating use of sentinel node biopsy as an accurate means of detecting metastatic disease in the axillary lymph nodes in patients with breast cancer.2–5
Almost simultaneously, reports appeared in the literature documenting the success of sentinel node localization using either isosulfan blue dye alone or using isosulfan blue dye in conjunction with technetium99m labeled sulfur colloid (99mTcSC). While most authors use a combination of blue dye and 99mTcSC, Guiliano et al continue to report excellent results using isosulfan blue dye alone.6 Conversely, Krag et al report similar excellent results using only radiotracer.2,3 Currently, the majority of breast surgeons prefer to use both dye and radiotracer for their evaluation of sentinel nodes. In recent years, others have published studies suggesting that sentinel node accuracy and yield could be duplicated with the use of methylene blue dye as opposed to isosulfan blue dye.7–9 This change in dye preference has found its way into the practice of a significant population of breast surgeons. Injection of small quantities (0.1–0.5 mL) of methylene blue into the breast during needle/wire breast cancer mammographic localization procedures has been done for years and has been associated with no reported adverse effects.10 In a similar fashion, injection of methylene blue in the web space between the toes has been used widely as a method to detect lymphatic channels for their cannulation for lymphangiograms.
With the development of the sentinel node biopsy method of evaluating axillary lymph node status in women with breast cancer came some unanticipated consequences for both surgeon and patient alike. Patients must undergo a separate preoperative injection of radiocolloid prior to their surgery. The radiocolloid injection is commonly performed a minimum of 2 hours preoperatively, often the afternoon prior to surgery or more typically the morning of surgery. Patients uniformly note that the radiocolloid injection is very painful. The injection is painful whether it is injected in small quantities intradermally or in larger quantities around the periphery of a tumor.11,12 With the advent of sentinel lymph node biopsy as an alternative standard of care for breast conservation candidates, there has been an increase in patient and referring-physician demand for this less invasive procedure. Surgeons have often been forced to deal with major delays in their surgical schedules based on the requirement for an additional preoperative procedure that is performed at the discretion of nonoperating room personnel, specifically nuclear medicine physicians and technicians.13
A single, simultaneous injection of blue dye and radiocolloid in the operating room at the time of breast cancer surgery would obviate the need for an additional preoperative procedure for the patients and would prevent delays in the surgical schedule. Unfortunately, 99mTc sulfur colloid has high-energy gamma emissions and a significant amount of activity (1–10 mCi) is commonly injected to ensure adequate node uptake. Much of this activity must clear from the injection site before the hand-held gamma probe can effectively discriminate individual nodes in the axilla from the injection site
We have devised a simple, economically feasible way to produce a sterile, pyrogen-free 125I-labeled methylene blue dye. Preliminary animal experimentation showed rapid transit to regional nodes and limited systemic biodistribution.14 When absorbed systemically, the radiolabeled dye is rapidly cleared in the urine. We hypothesized that admixing small quantities of the 125I labeled methylene blue dye (0.1–0.5 mL) in a much larger (4.5–4.9 mL) quantity of unlabeled methylene blue dye should offer the surgeon all the specificity of a 2-stage sentinel lymph node biopsy procedure, enhanced intraoperative 2-point discrimination based on the low energy of 125Iodine's gamma emissions, and potentially offer increased safety based on 125Iodine's low-energy (35 Kev) gamma emissions. More importantly, the use of a 1-step intraoperative procedure would be painless and would obviate the need for a second procedure performed outside of the operating room by nonsurgical personnel.
METHODS AND MATERIALS
To test these hypotheses, we performed a prospective phase I/II trial of 125I methylene blue for the intraoperative detection of sentinel nodes in 12 women with invasive breast cancer.
Drug Information
Methylene blue (1% USP) used in the protocol was obtained commercially as a sterile pyrogen-free product (Faulding Pharmaceutical Co., Paramus, NJ). This drug is commonly used in clinical practice and has documented low risk and incidence of adverse side effects. Allergic or adverse reactions attributed to methylene blue are rare and usually occur when much higher doses of this drug are administered. This blue dye was labeled with 125Iodine (125I) using a proprietary method, purified, and its sterility and pyrogenicity determined prior to distribution by Iso-Tex, Friendswood, TX. 125Iodine was commercially obtained from Nordion, Kanata, Ontario, Canada or from McMasters University, Hamilton, Ontario, Canada. The identity of 125I was confirmed on a multichannel analyzer. Purity of the final product was confirmed using mass spectrometry of 127I-labeled (by identical parallel reactions) product and HPLC. Each batch was tested for radiostability. The final product has a shelf-life of 120 days.
The decay-corrected radioactive content of the final admixture of cold (methylene blue) and hot (125I methylene blue) products ranged from 100 μCi/5 mL to 1000 μCi/5 mL. The physical half-life of 125I is 60 days; however in preliminary animal experiments, the biologic half-life of the radiolabeled dye is about 6 hours.14 This correlated well with the known plasma half-life of nonlabeled methylene blue (5.4 hours).
Clinical Study Design
Patients
Twelve women with pathologically proven invasive breast cancer were enrolled into this trial between December 2004 and May 2005. Clearance to use the experimental drug 125I methylene blue in this phase I/II pilot trial was granted following submission of an Investigational New Drug (IND #70627) application through the FDA and approval by the IRB for the Louisiana State University Health Sciences Center New Orleans, LA. Participating surgeons in this trial were considered to be experts in sentinel lymph node biopsy techniques for breast cancer. In general, the surgical techniques used for sentinel node biopsy in this series were based on the National Surgical Adjuvant Breast and Bowel Program (NSABP) guidelines. The inclusion criteria for this study included female subjects with stage 0, I, or II invasive breast cancer with a clinical axillary node status of N0. The exclusion criteria for this study precluded participation of subjects known to be pregnant or nursing, incarcerated prisoners, subjects under the age of 18 years, and subjects with a known allergy to shellfish, iodine, or methylene blue dye.
Preoperative Preparation
To reduce the potential for 125I uptake in the thyroid, all patients were given 10 drops a day of a saturated iodine solution (Lugol's solution) orally for 2 days prior to surgery, the day of surgery, and for 3 days thereafter.
Intraoperative Technique
Following induction of general anesthesia, patients were injected with a combination of unlabeled methylene blue and 125I methylene blue in doses ranging from 100 μCi to 1000 μCi. Two patients each received doses of 100, 200, 300, 400, 500, and 1000 μCi of 125I methylene blue dye combined with unlabeled methylene blue dye in a total volume of 5 mL. Patients were injected in the peritumoral or subareolar location using 1.25-mL aliquots in the 3-, 6-, 9-, and 12-o'clock positions of breast tissue surrounding the tumor or areola. Decisions regarding peritumoral or subareolar injection were at the discretion of the operating surgeon. Counting of the radioactive emissions at the primary injection site [counts per second (cps) averaged over 10 seconds] was performed and recorded immediately following injection. Manual massage and compression of the injected breast was then performed for 10 minutes following injection. A hand-held gamma detector (Neoprobe, Model 1000, Dublin, OH) was used to scan the axilla once each minute beginning at 15 minutes postinjection. Transcutaneous “hot spots” in the axilla were defined as radioactive counts (cps) consistently higher than the adjacent background. Failure to elicit a significant “hot spot” in the axilla within 20 minutes prompted us to perform “flushing” of the primary injection site with 25 to 50 mL of sterile NaCl solution per the NSABP B-32 protocol training manual. The time interval necessary for discovery of the “hot spot” following primary injection was also recorded. An incision was made in the axilla overlying the “hot spot.” The hand-held gamma detector was placed within the wound to facilitate more precise detection of the sentinel lymph node. Lymph nodes that were significantly higher in radioactive content than adjacent axillary tissue were considered “hot” sentinel lymph nodes and excised. In vivo and ex vivo nodal counts, and axillary background counts were performed and recorded before and following removal of the sentinel node. Lymph nodes with in vivo counts greater than 10% of the in vivo counts obtained with the first node identified were also considered sentinel lymph nodes. Lymph nodes that were stained blue and/or having afferent lymphatic channels stained blue were also considered to be sentinel lymph nodes and excised. In addition, lymph nodes that contained both significant radioactive counts and were stained blue were categorized as “hot and blue” sentinel lymph nodes. All sentinel lymph nodes identified by the established criteria were labeled, submitted individually, and delivered to pathology where they were stained with hematoxylin and eosin and for the presence of cytokeratin (immunohistochemistry) following serial step sectioning. All information regarding separate sentinel lymph nodes was reported individually.
Postoperative Procedure
All patients received standard postoperative care and continued to take oral Lugol's solution on the day of surgery and for 3 days thereafter. Patients were scanned postoperatively at 7 and 14 days with a hand-held gamma detector for detection of residual radioactivity in the primary injection site, the axilla, and the thyroid gland.
Statistical Analysis
This project was a pilot phase I/II study to determine the feasibility of this technique and to determine the specific doses (ratio of hot to cold dye) and timing from injection to surgical removal of the nodes required for a larger scale trial. Thus, formal statistical analysis of this data was not done.
RESULTS
Between December 2004 and May 2005, 12 patients were enrolled in this study. The mean age (±SD) of these women was 51 ± 8 years. Four of the women were white, 7 were black, and 1 was Hispanic. All patients were originally diagnosed with invasive breast cancer. Eleven patients underwent needle core biopsy and 1 underwent open incisional biopsy to establish a tissue diagnosis. All patients were staged clinically as stage I or II prior to surgery. Two patients underwent neoadjuvant chemotherapy prior to definitive surgery. All of the patients initially underwent lumpectomy and sentinel lymph node biopsy. Three of the patients were found to have metastatic disease in the sentinel nodes and underwent subsequent completion axillary dissection. No other metastatic nodal disease was discovered in these patients. All 3 findings of axillary node metastatic malignancy returned as invasive ductal carcinoma of the breast, which was congruent with the primary breast malignancy in all cases (Fig. 1).
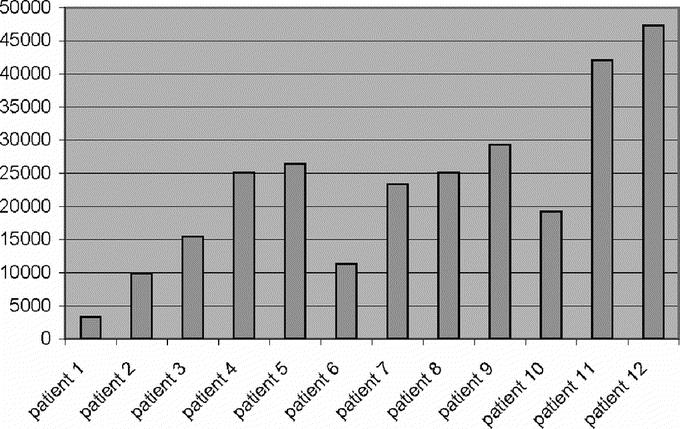
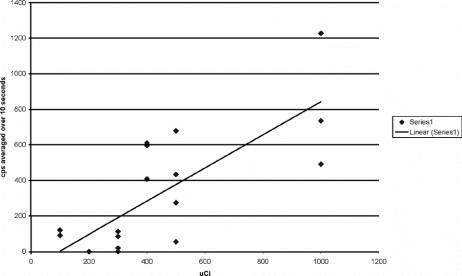
FIGURE 1. Mean cps (averaged over 10 seconds) at the primary tumor injection site.
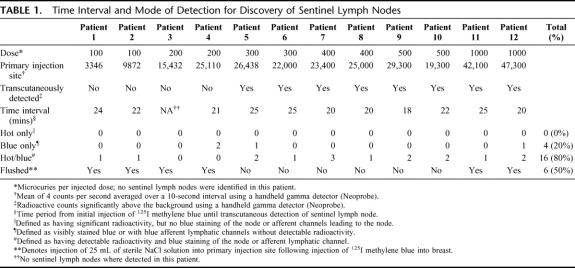
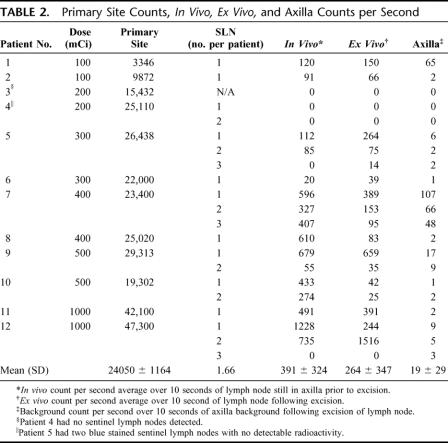
Four of the patients received peritumoral injections and 8 received subareolar injections. The mean radioactive count (cps ± SD) at the primary injection site was 24,050 ± 11,664 and ranged from 3346 to 47,300 (Table 1). The cps at the primary injection site correlated well with the dose injected (Fig. 2, R2 = P < 0.001). All patients were injected with the full 5 mL volume of 125I methylene blue solution. Six patients received a supplemental 25-mL sterile NaCl solution “flush” injection to facilitate lymphatic drainage according to NSABP guidelines. Sentinel nodes were detected in 11 of 12 patients. A low-dose 200-μCi patient was the single exception. The mean number of nodes per patient detected was 1.66. The mean time interval between injection and transcutaneous detection of the sentinel lymph node was 22 minutes. Hot spots in the axilla were transcutaneously identified in 11 of 12 patients. All hot spots involved nodes in the level I axillary node group. A sentinel node was found underneath the dermal hot spot in all instances. Of the 20 total sentinel lymph nodes discovered (Table 1), 16 nodes were classified as “hot and blue” and 4 nodes as “blue only.” No nodes met the criteria for “hot only.” The mean in vivo counts (±SD) for all “hot and blue” nodes (Table 2) were 391 ± 324 and ranged from 20 to 1228 cps; however, the mean in vivo (±SD) counts for patients receiving doses ranging from 400 μCi to 1000 μCi were 531 ± 289, with values ranging from 55 to 1228 cps. The mean ex vivo counts (±SD) for all “hot and blue” nodes were 264 ± 347 cps and ranged from 14 to 1516 cps; however, the ex vivo counts (±SD) for patients receiving doses ranging from 400 μCi to 1000 μCi (n = 6) were 310 ± 419 cps, with values ranging from 25 to 1516 cps. The mean axilla background counts were 19 ± 29 cps and ranged from 0 to 107 cps.
TABLE 1. Time Interval and Mode of Detection for Discovery of Sentinel Lymph Nodes
FIGURE 2. Mean in vivo nodal counts in cps (averaged over 10 seconds).
TABLE 2. Primary Site Counts, In Vivo, Ex Vivo, and Axilla Counts per Second
No adverse local, systemic, or anaphylactic reactions were noted during or following injection of the 125I methylene blue. No evidence of skin necrosis in the vicinity of the primary injection site was observed. One patient receiving a 200-μCi dose developed a local infection in the lumpectomy wound that resolved with local wound care. One patient receiving a dose of 300 μCi developed a seroma 1 week postoperatively. The radioactive count at the breast wound site was recorded as 3100 cps at that time. Drainage of the seroma resulted in dramatic decrease in wound counts (count 77 cps) 1 week later (day 14).
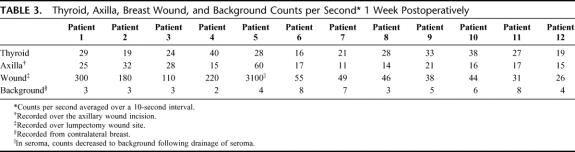
The mean radioactive counts (±SD) of the thyroid gland, axillary wound, breast lumpectomy wound, and background radioactive counts (Table 3) 1 week postoperatively were 26 ± 7, 22 ± 12, 349 ± 833, and 4 ± 2 cps, respectively.
TABLE 3. Thyroid, Axilla, Breast Wound, and Background Counts per Second 1 Week Postoperatively
DISCUSSION
The use of sentinel lymph node biopsy has become a standard technique for nodal staging of patients with early-stage breast cancer. The most common application of this technique involves a 2-stage procedure involving a preoperative injection of a 99mTc sulfur colloid radiotracer and the intraoperative injection of unlabeled isosulfan blue dye.
The use of isosulfan blue as the injectable dye in sentinel lymph node biopsy procedures stems from feline studies and studies comparing isosulfan blue to different dyes indicating effective lymphatic uptake and nodal binding of isosulfan blue in the primary node groups.15,16 Successful use in sentinel lymph node biopsy with melanoma patients made isosulfan blue a logical choice when the technique was extended to breast cancer patients.17 Subsequent clinical studies have shown that methylene blue is equivalent to isosulfan blue when used alone or in conjunction with a radiotracer.7–9 A review of the literature reveals an incidence of allergic reactions occurring in 1% to 3% of patients receiving isosulfan blue.18–20 These allergic reactions range from hives and wheals to anaphylactic shock.20 Others have documented cases of severe anaphylaxis in patients following administration of isosulfan blue.8,12,18,20 Isosulfan blue is a triphenylmethane-based dye and is one of the rosaniline dyes. Triphenylmethane dyes have been used for years in cosmetics, leather, medicine, and textile industries. Since many items used by the general population contain triphenylmethane-related dyes, sensitization to isosulfan blue can occur and may not be elicited by an allergy history or negative skin test.18,20 The exact mechanism for allergic reaction is unknown but has been theorized to be anaphylactic (ie, IgE-related) or anaphylactoid in nature.20 Conversely, serious allergic reactions to methylene blue injection are extremely rare, and there have been no reported anaphylactic reactions related to methylene blue use during sentinel lymph node biopsy.20 There have been several reports of local skin inflammation and even local necrosis associated with subdermal injection of methylene blue; however, peritumoral or subareolar injection, as performed in this study, can be performed at tissue depths that should lessen this risk.21
The use of the standard radiotracer 99Tc sulfur colloid is based on collaborative data from multiple studies demonstrating unfiltered 99mTc as having the highest success rate for labeling sentinel lymph nodes.2,3,22,23 Other commercially available radiopharmaceuticals; such as Cardiolyte, Dextran 40, or Microlite; have been found to be inferior to unfiltered 99mTc for detection of the sentinel lymph node.2,24–26 Although effective, the use of 99mTc sulfur colloid is not without drawbacks. In terms of patient experience, injection of the recommended 5 to 8 mL volume of 99mTc sulfur colloid into the breast is an extremely painful consequence of this “minimally invasive” procedure. Indeed, the pain related to the injection of the viscous sulfur colloid has been subjectively quoted as being “severe” by our patients. In addition to complaint of pain, vasovagal episodes following injection of 99mTc colloid have been reported.27 Although no concrete data exist on the rate of these complications and the degree of anxiety created, we theorized that the pain and anxiety experienced during injection of 99m Tc colloid should be similar to that reported for needle localization procedures. Kelly and Winslow reported the mean anxiety score of 5.3 (scale 0–10) in women undergoing needle localization procedures. Nine percent of patients fainted and other reported complications including pain, stinging, embarrassment, and dizziness.17 The usual 2-hour interval required for adequate transit of media to the sentinel nodes necessitates that this injection be performed while the patient is awake, often the day before or more typically the morning of surgery. The impetus behind the requisite waiting period revolves around the need for not only adequate time for proper nodal collection of the radioactive media, but also for adequate clearance of the primary injection site to prevent the phenomena known as “shine-through.” The relatively high radioactive dose of 99m Tc colloid (1–10 mCi) makes discrimination between primary injection site radioactivity and significant radioactivity in the sentinel nodes difficult, due to overlap in the radioactive signal from the primary injection site and the sentinel node(s) in the axilla (shine-through phenomenon).
Layeeque et al27 and Zogakis et al13 have both reported success with intraoperative subareolar injection of 99mTc colloid; however, more powerful comparative studies must be conducted before a final conclusion on this technique can be reached. The maximum dose of 125I methylene blue (1000 μCi) represents one tenth the radioactivity (10 mCi) for 99mTc sulfur colloid injected during lymphoscintigraphy procedures done the day before surgery. Obviously, less radioactive exposure provides a higher level of safety for patients, surgeons, and perioperative personnel. In addition, the energy of 99mTc is 140 Kev as compared with 35 Kev from 125I.
125I methylene blue as a single entity can be injected intraoperatively because the radiolabeled dye quickly transits through the lymphatics to the sentinel node groups. Animal studies using rabbits demonstrated significant radioactive uptake in regional lymph nodes within 10 minutes following injection.14 In our study at doses of 400 μCi and higher, transcutaneous detection of “hot spots” was achieved approximately 20 minutes postinjection. At lower doses (100–200 μCi), transcutaneous detection of “hot spots” was achieved within the same time period, but only following a 25-mL sterile NaCl “flushing” at the primary injection site to facilitate lymphatic drainage. Flushing procedures were used as an adjunct in 6 subjects. Regardless of the administered dose, 11 of 12 patients demonstrated transit of detectable radioactivity to the sentinel lymph nodes. The remaining subject had no sentinel lymph nodes detected by radiotracer or by the blue dye. The percentage of detected sentinel lymph nodes classified as “hot and blue” was 80%, with the remaining 20% of sentinel lymph nodes observed to be “blue only.” Wada et al have reported a similar percentage of “hot and blue” nodes (81%) in their analysis of the traditional combined technique using blue dye and radiotracer.28 Based on these results, it appears that conjugated dye and radiotracer effectively transit from the primary injection site to the sentinel lymph nodes. The lower emitted energy of 125I gamma particles necessitates an injected dose of at least 300 μCi to allow reliable transcutaneous detection, but injections of 1000 μCi were proven to be more effective. No significant difference in time interval for initial detection of “hot spot” or eventual detection of sentinel node was noted between the patients undergoing peritumoral versus subareolar injection. In general, patients with upper, lateral quadrant tumors were given subareolar injections. No adverse reactions were attributed in this study to injection of 125I methylene blue or unlabeled methylene blue dye. Superficial injections administered in a subareolar location exhibited no incidence of skin necrosis.
The 1- and 2-week postoperative radioactive counts using a hand-held gamma detector revealed no patients with significant thyroid radioactivity above background counts on the contralateral chest. All patients reported complete compliance with use of the thyroid-protective Lugol's solution. The 1 patient with a significant residual radioactive count 1 week postoperatively within the lumpectomy wound site had a large seroma at that time. Subsequent drainage of the seroma lowered the residual activity to background 2 weeks postoperatively. We theorized that the residual injected 125I methylene blue had become trapped in the seroma within the lumpectomy cavity.
Currently, there are no restrictions on intraoperative injection of radiodiagnostic agents listed by the Office of Safety and Health Administration guidelines or International Commission on Radiation Protection. Thus, there are no rules or guidelines that prohibit injection of radioisotope by surgeons in the operating room. Our hospital requires a 4-hour training class on radiation safety prior to handling of such materials. Indeed, injection of 125I methylene blue by a surgeon may be a billable event under the Injection of Contrast Code (CPT Code 38792).
The use of 125I methylene blue for labeling of sentinel lymph nodes in the axilla may prove to be an effective means of reliable detection of sentinel lymph nodes in selected breast cancer patients. Administration of the drug was proven to be safe for humans with no incidence of adverse reactions or residual radioactive uptake by the thyroid gland within this study. An effective dose of 1000 μCi was identified that allows for reliable transcutaneous detection of the sentinel lymph node.
CONCLUSION
This technique eliminates painful preoperative injections of 99mTc colloid, is performed by the surgeon in the operating room, is associated with lower radiation exposures for personnel, and avoids the delays caused by nonoperating room personnel. A prospective, adequately powered phase II study will be necessary to prove equivalency to the techniques that have become the current standard of care.
Footnotes
Reprints: Jason D. Cundiff, MD, 30 Chateau Pontet Canet, Kenner, LA 70065. E-mail: jcundi@lsuhsc.edu or jasoncundiff@cox.net.
REFERENCES
- 1.Glass LF, Fenske NA, Messina JL, et al. The role of selective lymphadenectomy in the management of patients with malignant melanoma. Dermatol Surg. 1995;21:979–983. [DOI] [PubMed] [Google Scholar]
- 2.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer: a multicenter validation study. N Engl J Med. 1998;339:941–946. [DOI] [PubMed] [Google Scholar]
- 3.Krag DN, Harlow S, Weaver D, et al. Radiolabeled sentinel node biopsy: collaborative trial with the National Cancer Institute. World J Surg. 2001;25:823–828. [DOI] [PubMed] [Google Scholar]
- 4.Kelley MC, Hansen N, McMasters KM. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Am J Surg. 2004;188:49–61. [DOI] [PubMed] [Google Scholar]
- 5.Newman LA. Lymphatic mapping and sentinel lymph node biopsy in breast cancer patients: a comprehensive review of variations in performance and technique. J Am Coll Surgeons. 2004;199:804–816. [DOI] [PubMed] [Google Scholar]
- 6.Giuliano AE, Kirgan DM, Guenther JM, et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–398; discussion 398–401. [DOI] [PMC free article] [PubMed]
- 7.Eldrageely K, Vargas MP, Khalkhali I, et al. Sentinel lymph node mapping of breast cancer: a case-control study of methylene blue tracer compared to isosulfan blue. Am Surgeon. 2004;10:872–875. [PubMed] [Google Scholar]
- 8.Simmons R, Thevarajah S, Brennan MB, el al. Methylene blue dye as an alternative to isosulfan blue dye for sentinel lymph node localization. Ann Surg Oncol. 2003;10:342–347. [DOI] [PubMed] [Google Scholar]
- 9.Blessing WD, Stolier AJ, Teng SC, et al. A comparison of methylene blue and lymphazurin in breast cancer sentinel node mapping. Am J Surg. 2002;184:341–345. [DOI] [PubMed] [Google Scholar]
- 10.Choudhury A, Wengert PA Jr, Smith JS Jr. A new surgical localization technique for biopsy in patients with nipple discharge. Arch Surg. 1989;124:874–875. [DOI] [PubMed] [Google Scholar]
- 11.Kern KA. Concordance and validation study of sentinel lymph node biopsy for breast cancer using subareolar injection of blue dye and technetium 99m sulfur colloid. J Am Coll Surg. 2002;195:467–475. [DOI] [PubMed] [Google Scholar]
- 12.Reitsamer R, Peintinger F, Rettenbacher L, et al. Subareolar subcutaneous injection of blue dye versus peritumoral injection of technetium-labeled human albumin to identify sentinel lymph nodes in breast cancer patients. World J Surg. 2003;27:1291–1294. Epub 2003 Oct 28. [DOI] [PubMed]
- 13.Zogakis TG, Wetherill RE, Christensen RD, et al. Intraoperative subareolar injection of 99mTc-labeled sulfur colloid results in consistent sentinel lymph node identification. Ann Surg Oncol. 2005;12:167–172. [DOI] [PubMed] [Google Scholar]
- 14.Stafford SJ, Wright JL, Schwimer J, et al. A novel injectible for sentinel lymph node biopsy: development of 125I methylene blue. J Surg Res. In press. [DOI] [PubMed]
- 15.Leidenius MH, Leppanen EA, Krogerus LA, et al. The impact of radiopharmaceutical particle size on the visualization and identification of sentinel nodes in breast cancer. Nucl Med Commun. 2004;25:233–238. [DOI] [PubMed] [Google Scholar]
- 16.Peintinger F, Reitsamer R, Stranzl H, et al. Comparison of quality of life and arm complaints after axillary lymph node dissection vs. sentinel lymph node biopsy in breast cancer patients. Br J Cancer. 2003;89:648–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly P, Winslow EH. Needle wire localization for nanopalpable breast lesions: sensations anxiety levels, and informational needs. Oncol Nurs Forum. 1996;23:639–645. [PubMed] [Google Scholar]
- 18.Cimmino VM, Brown AC, Azocik JF, et al. Allergic reaction to isosulfan blue during sentinel node biopsy: a common event. Surgery. 2001;130:439–432. [DOI] [PubMed] [Google Scholar]
- 19.Albo D, Wayne JD, Hunt KK, et al. Anaphylactic reactions to isosulfan blue dye during sentinel lymph node biopsy for breast cancer. Am J Surg. 2001;182:393–398. [DOI] [PubMed] [Google Scholar]
- 20.Thevarajah S, Huston TL, Simmons RM. A comparison of the adverse reactions associated with isosulfan blue versus methylene blue dye in sentinel lymph node biopsy for breast cancer. Am J Surg. 2005;189:236–239. [DOI] [PubMed] [Google Scholar]
- 21.Stradling B, Aranha G, Gabram S, et al. Adverse skin lesions after methylene blue injections for sentinel lymph node localization. Am J Surg. 2002;184:350–352. [DOI] [PubMed] [Google Scholar]
- 22.McMasters KM, Tuttle TM, Carlson DJ, et al. Sentinel lymph node biopsy for breast cancer: a suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. J Clin Oncol. 2000;18:2560–2566. [DOI] [PubMed] [Google Scholar]
- 23.Tafra L, Lannin DR, Swanson MS, et al. Multicenter trial of sentinel node biopsy for breast cancer using both technetium sulfur colloid and isosulfan blue dye. Ann Surg. 2001;233:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathanson SD, Anaya P, Karvelis KC, et al. Sentinel lymph node uptake of two different technetium-labeled radiocolloids,. Ann Surg Oncol. 1997;4:104–110. [DOI] [PubMed] [Google Scholar]
- 25.Ege GN, Warbick A. Lymphoscintigraphy: a comparison of 99Tc(m) antimony sulphide colloid and 99Tc(m) stannous phytate. Br J Radiol. 1979;52:124–129. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan WD, Davis MA, Rose CM. A comparison of two technetium-99m-labeled radiopharmaceuticals for lymphoscintigraphy: concise communication. J Nucl Med. 1979;20:933–937. [PubMed] [Google Scholar]
- 27.Layeeque R, Kepple J, Henry-Tillman RS, et al. Intraoperative subareolar radioisotope injection for immediate sentinel lymph node biopsy. Ann Surg. 2004;239:841–845; discussion 845–848. [DOI] [PMC free article] [PubMed]
- 28.Wada N, Imoto S, Yamauchi C, et al. Correlation between concordance of tracers, order of harvest, and presence of metastases in sentinel lymph nodes with breast cancer. Ann Surg Oncol. 2005;12:497–503. [DOI] [PubMed] [Google Scholar]