Abstract
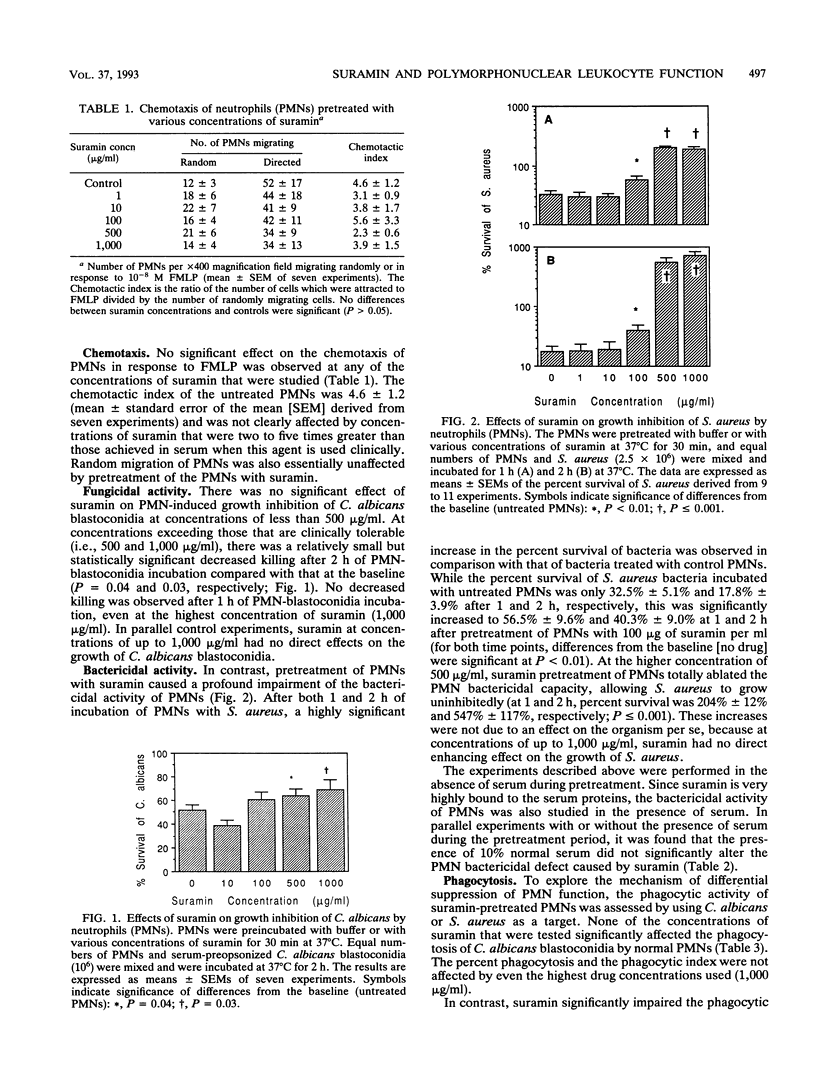
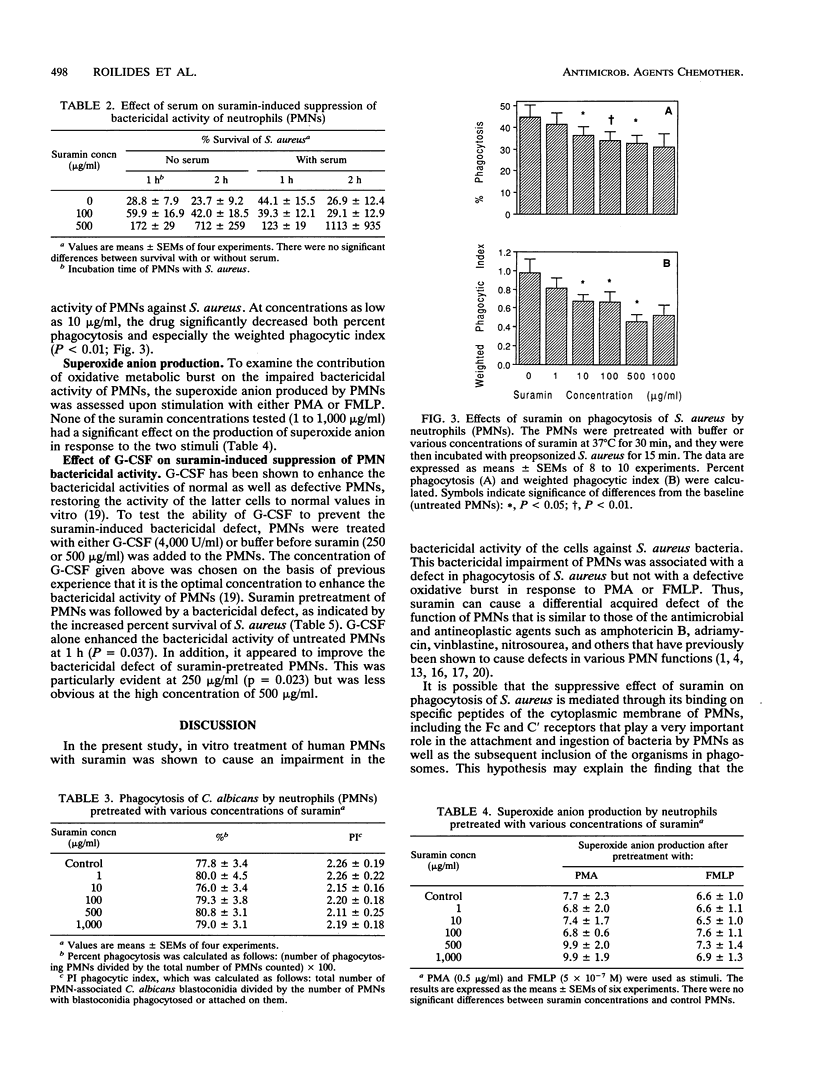
Suramin is a polyanionic compound with potent antineoplastic properties. Because polymorphonuclear leukocytes (PMNs) are a crucial component of host defenses against bacteria and fungi, the effects of suramin on PMN function were studied in vitro. PMNs from healthy donors were incubated with concentrations of suramin of 1 to 1,000 micrograms/ml (within and exceeding the therapeutic range) for 30 min, and PMN functional parameters were subsequently assessed. Suramin had no effect on viability, chemotaxis to N-formylmethionyl leucyl phenylalanine, phagocytosis of Candida albicans, or superoxide anion production in response to phorbol myristate acetate and formylmethionyl leucyl phenylalanine. Fungicidal activity against C. albicans blastoconidia was unaffected at a suramin concentration of < 500 micrograms/ml, whereas at higher concentrations a slight suppression was observed (P = 0.04). Bactericidal activity against Staphylococcus aureus was significantly suppressed by concentrations of > or = 100 micrograms/ml (P < 0.01). Phagocytosis of S. aureus was also significantly impaired at > or = 10 micrograms/ml (P < 0.05). The presence of 10% human serum during pretreatment did not abrogate the suramin-induced suppression of bactericidal activity. Treatment of PMNs with granulocyte colony-stimulating factor (4,000 U/ml) for 30 min prior to the addition of suramin (250 micrograms/ml) improved the bactericidal defect (P = 0.02). The PMN functional impairment may be related to increased susceptibility to bacterial infections, and granulocyte colony-stimulating factor may improve the defect.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Daschner F. D. Antibiotics and host defence with special reference to phagocytosis by human polymorphonuclear leukocytes. J Antimicrob Chemother. 1985 Aug;16(2):135–141. doi: 10.1093/jac/16.2.135. [DOI] [PubMed] [Google Scholar]
- Fleischmann J., Golde D. W., Weisbart R. H., Gasson J. C. Granulocyte-macrophage colony-stimulating factor enhances phagocytosis of bacteria by human neutrophils. Blood. 1986 Sep;68(3):708–711. [PubMed] [Google Scholar]
- Goren M. B. Phagocyte lysosomes: interactions with infectious agents, phagosomes, and experimental perturbations in function. Annu Rev Microbiol. 1977;31:507–533. doi: 10.1146/annurev.mi.31.100177.002451. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Interference with normal phagosome-lysosome fusion in macrophages, using ingested yeast cells and suramin. Nature. 1975 Jul 3;256(5512):47–49. doi: 10.1038/256047a0. [DOI] [PubMed] [Google Scholar]
- Harvath L., Falk W., Leonard E. J. Rapid quantitation of neutrophil chemotaxis: use of a polyvinylpyrrolidone-free polycarbonate membrane in a multiwell assembly. J Immunol Methods. 1980;37(1):39–45. doi: 10.1016/0022-1759(80)90179-9. [DOI] [PubMed] [Google Scholar]
- Hawking F. Suramin: with special reference to onchocerciasis. Adv Pharmacol Chemother. 1978;15:289–322. doi: 10.1016/s1054-3589(08)60486-x. [DOI] [PubMed] [Google Scholar]
- Kielian M. C., Steinman R. M., Cohn Z. A. Intralysosomal accumulation of polyanions. I. Fusion of pinocytic and phagocytic vacuoles with secondary lysosomes. J Cell Biol. 1982 Jun;93(3):866–874. doi: 10.1083/jcb.93.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa S., Yuo A., Souza L. M., Saito M., Miura Y., Takaku F. Recombinant human granulocyte colony-stimulating factor enhances superoxide release in human granulocytes stimulated by the chemotactic peptide. Biochem Biophys Res Commun. 1987 May 14;144(3):1143–1146. doi: 10.1016/0006-291x(87)91430-6. [DOI] [PubMed] [Google Scholar]
- La Rocca R. V., Stein C. A., Danesi R., Cooper M. R., Uhrich M., Myers C. E. A pilot study of suramin in the treatment of metastatic renal cell carcinoma. Cancer. 1991 Mar 15;67(6):1509–1513. doi: 10.1002/1097-0142(19910315)67:6<1509::aid-cncr2820670608>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Levine A. M., Gill P. S., Cohen J., Hawkins J. G., Formenti S. C., Aguilar S., Meyer P. R., Krailo M., Parker J., Rasheed S. Suramin antiviral therapy in the acquired immunodeficiency syndrome. Clinical, immunological, and virologic results. Ann Intern Med. 1986 Jul;105(1):32–37. doi: 10.7326/0003-4819-105-1-32. [DOI] [PubMed] [Google Scholar]
- Malawista S. E. Vinblastine: colchicine-like effects on human blood leukocytes during phagocytosis. Blood. 1971 May;37(5):519–529. [PubMed] [Google Scholar]
- Morstyn G., Lieschke G. J., Sheridan W., Layton J., Cebon J., Fox R. M. Clinical experience with recombinant human granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor. Semin Hematol. 1989 Apr;26(2 Suppl 2):9–13. [PubMed] [Google Scholar]
- Pesanti E. L. Suramin effects on macrophage phagolysosome formation and antimicrobial activity. Infect Immun. 1978 May;20(2):503–511. doi: 10.1128/iai.20.2.503-511.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering L. K., Ericsson C. D., Kohl S. Effect of chemotherapeutic agents on metabolic and bactericidal activity of polymorphonuclear leukocytes. Cancer. 1978 Oct;42(4):1741–1746. doi: 10.1002/1097-0142(197810)42:4<1741::aid-cncr2820420412>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Pruzanski W., Saito S., DeBoer G. Modulatory activity of chemotherapeutic agents on phagocytosis and intracellular bactericidal activity of human polymorphonuclear and mononuclear phagocytes. Cancer Res. 1983 Mar;43(3):1420–1425. [PubMed] [Google Scholar]
- Roilides E., Mertins S., Eddy J., Walsh T. J., Pizzo P. A., Rubin M. Impairment of neutrophil chemotactic and bactericidal function in children infected with human immunodeficiency virus type 1 and partial reversal after in vitro exposure to granulocyte-macrophage colony-stimulating factor. J Pediatr. 1990 Oct;117(4):531–540. doi: 10.1016/s0022-3476(05)80684-5. [DOI] [PubMed] [Google Scholar]
- Roilides E., Walsh T. J., Pizzo P. A., Rubin M. Granulocyte colony-stimulating factor enhances the phagocytic and bactericidal activity of normal and defective human neutrophils. J Infect Dis. 1991 Mar;163(3):579–583. doi: 10.1093/infdis/163.3.579. [DOI] [PubMed] [Google Scholar]
- Roilides E., Walsh T. J., Rubin M., Venzon D., Pizzo P. A. Effects of antifungal agents on the function of human neutrophils in vitro. Antimicrob Agents Chemother. 1990 Feb;34(2):196–201. doi: 10.1128/aac.34.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. A., LaRocca R. V., Thomas R., McAtee N., Myers C. E. Suramin: an anticancer drug with a unique mechanism of action. J Clin Oncol. 1989 Apr;7(4):499–508. doi: 10.1200/JCO.1989.7.4.499. [DOI] [PubMed] [Google Scholar]
- Stute N., Santana V. M., Rodman J. H., Schell M. J., Ihle J. N., Evans W. E. Pharmacokinetics of subcutaneous recombinant human granulocyte colony-stimulating factor in children. Blood. 1992 Jun 1;79(11):2849–2854. [PubMed] [Google Scholar]


