Abstract
Objectives:
A considerable percentage of morbidity and mortality after esophagectomy and gastric pull-up is due to leakage of the esophagogastrostomy, which is mainly caused by ischemia of the gastric fundus. Previous clinical studies demonstrated that impaired microcirculation of the gastric conduit almost recovers within the first 5 postoperative days. Therefore, this study was designed to improve gastric perfusion by laparoscopic ischemic conditioning of the stomach.
Methods:
The study group consisted of 83 patients with 44 esophageal adenocarcinomas and 39 squamous cell carcinomas. A total of 51% received neoadjuvant radiochemotherapy. First, all patients underwent laparoscopic mobilization of the stomach including the cardia and preparation of the gastric conduit. After a mean delay of 4.3 days (range, 3–7 days), a conventional right-sided transthoracic en bloc esophagectomy was performed. Reconstruction was done by gastric pull-up and high intrathoracic esophagogastrostomy.
Results:
Three conversions (3.6%) to open surgery were necessary during laparoscopic mobilization of the stomach. The reoperation rate was 2.4% (one relaparoscopy for control of a bleeding of the stapler line, one rethoracotomy for chylothorax). Two patients showed circumscribed necroses of the upper part of the fundus after gastric pull-up into the chest. These necroses were resected for reconstruction by esophagogastrostomy. Five patients (6.0%) developed small anastomotic leakages with minor clinical symptoms; however, the gastric conduits were well vascularized. All leakages healed after endoscopic stenting. Major postoperative complications were observed in 13.3% of the patients and the 90-day mortality was 0%.
Conclusion:
Laparoscopic ischemic conditioning of the gastric conduit is feasible and safe and may contribute to the reduction of postoperative morbidity and mortality after esophagectomy and gastric pull-up.
To reduce postoperative morbidity and mortality after transthoracic esophagectomy, 83 patients with esophageal carcinoma underwent laparoscopic ischemic conditioning of the gastric tube. After a mean delay of 4.3 days, this was followed by transthoracic en bloc esophagectomy and high intrathoracic esophagogastrostomy. A mortality of 0% favors this concept.
Esophagectomy and reconstruction by gastric pull-up still is associated with a postoperative morbidity of 40% to 50% and mortality of 5% to 10%.1–8 Neoadjuvant radiochemotherapy increases the frequency of postoperative morbidity and mortality.9–12 Besides cardiopulmonary problems, severe postoperative complications are mainly due to anastomotic leakage and its sequelae.4,6,7,13,14 Insufficiency of the esophagogastrostomy is primarily caused by reduced vascularization and consecutive ischemia of the anastomosed gastric fundus.15–20 Anatomic studies focusing on the gastric vascularization demonstrated a rarefaction of the intramural vessels of the upper part of the gastric conduit.15,16
Experiments in animals and clinical studies have shown that, after ligation of the left gastric and the left gastroepiploic artery, mucosal pCO2 as indicator of microcirculation significantly rises, and gastric blood flow is reduced to about 50%.19,21–25 Our own studies demonstrated that mucosal pCO2 of the stomach initially rises and declines to basic values 4 to 5 days after devascularization and gastric pull-up.26 Several experimental settings have tried to improve tissue perfusion and oxygenation of the gastric conduit; one of these was described as ischemic conditioning (delay phenomenon). Urschel et al21,22 and Reavis et al25 have demonstrated in animals that ischemic conditioning of the gastric conduit is possible and improves anastomotic healing. In patients, preoperative embolization of gastric arteries for conditioning resulted in significantly less decrease of blood flow than in controls.24,27
Based on these data, our study was conducted to investigate ischemic conditioning of the stomach prior to esophagectomy and gastric pull-up. The purpose was to evaluate the results of this procedure concerning postoperative morbidity, especially anastomotic healing and postoperative mortality.
METHODS
Patients
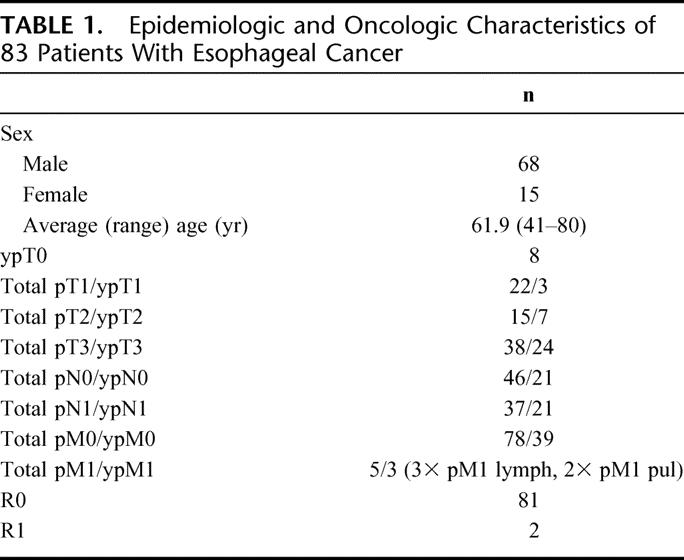
Between December 2003 and September 2005, 83 consecutive patients were included in the study (Table 1). 44 patients had esophageal adenocarcinoma and 39 squamous cell carcinoma of the esophagus. 42 patients (50.6%) with uT3 carcinomas according to endosonography received neoadjuvant radiochemotherapy with 36 Gy and 5-fluorouracil/cisplatin according to a standardized protocol.28 The average ASA score was 2.2 with a range of 1 to 3. A total of 73 patients (88%) had a peridural catheter before thoracotomy for perioperative pain control. Technical difficulties were the cause for not placing a peridural catheter in 10 patients. All 83 patients underwent gastric devascularization (gastrolysis) and delayed esophagectomy with reconstruction. A total of 82 patients had a laparoscopic approach, whereas 1 patient had primary open gastrolysis because of several preceding abdominal operations.
TABLE 1. Epidemiologic and Oncologic Characteristics of 83 Patients With Esophageal Cancer
During the same time period, 9 other patients underwent conventional one-stage esophagectomy without mortality and were not included in the study group. In 7 of these patients, resectability had to be clarified first by thoracotomy before gastric pull-up. Two patients needed colon interposition because of previous gastric surgery.
Operative Techniques
The laparoscopic procedure is performed in supine position of the patient with straddled legs. The surgeon stands between the patient's legs. Five abdominal ports (one, 5 mm; and four, 11 mm) are used for the dissection. The gastrohepatic ligament is divided and the right and left crura of the diaphragm are dissected. The phrenoesophageal membrane is divided and the lower mediastinum is entered to dissect the lower esophagus. In case of distal esophageal carcinoma in advanced stage, superficial parts of the right and left crura are dissected that remain as a cover of the esophagus. The pericardium is prepared and the right and left pleura are not opened. If the pleura is opened on one side, a chest tube is inserted. The stomach is freed by dividing the short gastric vessels using the Ligasure device (Autosuture, Tyco Healthcare). The gastrocolic omentum is carefully divided preserving the right gastroepiploic arcade (Fig. 1A, B). The right colonic flexure is detached, and a Kocher maneuver is performed (Fig. 1C). Retrogastric adhesions are dissected, and the gastroduodenal artery as well as the right gastroepiploic artery and vein are visualized. The stomach is retracted superiorly and the superior edge of the pancreas is exposed. The common hepatic artery and the central part of the splenic artery are cleared of the surrounding lymphatic tissue (Fig. 1D). The fatty tissue around the origin of the left gastric artery and vein is dissected in order that the lymph nodes of group 7 remain at the left gastric artery.29 The left gastric vein is dissected with the Ligasure. The left gastric artery is closed with 2 laparoclips and cut in between (Fig. 1E). The right gastric artery is preserved. The fat tissue of the lesser curvature between the middle and distal third is dissected with the Ligasure in oral direction for 3 cm. A 60-mm Endo Stapler is inserted from the right side and a staple line along the lesser curvature of the stomach is placed in direction of the gastric fundus (Endo GIA, Autosuture, Tyco Healthcare) (Fig. 1F). The reason is technical and not a fundamental part of the conditioning process. It is a lot easier to get this lesser curvature stapling started in the abdomen than in the chest. No pyloroplasty is performed during laparoscopy. In case of a narrow hiatus, the right crura are partially divided to allow an easy passage later on of the gastric tube through the hiatus and prevent gastric outlet obstruction. The 5 port sites are closed. The patient is usually extubated in the operation room and transferred to the normal ward. He is allowed to drink liquids and soup as well as caloric drinks starting on the evening of the day of operation.
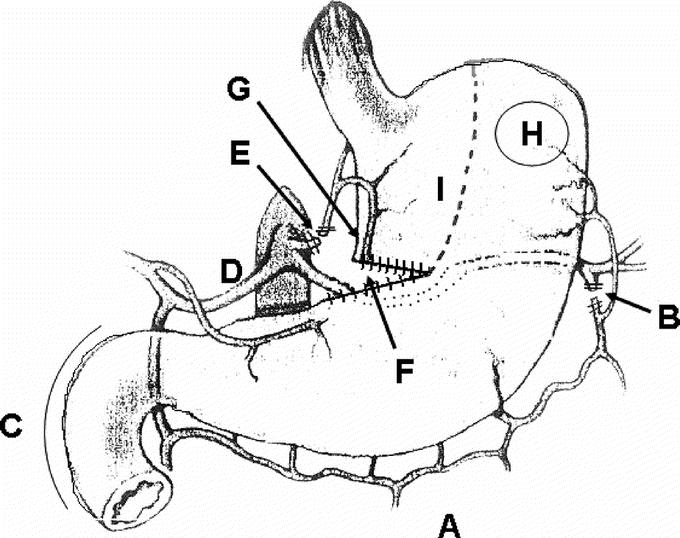
FIGURE 1. Final aspect of the gastric conduit after laparoscopic ischemic conditioning A, Divided gastroepiploic omentum preserving the right gastroepiploic arcade. B, Left gastroepiploic artery cut. C, Kocher maneuver performed. D, Common hepatic and splenic artery cleared. E, Left gastric artery and vein cut. F, 60-mm endostapling between the distal and middle third of the lesser curvature. G, Intended introduction site of the circular stapler in the chest. H, Intended location for esophagogastrostomy. I, Intended linear stapling for completion of the conduit in the chest.
Five days after the laparoscopic procedure, a right-sided transthoracic en bloc esophagectomy is performed.4,28,30 The azygos vein and the intercostal veins are divided preserving the intercostal arteries. The en bloc resection comprises the azygos vein and the thoracic duct. The esophageal hiatus is dissected from the chest gaining access to the abdominal esophagus, which had been freed during the laparoscopic procedure. After dissection along the adventitia of the aorta with clip closure of small aortic branches, the left pleura is opened in the lower part of the mediastinum. All bifurcational lymph nodes as well as the lymph nodes along the right and the left main bronchus are dissected. The vagal branches to the heart and the lung are preserved while the vagus branches to the esophagus are cut. The pleura covering the esophagus in the upper mediastinum is cut longitudinally and dissected to both sides. The esophagus is dissected together with the lymph nodes of the upper mediastinum. The membranous part of the trachea is freed. The right and left recurrent laryngeal nerves are cleared from lymph nodes and the cervical esophagus is exposed from below. This dissection is performed with a 36-French bougie placed in the thoracic esophagus. The esophagus below the junction between thoracic and cervical part is closed with a 45-mm Purse string clamp (Autosuture, Tyco Healthcare) and cut. A 28-mm circular stapler, as an exception size 25 mm, is introduced in the cervical esophageal stump and the Purse string suture is tied (PCEEA Stapler, Autosuture, Tyco Healthcare). Now the gastric conduit is gently pulled into the right pleural cavity through the hiatus with the greater curvature to the left side. The most appropriate place for anastomosis is marked by a stay-suture on the left anterior wall of the gastric fundus (Fig. 1H). The anterior wall of the stomach close to the lesser curvature in the subcardia region is opened, and the stapler is inserted and perforated with the sharp tip through the marked area at the fundic front wall (Fig. 1G, H). After connection and closure of the stapler, the anastomosis is performed. After retraction of the stapler, the integrity of both rings is controlled. The intraesophageal bougie is passed into the anastomosed gastric conduit. The esophagus, the right part of the fundus, and the upper two thirds of the lesser curvature, including the former introduction site of the stapler, are resected after placing a TA 90 linear stapler (Autosuture, Tyco Healthcare) (Fig. 1I). The specimen is opened and controlled for complete resection of the esophageal cancer. The lesser curvature stapler line is oversewn with interrupted 3.0 Vicryl sutures because of bleeding. The bougie is retracted from the gastric conduit and replaced by a double-lumen gastric tube. The esophagogastrostomy at the cervicothoracic junction is covered by the parietal pleura. After inserting a chest tube, the thorax is closed.
Statistics
For analysis, only descriptive means were used (mean ± SD).
RESULTS
In the beginning of the series, conversions during laparoscopic gastrolysis were necessary in 3 patients (3.6%) because of adhesions (n = 2) and one bleeding of the spleen (open splenectomy). One relaparoscopy was performed on postoperative day 1 after gastrolysis to control bleeding of the stapler resection line. One rethoracotomy on postoperative day 24 was necessary for closure of the thoracic duct because of chylothorax. The further course of this patient was uneventful. The total reoperation rate was 2.4%. Blood transfusions for laparoscopic gastrolysis were performed in 3 patients; 2 patients needed 2 units and 1 patient needed 6 units. Blood transfusions for esophagectomy were performed in 35 patients (average, 2.5 units; range, 1–5 units). The R0 resection rate was 97.6% (81 of 83 patients). On the average, 27 lymph nodes were dissected per patient (range, 7–54 lymph nodes).
The mean interval between gastrolysis and esophagectomy was 4.3 days with a range of 3 to 7 days (5 days, n = 33; 4 days, n = 34; 3 days, n = 13; 6 days, n = 2; 7 days, n = 1). The reasons for deviation from the intended interval of 5 days were organizational, main ones being weekends, holidays, and nonavailability of surgeons. Clinical assessment of the vascularization at the final stapler line on the lesser curvature showed definitely more intense bleeding up to the top of the conduit compared with experience in patients with one stage reconstruction. Five surgeons were involved in the procedures.
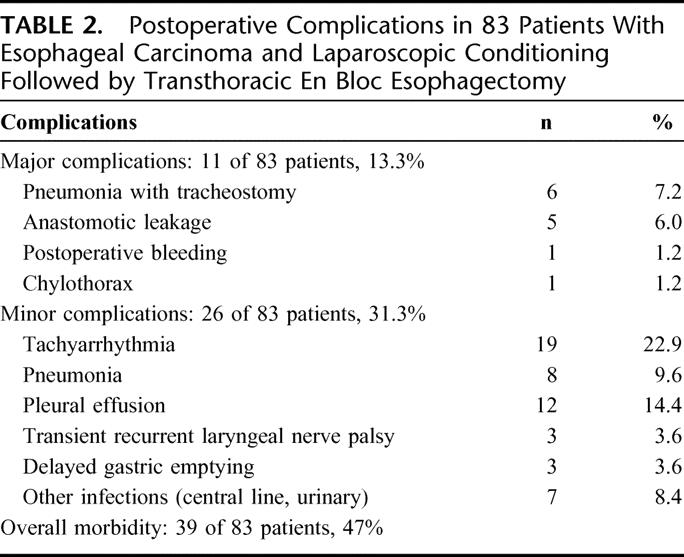
Pulmonary problems including pneumonia and prolonged pleural effusions accounted to 26 minor complications (Table 2). Endoscopic tracheostomies were necessary in 6 patients (7.2%) due to of pulmonary insufficiency and pneumonia and the necessity of prolonged mechanical ventilation after thoracotomy. Two patients had circumscribed necroses of the upper part of the fundus after gastric pull-up. These were resected during construction of the gastric tube. Five leakages were diagnosed at the esophagogastrostomy (6.0%), including 1 of the patients with previously resected fundic necrosis. The 5 patients had only minor clinical symptoms, and endoscopy showed very well-vascularized resection lines of the esophagus and stomach. All 5 leakages were successfully managed by endoscopic self-expanding metal stents (SEMS, Boston Scientific) at the day of detection of the leakage (postoperative day 7, 9, 11, 16, and 20). The stents were removed without problems 4 to 6 weeks after insertion. None of the patients with fundic necroses or anastomotic leak had preoperative chemoradiation. In the subgroup of 42 patients with induction therapy, only 3 developed postoperative pneumonia compared with 11 of 41 patients without chemoradiation. During postoperative hospital stay, endoscopic pneumatic dilatation of the pylorus was performed on 3 patients due to delayed gastric emptying. The further course of these patients was uneventful. None of the patients died during the postoperative course. The in-hospital mortality and the 90-day mortality rates were 0%. The median time of follow-up was 7.2 months and the 1-year actuarial survival rate was 91%.
TABLE 2. Postoperative Complications in 83 Patients With Esophageal Carcinoma and Laparoscopic Conditioning Followed by Transthoracic En Bloc Esophagectomy
DISCUSSION
Our results show that laparoscopic ischemic conditioning of the stomach for esophageal replacement is feasible and safe. The laparoscopic technique of gastric devascularization with preservation of the right gastroepiploic artery and the right gastric artery is demanding and requires experience in esophageal as well as minimal invasive surgery. However, several large series have proved similar results compared with the open technique, but with the advantage of less invasiveness.31–34 The conversion and reoperation rates in our series were very low, and the number of dissected abdominal lymph nodes was comparable to conventional surgery.3,4,35 However, the focus of the present paper is more on the gastric conditioning than the laparoscopic approach.
The mean delay of 4.3 days between gastric devascularization and reconstruction did not have disadvantages for the patients. It was always possible to pull up the prepared gastric conduit through the hiatus into the right pleural cavity, and the length was always sufficient. Therefore, all procedures could be completed as planned. The delay had advantage in 2 patients with circumscribed necroses of the fundus because the demarcation line could definitely be judged and taken into account during final construction of the conduit.
Data on real long-term survival of the present series are not yet available. However, the 1-year actuarial survival rate of 91% reassures that gastric delay does not result in early demise for some unrecognized reason. In a patient with malignancy, a potential increase in VEGF and angiogenesis in association with the delay has to be discussed. However, the surgical trauma of partial gastric devascularization by minimal invasive technique was limited and the time of delay was short. Therefore, provocation of tumor growth by this mechanism seems to be unlikely, although no data are available.
There was no selection bias as the material represents a continuous series except the 9 patients mentioned above. The general status of the patients documented by the ASA classification is comparable to other series of esophagectomy. Some centers with great esophageal surgery expertise have reported non delay series with comparable postoperative morbidity as our series.2,8,30,33 However, all of them had a lower percentage of patients with preoperative induction therapy. Because 51% of our group had neoadjuvant radiochemotherapy, the patients were even more at risk than in other reports. Four meta-analyses have shown that induction radiochemotherapy is linked to increased postoperative morbidity and mortality.9–12 All our patients had transthoracic esophagectomy, which is associated with a higher postoperative morbidity and mortality than transhiatal esophagectomy.35 The institution's hospital mortality for transthoracic esophagectomy in the recent past was 5.5% in a series of 109 consecutive patients.4
The question arises if delay improves outcome. Compared with the conventional technique of one-stage esophagectomy, 3 important steps have been modified. Not only the conditioning of the stomach was introduced but also the operative stress of the patient was reduced by 2 means: first by the minimal invasiveness of the abdominal procedure and second by dividing a long surgical procedure into 2 shorter parts with the possibility of intermediate recovery. Therefore, the favorable outcome in this series cannot exclusively be attributed to gastric conditioning.
Ischemic conditioning also described as delay phenomenon is derived from the creation of skin flaps.36–38 In animal experiments, the effect of conditioning is also well analyzed for the gastric conduit and esophagogastrostomy.21,22,25 The theoretical background is that after partial devascularization the gastric conduit should recover, and gastric tissue perfusion should be improved prior to pull-up and anastomosis to the esophagus.21 This should result in better prerequisites for anastomotic healing. Our own studies have demonstrated microcirculatory changes associated with gastric tube formation either in pigs but also in humans.19,20,26 In patients, Schilling et al reported a reduction of 69% of the gastric tissue perfusion immediately after surgical devascularization of the stomach.23 Elongation and transformation of the stomach to a gastric tube further reduced blood flow, especially at the fundus. Akiyama et al showed in patients a 69% reduction of gastric tissue blood flow in the upper fundus after construction of the gastric conduit;24 in that study, 24 patients underwent ischemic conditioning by angiographic embolization of the right and left gastric artery and the splenic artery. The average interval was 11.9 days prior to esophagectomy.
Urschel et al found in rats a decreased gastric tissue perfusion of 66% in the fundus after gastric devascularization with a complete recovery after 3 weeks.22 Furthermore, fewer anastomotic leaks and higher anastomotic wound-breaking strengths of the intra-abdominal esophagogastric anastomosis were found in comparison with a control group without ischemic conditioning. Similar results in opossums were reported by Reavis et al, comparing a delay group of 28 days after gastric devascularization to a group of immediate esophagogastrostomy.25 The initial blood flow in the fundus decreased by 73% in both groups, but the delay group had more than 3 times the gastric blood flow of the immediate group at the time of anastomosis.
The time interval between partial gastric devascularization and reconstruction depends on 2 variables: first, the onset of the effect of ischemic conditioning, the so-called delay phenomenon; and second, the development of intra-abdominal adhesions of the gastric conduit. The slot for the optimal time of reconstruction is short. As is known from relaparotomies, adhesions become more difficult for surgical dissection with time, especially from postoperative day 6 to 7 on. In 3 patients, organizational problems caused a delayed reconstruction up to day 6 (2 time) or 7 (1 time). We experienced that detaching and pulling up the gastric conduit through the chest are still possible but get more difficult with increasing time interval. In the second step of the procedure, the gastric conduit is pulled up from the right pleural cavity through the hiatus without seeing it. This blind intra-abdominal procedure becomes dangerous in case of severe adhesions, which develop after 6 to 7 days. For these reasons, the time interval between gastrolysis and reconstruction cannot exceed more than 5 to 6 days to avoid damage of the stomach. On the other hand, mucosal pCO2 measurement of the gastric conduit demonstrated a progressive recovery after the second postoperative day, almost reaching baseline values on the fifth postoperative day.26 Accordingly, regarding skin flaps, Dhar and Taylor demonstrated in conditioning the most dramatic increase of blood flow from day 3 to 5.37 Based on all these considerations, postoperative day 5 represents the optimal time for the delayed gastric pull-up and anastomosis. To take advantage of the delay phenomenon by more extended intervals, as in the animal experiments by Urschel et al (3 weeks in rats)22 or Reavis et al (28 days in opossum),25 appears not to be applicable for the clinical setting.
The delay of 5 days between laparoscopy and esophagectomy also has the effect of timely dividing the double stress for the stomach-devascularization and pull-up.23 Consequently, the blood flow of the devascularized stomach gains time to recover in its normal anatomic position. Afterward, it may be better prepared to stand the second stress of stretching and transposition to the upper thorax.
Many patients with esophageal cancer suffer from an elevated risk of surgery because of nicotine and alcohol abuse, especially those with squamous cell carcinoma. Cardiovascular problems and adipositas are more obvious in patients with adenocarcinoma.39 The one-stage, 2-cavity operation is a severe stress for a high percentage of these patients, resulting in a high postoperative morbidity, especially concerning cardiopulmonary problems.39,40 The risk is further increased in patients with neoadjuvant radiochemotherapy.9–12 The prospective randomized trial comparing definite radiochemotherapy with neoadjuvant radiochemotherapy plus surgery for esophageal cancer has shown a significantly better local tumor control in the surgical group.41 The survival data at 2 years, however, were not significantly different, although the surgical group showed increased survival up to 5 years. The main reason for the lacking statistical significance in this trial was the higher treatment-related mortality in patients after induction therapy and surgery compared with definite radiochemotherapy (13% vs. 3.5%). Therefore, all efforts should aim to reduce postoperative complications and mortality after surgery. Our patients with preoperative chemoradiation had a low rate of major complications and no higher overall postoperative morbidity than the patients without induction therapy. A recent paper has shown that postoperative complications are also an important determinant of long term patient survival after major surgery.42 Consequently, the presented concept could lead to better long-term survival rates with improved results compared with definite radiochemotherapy of esophageal cancer.
An alternative concept with the intention to reduce morbidity and mortality after esophagectomy is the initial transthoracic esophagectomy and delayed reconstruction after 8 to 10 days. This two-stage procedure has been described for high-risk patients, especially with poor functional status or after neoadjuvant radiochemotherapy.43–45 For these patients, a mortality of 6.3% or 3.3% has been described in 2 series of 32 or 61 patients.45 Two very small series from Japan with 10 or 18 patients reported a mortality of 0% or 5.5%.43,44 However, the disadvantages of this principle are the need for a temporary cervical esophagostoma, no possibility of intrathoracic anastomosis, and the reconstruction always through the longer route of the anterior mediastinum.
The disadvantage of the delay procedure seems to be the investment of time and costs due to longer hospitalization prior to esophagectomy. This could, however, be compensated by a shorter postoperative stay due to a lower complication rate. In summary, the advantages of laparoscopic ischemic conditioning of the stomach are simple application, minimal invasive abdominal approach, recovery of the patient prior to thoracotomy, and potential recovery of the gastric blood flow prior to pull-up of the stomach. The current data show that this approach may contribute to the reduction of postoperative morbidity, especially mortality in patients who require esophagectomy for esophageal cancer. However, the data provide no direct evidence (yet) to support a causal relationship between delay, improved anastomotic healing, and decreasing mortality. The next step after showing feasibility and safety of this method needs to be a prospective controlled trial to demonstrate effectiveness.
ACKNOWLEDGMENT
Dedicated to Professor J. R. Siewert, our clinical and scientific teacher.
Footnotes
Reprints: Arnulf H. Hölscher, MD, Department of Visceral and Vascular Surgery, Kerpener Str. 62, 50937 Cologne, Germany. E-mail: Arnulf.Hoelscher@uk-koeln.de.
REFERENCES
- 1.Orringer MB, Marshall B, Jannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg. 1999;230:392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando N, Ozawa S, Kitagawa Y, et al. Improvement in the results of surgical treatment of advanced squamous cell esophageal carcinoma during 15 consecutive years. Ann Surg. 2000;232:225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siewert JR, Stein HJ, Feith M, et al. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the western world. Ann Surg. 2001;234:360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hölscher AH, Schröder W, Bollschweiler E, et al. How safe is high intrathoracic esophagogastrostomy? Chirurg. 2003;74:726–733. [DOI] [PubMed] [Google Scholar]
- 5.McCulloch P, Ward J, Tekkis PP. Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicenter prospective cohort study. BMJ. 2003;327:1192–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rentz J, Bull D, Harpole D, et al. Transthoracic versus transhiatal esophagectomy: a prospective study of 945 patients. J Thorac Cardiovasc Surg. 2003;125:1114–1120. [DOI] [PubMed] [Google Scholar]
- 7.Schäfer H, Engert A, Grass G, et al. Perioperative granulocyte colony-stimulating factor does not prevent severe infections in patients undergoing esophagectomy for esophageal cancer: a randomized placebo-controlled clinical trial. Ann Surg. 2004;240:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lerut T, Nafteux P, Moons J, et al. Three-field lymphadenectomy for carcinoma of the esophagus and gastroesophageal junction in 174 R0 resections: impact on staging, disease-free survival and outcome. Ann Surg. 2004;240:962–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaklamanos I, Walker G, Ferry K, et al. Neoadjuvant treatment for resectable cancer of the esophagus and the gastroesophageal junction: a meta-analysis of randomized clinical trials. Ann Surg Oncol. 2003;10:754–761. [DOI] [PubMed] [Google Scholar]
- 10.Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185:538–543. [DOI] [PubMed] [Google Scholar]
- 11.Fiorica F, Di BD, Schepsis F, et al. Preoperative chemoradiotherapy for oesophageal cancer: a systemic review and meta-analysis. Gut. 2004;53:925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greer SE, Goodney PP, Sutton JE, et al. Neoadjuvant chemoradiotherapy for esophageal carcinoma: a meta-analysis. Surgery. 2005;137:172–177. [DOI] [PubMed] [Google Scholar]
- 13.Valverde A, Hay JM, Fingerhut A, et al. Manual versus mechanical esophagogastric anastomosis after resection for carcinoma: a controlled trial. Surgery. 1996;120:476–483. [DOI] [PubMed] [Google Scholar]
- 14.Briel JW, Tamhankar AP, Hagen JA, et al. Prevalence and risk factors for ischemia, leak and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg. 2004;198:536–541. [DOI] [PubMed] [Google Scholar]
- 15.Liebermann-Meffert DMI, Meier R, Siewert JR. Vascular anatomy of the gastric tube used for esophageal reconstruction. Ann Thorac Surg. 1992;54:1110–1115. [DOI] [PubMed] [Google Scholar]
- 16.Pierie JP, De Graaf PW, Poen H, et al. Impaired healing of cervical esophagogastrostomies can be predicted by estimation of gastric serosal blood perfusion by laser Doppler flowmetry. Eur J Surg. 1994;160:599–603. [PubMed] [Google Scholar]
- 17.Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg. 1995;169:634–640. [DOI] [PubMed] [Google Scholar]
- 18.Boyle NH, Pearce A, Hunter D, et al. Scanning laser Doppler flowmetry and intraluminal recirculating gas tonometry in the assessment of gastric and jejunal perfusion during oesophageal resection. Br J Surg. 1998;85:1407–1411. [DOI] [PubMed] [Google Scholar]
- 19.Schröder W, Stippel D, Lacher M, et al. Intraoperative changes of mucosal pCO2 during gastric tube formation. Langenbecks Arch Surg. 2001;386:324–327. [DOI] [PubMed] [Google Scholar]
- 20.Schröder W, Beckurts KTE, Stähler D, et al. Microcirculatory changes associated with gastric tube formation in the pig. Eur Surg Res. 2002;34:411–417. [DOI] [PubMed] [Google Scholar]
- 21.Urschel JD. Ischemic conditioning of the rat stomach: implications for esophageal replacement with stomach. J Cardiovasc Surg. 1995;36:191–193. [PubMed] [Google Scholar]
- 22.Urschel JD, Antkowiak JG, Delacure MD, et al. Ischemic conditioning (delay phenomenon) improves esophagogastric anastomotic wound healing in the rat. J Surg Oncol. 1997;66:254–256. [DOI] [PubMed] [Google Scholar]
- 23.Schilling MK, Redaelli C, Maurer C, et al. Gastric microcirculatory changes during gastric tube formation: assessment with laser Doppler flowmetry. J Surg Res. 1996;62:125–129. [DOI] [PubMed] [Google Scholar]
- 24.Akiyama S, Ito S, Segikuchi H, et al. Preoperative embolisation of gastric arteries for esophageal cancer. Surgery. 1996;120:542–546. [DOI] [PubMed] [Google Scholar]
- 25.Reavis KM, Chang EY, Hunter JG, et al. Utilization of the delay phenomenon improves blood flow and reduces collagen deposition in esophagogastric anastomoses. Ann Surg. 2005;241:736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schröder W, Stippel D, Gutschow C, et al. Postoperative recovery of microcirculation after gastric tube formation. Langenbecks Arch Surg. 2004;389:267–271. [DOI] [PubMed] [Google Scholar]
- 27.Isomura T, Itoh S, Endo T, et al. Efficacy of gastric blood supply redistribution by transarterial embolisation: preoperative procedure to prevent postoperative anastomotic leaks following esophagoplasty for esophageal carcinoma. Cardiovasc Intervent Radiol. 1999;22:119–123. [DOI] [PubMed] [Google Scholar]
- 28.Schneider PM, Baldus SE, Metzger R, et al. Histomorphologic tumor regression and lymph node metastasis determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer: implications for response classification. Ann Surg. 2005;242:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Japanese Gastric Cancer Association. Japanese Classification of Gastric Cancer, 2nd English ed. Gastric Cancer. 1998;1:10–24. [DOI] [PubMed] [Google Scholar]
- 30.Altorki N, Kent M, Ferrera C, et al. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg. 2002;236:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swanstrom LL, Hanson P. Laparoscopic total esophagectomy. Arch Surg. 1997;132:943–949. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen NT, Follette DM, Wolfe BM, et al. Comparison of minimally invasive esophagectomy with transthoracic and transhiatal esophagectomy. Ann Surg. 2000;135:920–925. [DOI] [PubMed] [Google Scholar]
- 33.Luketich JD, Alvelo-Rivera M, Buenaventura O, et al. Minimal-invasive esophagectomy: outcomes in 222 patients. Ann Surg. 2003;238:486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osugi H, Takemura M, Higashino M, et al. A comparison of video-assisted thoracoscopic oesophagectomy and radical lymph node dissection for squamous cell cancer of the oesophagus with open operation. Br J Surg. 2003;90:108–113. [DOI] [PubMed] [Google Scholar]
- 35.Hulscher JB, Van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–1669. [DOI] [PubMed] [Google Scholar]
- 36.Cederna PS, Chang P, Pittet-Cuenod BM, et al. The effect of the delay phenomenon on the vascularity of rabbit rectus abdominis muscles. Plast Reconstr Surg. 1997;99:194–205. [DOI] [PubMed] [Google Scholar]
- 37.Dhar SC, Taylor GI. The delay phenomenon: the story unfolds. Plast Reconstr Surg. 1999;104:2079–2091. [DOI] [PubMed] [Google Scholar]
- 38.Lineaweaver WC, Lei MP, Mustain W, et al. Vascular endothelium growth factor, surgical delay and skin flap survival. Ann Surg. 2004;239:866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bollschweiler E, Schröder W, Hölscher AH, et al. Preoperative risk analysis in patients with adenocarcinoma or squamous cell carcinoma of the oesophagus. Br J Surg. 2000;87:1106–1110. [DOI] [PubMed] [Google Scholar]
- 40.Bartels H, Stein HJ, Siewert JR. Preoperative risk analysis and postoperative mortality of oesophagectomy for respectable esophageal cancer. Br J Surg. 1998;85:840–4. [DOI] [PubMed] [Google Scholar]
- 41.Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–2317. [DOI] [PubMed] [Google Scholar]
- 42.Khuri SF, Henderson WG, DePalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugimachi K, Kitamura M, Maekawa S, et al. Two-stage operation for poor risk patients with carcinoma of the esophagus. J Surg Oncol. 1987;36:105. [DOI] [PubMed] [Google Scholar]
- 44.Saito T, Shimoda K, Shigemitsu Y, et al. Extensive lymphadenectomy for thoracic esophageal carcinoma: a two-stage operation for high risk patients. Surg Today. 1994;24:610. [DOI] [PubMed] [Google Scholar]
- 45.Stein HJ, Bartels H, Siewert JR. Esophageal cancer: indications for two-stage procedures. Chirurg. 2001;72:881–886. [DOI] [PubMed] [Google Scholar]


