Abstract
Objective:
To assess the long-term results of the duodenal switch operation made for pathologic transpyloric duodenogastric reflux (DGR).
Summary Background Data:
DGR symptoms and lesions are poorly responsive to medical treatment.
Methods:
A duodenal switch operation was made on 48 patients suffering from pathologic transpyloric DGR either unrelated (n = 28) or secondary (n = 20) to previous upper gastrointestinal (GI) surgery, including cholecystectomy or vagotomy. The diagnosis was based on the combination of several objective arguments: a long history of gastric symptoms (ie, nausea, epigastric pain, and/or bilious vomiting) poorly responsive to medical treatment (48 of 48), gastroesophageal reflux symptoms unresponsive to proton-pump inhibitors (PPI) (23 of 29), gastritis on upper GI endoscopy (37 of 48) and/or at histology (28 of 41), presence of a bilious gastric lake at >1 upper GI endoscopy (30 of 48), DGR at diisopropyl iminodiacetic acid (DISIDA) scintigraphy scanning (7 of 13), pathologic 24-hour intragastric bile monitoring with the Bilitec device (40 of 41), and absence of Helicobacter pylori antral infection (39 of 41).
Results:
At follow-up (median, 81 months), gastric symptoms were nil, had improved, and remained unchanged in 29 (60.4%), 16 (33.3%), and 2(4.2%) patients, respectively, and 1 patient experienced symptomatic recurrence after a 92-month symptom-free period (2.1%). Among the 44 patients who had postoperative upper GI endoscopy, 42 (95.5%) had no gastritis whereas 5 (11.3%) had an ulcer at the duodenojejunostomy. Gastric exposure to bile at postoperative 24-hour intragastric Bilitec test in 36 patients was nil, within the normal range, and still slightly pathologic in 15 (41.7%), 19 (52.8%), and 2 (5.5%), respectively.
Conclusions:
The duodenal switch operation made on patients in whom diagnosis of pathologic transpyloric DGR is supported by several objective arguments provides most of them with symptomatic and endoscopic improvement parallel to abolishment or normalization of gastric exposure to bile. Postoperative PPI therapy during a 2-month period is to be recommended to prevent the development of an anastomotic ulcer.
A duodenal switch operation was performed on 48 patients having gastric symptoms and/or lesions suggestive of pathologic transpyloric duodenogastric reflux with a good symptomatic outcome in 44 patients and abolishment or normalization of gastric exposure to bile at 24-hour intragastric bile monitoring with the Bilitec device in 34 of the 36 patients investigated postoperatively.
Duodenogastric reflux (DGR) and its clinical relevance remain intriguing for most of surgeons and physicians. However, the introduction of the Bilitec device,1 an instrument that allows the monitoring of bile in the foregut lumen over a 24-hour period, has broadened our knowledge of the implication of DGR in the genesis of upper gastrointestinal (GI) symptoms and lesions. We now know indeed that DGR is a physiologic event,2 but also that the pathologic presence of duodenal juice in the foregut lumen may account for the development of Barrett's metaplasia and dysplasia,3 and for that of gastric polyps,4 as well. Moreover in a subset of patients, the contribution of duodenal juice to the composition of gastric contents may be more substantial than usual,5 so as to produce deleterious effects on the gastric mucosa itself and generate gastric symptoms such as epigastric pain, nausea, and bilious vomiting.6–9 So, pathologic transpyloric DGR may be detected by 24-hour intragastric bile monitoring in up to 40% of patients primarily referred for surgical management of gastroesophageal reflux disease (GERD), but having in fact, gastric symptoms in addition to heartburn, regurgitation, and erosive esophagitis.10
As medical management of these gastric symptoms is far from satisfactory,11,12 a duodenal diversion procedure such as the duodenal switch operation,13,14 is to be considered to improve gastric symptomatology and inflammation caused by the reflux of duodenal contents into the stomach (Fig. 1).
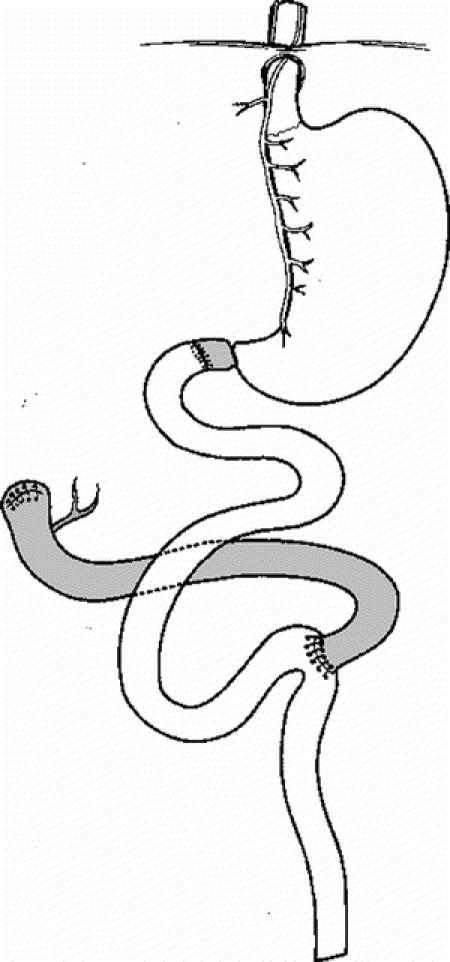
FIGURE 1. Duodenal switch procedure.
The present paper reports a 15-year experience of the duodenal switch operation made on patients having pathologic DGR either without any history of previous upper GI surgery or with a history of a previous surgical operation promoting transpyloric DGR such as cholecystectomy15 or vagotomy.16
METHODS
Study Population
Over a 15-year period, ie, from 1990 to 2005, a duodenal switch operation13,14 was performed by the senior author (J.M.C.) on 48 patients, ie, 14 males and 34 females, aged 14 to 74 years. All patients had been complaining of foregut symptoms suggestive of pathologic exposure of the gastric mucosa to bile,8 ie, nausea (n = 41), epigastric pain (n = 45), and bilious vomiting (n = 34) for a period ranging from 9 to 600 months (mean, 91.9 months).
The 48 patients belonged to 2 different groups: group I (n = 28), ie, 26 patients without any history of previous upper GI surgery, and 2 patients having the gastric symptoms unchanged after cholecystectomy (n = 1) or vertical gastroplasty (n = 1); and group II, 20 patients who developed gastric symptoms after upper GI surgery, ie, cholecystectomy (n = 14), cholecystectomy plus fundoplication (n = 1), vagotomy (n = 1), vagotomy plus pyloric dilation (n = 1), vagotomy plus fundoplication (n = 1), subtotal esophagectomy, vagotomy and gastric pull-up (n = 1), or distal esophagectomy, vagotomy and colonic interposition (n = 1).
The possible implication of DGR in the genesis of gastric symptoms in 32 patients (66.6%) had not been hypothesized by the referring physicians or surgeons, whereas 16 patients (33.3%) were referred to our Unit after DGR had been suspected. Eighteen patients (37.5%) had been told they were suffering from a psychologic disorder without any organic substratum. All patients had been given prokinetics, mucosa-protective medicines, H2-blockers, and/or proton-pump inhibitors (PPI) that had been poorly effective on gastric symptomatology.
Initial Interview
All patients were interviewed by the senior author (J.M.C.) for the presence of gastric symptoms suggestive of pathologic transpyloric DGR, ie, epigastric pain, nausea and bilious vomiting.8 Severity of each gastric symptom was scored depending on whether it was absent (0 point), it was occasional requiring no specific treatment (1 point), it occurred more than once a week requiring specific treatment such as prokinetics, mucosa-protective medicines, H2-blockers, or PPI (2 points), or it interfered with daily activity (3 points). In addition to gastric symptoms, 29 patients, ie, 18 of group I and 11 of group II, (60.4%) also had esophageal symptoms suggestive of pathologic gastroesophageal reflux with a history of esophagitis on upper GI endoscopy.
Preoperative Workup
The diagnosis of pathologic transpyloric DGR was based on the presence of a combination of the following arguments: a long history of gastric symptoms poorly responsive to prokinetics, mucosa-protective medicines, H2-blockers and/or PPI, gastroesophageal reflux (GER) symptoms unresponsive to PPI, gastritis on upper GI endoscopy, and/or at pathologic examination of gastric biopsy samples, presence of a large amount of bile in the gastric cavity at >1 endoscopic examination, excessive alkaline shifts (pH >7) at 24-hour esophageal pH monitoring, and DGR at technetium-99m diisopropyl iminodiacetic acid (99mTc-DISIDA) scintigraphy scanning. Patients operated on from December 1993 on (n = 41) were also investigated by 24-hour intragastric bile monitoring with the Bilitec device1,17 (Medtronic, Skovlunde, Denmark). Should the Bilitec test be normal, the patient was investigated further by overnight, intermittent aspiration of gastric juice through the lumen of a nasogastric catheter to detect the eventual presence of high-concentration lipase peaks in the stomach. Technetium-labeled gastric emptying study was performed in 21 patients.
The gastric lake was defined as bilious whenever a large amount of greenish-yellowish fluid was present in the stomach at upper GI endoscopy. Mucosal erythema, erosions, or ulcerations of the gastric wall were considered endoscopic signs of gastric inflammation. Pinch biopsy specimens were taken from the antral mucosa and standard hematoxylin-eosin sections were analyzed. Chemical gastritis was diagnosed according to the criteria of Dixon et al,18 [ie, elongation and tortuosity of the gastric glands (foveolar hyperplasia), interstitial edema, vascular congestion, paucity of chronic inflammatory cells, and prominence of smooth muscle cells in the lamina propria]. Chronic nonspecific gastritis was diagnosed when more lymphocytes and plasma cells, with or without neutrophils, were seen in the lamina propria. Antral biopsy specimens were also stained in search of Helicobacter pylori (Hp) organisms using Giemsa method. The Bilitec test was performed according to a study protocol published elsewhere.10 It was defined as abnormal when gastric bile exposure exceeded the 95th percentile of a set of 25 healthy volunteers investigated at the Saint-Luc Academic Hospital, Brussels, Belgium in 1 of the 3 study periods (ie, total, upright, or supine) at one of the following thresholds: 0.25, 0.30, 0.40, 0.50, and 0.60.10
Preoperative work-up data are summarized in Table 1.
TABLE 1. Symptoms, Signs, and Clinical Findings Supporting the Diagnosis of Pathologic DGR
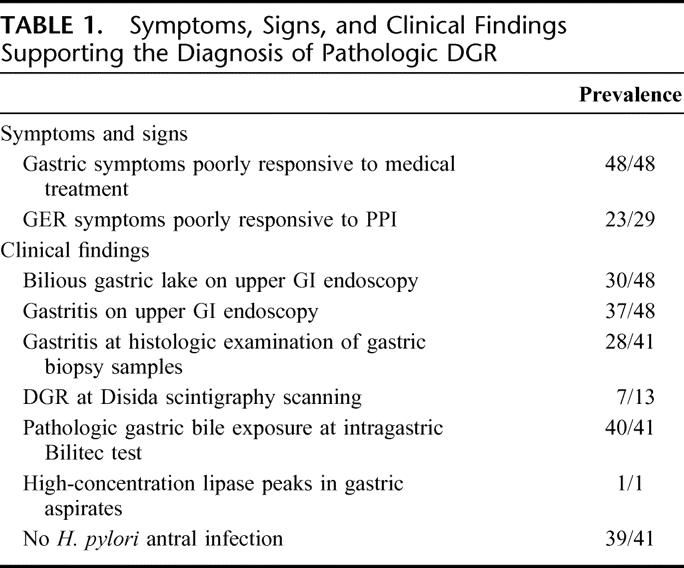
Thirty patients (62.5%) had a bilious gastric lake while 37 patients (77%) had endoscopic signs of gastritis (ie, mucosal erythema in 15, erosions in 8, and ulcerations in 14). Antral biopsies were analyzed in 41 patients showing chemical gastritis in 13 (32%), chronic nonspecific gastritis in 15 (36%), and no signs of gastritis in 13 (32%). Among the 13 patients with histologic signs of chemical gastritis, the number of Dixon's criteria18 was 1, 2, 3, 4, and 5 in 0, 2, 5, 5, and 1 patients, respectively. Antral Hp infection was absent in all but 2 of the 41 patients investigated. However, bacterial concentration in these 2 patients was low. Gastric exposure to bile (Fig. 2) was pathologic in 40 of the 41 patients investigated (97.6%). The only patient with a normal intragastric bile profile had high-concentration lipase peaks at overnight aspiration of gastric contents. Preoperative Bilitec test results in group I did not significantly differ from those in group II: the mean percentage of time that bile absorbance was above the 0.25 threshold was 25.7% versus 25.6% for the total period (P = 0.6816), 12.7% versus 17.4% for the upright period (P = 0.1314), and 41.6% versus 31.8% for the supine period (P = 0.2040). Gastric emptying at technetium-labeled gastric emptying study in 21 patients was delayed in 13 (61.9%), whereas it was normal in 8. No patient with delayed gastric emptying had a history of previous vagotomy.
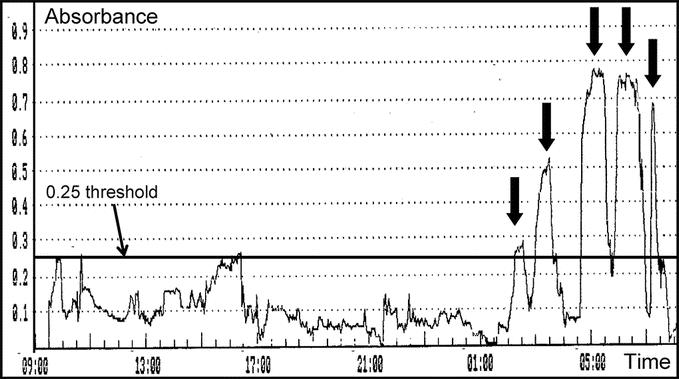
FIGURE 2. Intragastric bile monitoring tracing showing 5 nocturnal absorbance peaks of high amplitude (black arrows) attesting to the existence of pathologic gastric exposure to bile.
Surgical Procedure
The duodenal switch procedure was described by Tom Demeester 19 years ago13 (Fig. 1) and was taught by him and his coworker, Ron Hinder14 to the senior author (J.M.C.) at the Creighton University, Omaha, Nebraska in 1989. The procedure includes19,20 Kocher maneuver, stapling division and closure of the duodenum as far from the pylorus as possible (usually 3 to 4 cm more distally) but proximal to the papilla, reopening of the stapled proximal duodenal stump, and performance of a duodenojejunostomy between the proximal duodenum and the upper end of a 60-cm transmesocolic Roux-en-Y jejunal loop through an upper midline incision. In the presence of any abnormal adhesion between the proximal duodenum and pancreatic head, separation of the duodenum from the pancreatic head was performed very carefully bearing in mind possible early branching of the papilla or the presence of a Santorini duct, which enters the duodenal wall proximal to the papilla. Whenever finger identification of the papilla on the duodenum was difficult despite information given by preoperative cholangiography, the duodenum was opened at the end of its second segment for digital palpation of the duodenal mucosa or the common bile duct was catheterized through the cystic duct and cholecystectomy done. Division of the proximal duodenum was performed with maintenance of the lower small branches of the right gastric artery and vein to the duodenum to prevent intraoperative ischemia of the proximal duodenum and, subsequently, postoperative fistula. The staple line closing the distal duodenal stump was oversewn using an indwelling seroserosal continuous suture technique. The duodenojejunostomy was performed end-to-end using a single-layered extramucosal running suture technique with 3-0 nonabsorbable stitches. Omental fringes were interposed between the duodenojejunal anastomosis and the distal duodenal stump at the end of the operation, a maneuver that prevents the development of an internal fistula between the duodenojejunostomy and the distal duodenal stump. A nasogastric tube was left in the gastric cavity until resumption of bowel movements, and both gastric emptying and passage of a water-soluble contrast medium such as gastrografin through the anastomosis were checked roentgenographically before starting oral feeding again.
In practice, 29 patients, ie,18 of group I and 11 of group II, with concomitant pathologic gastroesophageal and DGR underwent combined fundoplication and duodenal switch operation during the same operating session while 19 patients underwent a duodenal switch operation alone. A cholecystectomy was performed in 3 patients, and 1 patient had a wedge gastric resection for an extramucosal tumor that revealed to be a leiomyosarcoma.
From 1997 on, omeprazole (20 mg daily) was given during a 2-month period after the operation to reduce exposure of the fresh suture line to gastric acid until completion of the mucosal healing process.
Postoperative Workup
Three months after the operation, upper GI endoscopy was performed in 44 patients (91.7%) to evaluate the presence of a bilious gastric lake and of residual signs of gastric inflammation (mucosal erythema, erosions, or ulcerations). Pinch biopsy specimens were taken from the antral mucosa for histologic examination in 33 of these 44 patients. Gastric exposure to bile was investigated using the 24-hour intragastric Bilitec test in 36 of the 41 patients operated on in the Bilitec era. Six patients with preoperative delayed gastric emptying had postoperative technetium-labeled gastric scintigraphy scanning. Four of them had had concomitant duodenal switch and fundoplication.
Long-term Interview
For the purpose of the present study, all the 48 patients were interviewed by the first author (P.S.), a resident coming from Turin, Italy, who acted as an independent observer. Patients were asked for the presence of residual gastric symptoms8 (epigastric pain, nausea, and bilious vomiting) according to the same symptom score as the one used prior to the operation.
Statistical Analysis
Categorical variables were analyzed using Fisher exact or χ2 tests, and continuous variables using Mann-Whitney U and Kruskal-Wallis nonparametric rank sum tests. A P value <0.05 was considered statistically significant.
RESULTS
Intraoperative Course
Ischemia of the proximal duodenum after duodenal division developed in 1 patient secondary to inadvertent division of the terminal duodenal rami of the right gastric vessels. The proximal duodenal stump was resected and the anastomosis performed between the duodenal aspect of the pylorus and the upper end of the Roux-en-Y jejunal loop.
Identification of the papilla by simple digital palpation of the duodenal wall was impossible in 6 patients. This led to opening of the duodenal wall for internal palpation of the papilla in 3 patients and to transcystic catheterization of the common bile duct with subsequent cholecystectomy in 3 others.
Postoperative Radiologic Outcome
Five to seven days after the operation, gastric emptying of the contrast medium was delayed in 6 patients (ie, 3 of group I and 3 of group II) so that prolonged gastric aspiration was needed. An asymptomatic minute blind fistula from the duodenojejunostomy was diagnosed in 1 patient.
Symptomatic Outcome
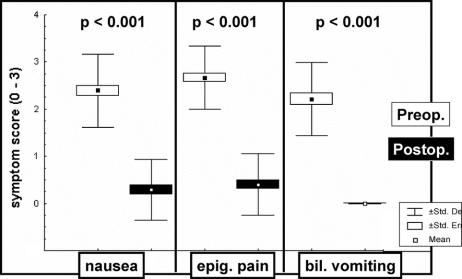
After a follow-up period ranging from 3 to 174 months (median, 81 months), 29 patients (60.4%) had no residual gastric symptoms, preoperative gastric symptoms were improved in 16 patients (33.3%), 2 patients (4.2%) were not improved at all, and 1 patient had experienced symptomatic recurrence after a 92-month symptom-free period (2.1%). These percentages in the 28 patients of group I and the 20 patients of group II were 60.7% versus 60%, 32.1% versus 35%, 3.6% versus 5%, and 3.6% versus 0%, respectively (P > 0.05). As shown Figure 3, the score for each symptom (ie, nausea, epigastric pain, and bilious vomiting) was significantly (P < 0.001) lower at follow-up than prior to the operation. Dumping symptoms (ie, postprandial diarrhea and sweating) that did not exist before the operation had developed in one patient. Body weight (mean ± SEM) was 67.0 ± 1.9 kg preoperatively versus 68.6 ± 1.8 kg at follow-up (P = 0.091). Forty-four patients (91.7%) were satisfied with the operation so that they would make the decision of an operation again. Four patients (8.3%) were dissatisfied because of the acquisition of dumping symptoms (n = 1), the persistence of disabling gastric symptoms (n = 2), or the recurrence of gastric symptoms at long-term follow-up. In the latter patient, symptomatic recurrence was substantiated by the reappearance of a pathologic exposure of the gastric mucosa to bile with the percentage of total time that bile absorbance exceeded the threshold of 0.25 increasing from 2% in the immediate postoperative period to 29.4% 92 months postoperatively. All this led to reoperation of the patient so that the 60-cm Roux-en-Y jejunal loop was lengthened to 110 cm21 with subsequent symptomatic relief.
FIGURE 3. Gastric symptom score before (blank boxes) and after (black boxes) duodenal switch operation (left: nausea; center: epigastric pain; right: bilious vomiting).
Endoscopic Outcome
Upper GI endoscopy performed 3 months postoperatively showed no bile in the gastric cavity of any patient. No gastric lesion was found in 42 among the 44 patients (95.5%) investigated, whereas there still was antral erythema in 1 patient and antral erosions in another. An ulcer was found at the level of the duodenojejunostomy in 5 patients (11.3%), ie, 3 of group I and 2 of group II, all belonging to our early experience. Actually, 2 patients were readmitted to the hospital for upper GI bleeding in relation to an ulcer that had developed at the level of the duodenojejunal suture line. Bleeding stopped spontaneously and PPI therapy (omeprazole, 40 mg daily) was initiated. However, both patients were reoperated on by proximal gastric vagotomy a few months later because of the persistence of the anastomotic ulcer in spite of continuous PPI intake. In retrospect, 1 of these 2 patients had an asymptomatic minute blind fistula from the duodenojejunostomy at early postoperative upper GI series. The 3 other patients had an asymptomatic ulcer that was no longer observed at an endoscopic control made after 2-month PPI therapy.
Among the 22 patients with preoperative gastritis at histology who had postoperative gastric biopsies, histology made 3 months postoperatively had normalized in 15 patients, whereas chemical gastritis and nonspecific chronic gastritis were found in 0 and 7 patients, respectively.
Intragastric Bile Monitoring Outcome
Postoperative Bilitec test was performed in 36 patients, 23 belonging to group I and 13 to group II. Exposure of the gastric mucosa to bile at 24-hour gastric bile monitoring was nil in 15 patients (41.7%) whereas 21 patients (58.3%) still had bile in their gastric cavity. In the latter instance, bile exposure remained within the range of controls in all but 2 patients. These 2 patients were asymptomatic and had no residual gastritis, but exposure of their gastric mucosa to bile still was just above the 95th percentile of controls. In 1 of these 2 patients, a second Bilitec test performed 27 months postoperatively was similar to the one done just after the operation. No fistula between the duodenojejunostomy and the distal duodenal stump was observed on contrast medium series.
As shown in Figure 4, postoperative gastric exposure to bile was significantly (P < 0.001) lower after than before the operation in the 3 periods of recording. The mean percentage of time that bile absorbance was above the 0.25 threshold dropped from 25.64% to 3.09% for the total period, from 14.65% to 1.54% for the upright period, and from 37.53% to 4.45% for the supine period.
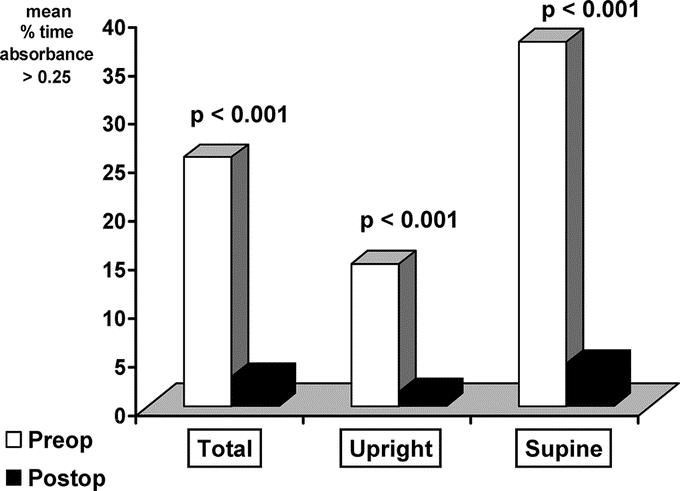
FIGURE 4. Exposure of the gastric mucosa to bile before (blank columns) and after (black columns) duodenal switch operation expressed as the percentage of time that bile absorbance >0.25 for the total (left), upright (center), and supine (right) periods of recording.
Gastric Emptying Outcome
Three months after operation, delayed gastric emptying improved substantially in 4 of the 6 patients who underwent both preoperative and postoperative technetium-labeled gastric emptying scintigraphy scanning whereas it didn't in 2. Three of the 4 patients with a speeding of gastric emptying had had concomitant duodenal switch and fundoplication.
DISCUSSION
The study confirms that a subset of patients, among those numerous patients who seek consultation for so-called dyspeptic symptoms,8,10 has the pathologic presence of duodenal juice in the gastric lumen, a condition that is best revealed by 24-hour intragastric bile monitoring with the Bilitec device.1,17 Dyspeptic patients who are at the highest risk for the presence of pathologic gastric exposure to duodenal juice are those having no Hp infection in the antrum. We know indeed that the 2 phenomena are mutually exclusive,10 owing to the fact that bile acids inhibit both the in vitro and in vivo growth of the bacterium.22–24 This is confirmed in the present study where 39 of the 41 patients who were checked for the presence of Hp in the antrum were Hp negative, the 2 remaining patients having rare bacteria evidenced only. In any event, nonperformance of the intragastric Bilitec test in dyspeptic patients without Hp organisms in the antrum enables the clinician to apprehend the relation between symptoms and DGR. Inaccurate diagnosis together with the relative resistance of these symptoms to prokinetics and antacids explain the usually long (ie, as long as 600 months in 1 of our patients) clinical course of these patients. Even, some of them (ie, 3 in 8 in the present series) may be told having a psychologic disturbance rather than an organic disorder according to the well-known principle that obscurantism starts where science ends. It thus appears that dyspeptic patients with a long history of gastric symptoms poorly responsive to optimal medical treatment, with pathologic antral exposure to bile at 24-hour intragastric bile monitoring, and without Hp antral infection should be considered for a duodenal switch operation.
Diversion of duodenal juice from the gastric cavity in such patients can afford substantial symptomatic and objective improvement. Indeed, more than 9 patients in 10 experienced a good symptomatic relief as attested to by the dramatic drop in the symptom score recorded in the long-term. This confirms Demeester's initial data13 and those from Klingler,20 the latter reported a 94% clinical success rate in a series of 32 operations. Unlike those operated on by a classic duodenal diversion procedure, including vagotomy and distal gastrectomy,25,26 very few patients acquire disabling symptoms such as postprandial sweating or diarrhea after the operation, probably because the duodenal switch operation maintains the gastric cavity, pyloric sphincter, and vagus nerves intact.16 Moreover, the minor effect of the procedure on the global functioning of the alimentary tract is suggested by the long-term maintenance of the preoperative body weight.
As early as 3 months after the operation, endoscopic gastritis had disappeared in almost all patients, and no patient had residual chemical gastritis at histology. Patients with nonspecific gastritis emphasize the need for the redefinition of so-called chemical gastritis in the light of Bilitec data. Indeed, the histologic pattern we now name “chemical gastritis” was described by Dixon et al 20 years ago18 from patients with a history of previous gastrectomy promoting the reflux of a huge amount of jejunal juice into the residual stomach. In this respect, unpublished personal data (Collard J-M. Jejunogastric bile reflux after B II gastrectomy. Personal communication, September 2003) indicate that, compared with jejunogastric bile reflux we have observed after BII gastrectomy, transpyloric DGR in patients with an intact stomach is much less important so that histologic changes, although still to be defined, are probably more subtle than the classic pattern.
Improvement of gastric symptoms and lesions after duodenal switch operation correlates with the dramatic drop in gastric exposure to bile at the postoperative Bilitec test compared with preoperative results. In two thirds of patients, however, there was normalization to physiologic values rather than complete abolishment of gastric exposure to bile. This is not surprising, bearing in mind that a long Roux-en-Y loop does not necessarily oppose any reflux of jejunal juice to the organ situated more proximally,27,28 but usually protects this organ against pathologic exposure to jejunal contents, so that reflux symptoms and lesions are unlikely. Long-term reappearance of gastric symptoms and lesions parallel to alteration of the Bilitec test results achieved just after the operation in 1 patient confirms our previous observation29 that in some rare patients, the functioning of the Roux-en-Y loop may deteriorate with time, without any rational explanation given to date. In such an instance, lengthening of the already long Y-loop by an additional length of 50 cm21,29 has been shown to be effective on the reflux of jejunal juice to the more proximal organ with, as was observed in this particular patient, subsequent symptomatic relief.
Little is known about the motor and secretory disorders that underlie pathologic exposure of the stomach to bile. The study indicates that a substantial number of patients without any history of previous vagotomy have primary delayed gastric emptying, a condition that may impair the re-expulsion of duodenal juice from the stomach to the duodenum.16 Postoperative speeding of gastric emptying, as was observed in 4 of the 6 patients investigated, is likely due to the adjunction in 3 of them of a fundoplication, a procedure that was shown by Hinder et al30 to improve gastric emptying. However, we have to be cautious with the interpretation of technetium test results in DGR patients because postprandial gastric emptying does not reflect what happens at night, ie, when bile is the most often found in the stomach. In any event, further functional studies are needed to determine the respective implication of the duodenum, pylorus, and stomach in the genesis of DGR.
The main drawback of the duodenal switch procedure is the risk for the development of an ulcer at the level of the duodenojejunostomy13 early after the operation. This was observed in 5 of our 48 patients. As no patient developed any ulcerative lesion in the long term, early ulcers are likely related to direct contact between acidic gastric outflow and the fresh postpyloric suture line. Although gastric acid output is not significantly increased after a duodenal switch operation,31 immediate administration of PPI for a 2-month period, ie, until completion of the mucosal healing process, should be recommended.
Achievement of a satisfactory postoperative outcome requires the observance of some technical rules during the operation.19 The most critical step is the isolation of the duodenal conduit from the head of the pancreas a few centimeters distal to the pylorus. This maneuver may be facilitated checking the anatomy of the common bile duct roentgenographically prior to the operation to identify patients having proximal duodenal implantation of the papilla. Another critical step is the maintenance of an adequate blood supply to the proximal duodenal stump, not dividing the terminal rami of the right gastric vessels to the postpyloric region because of the existence of a parietal vascular barrier at the pylorus. A third important step is the interposition of omental fringes between the duodenojejunal anastomosis and the suture line on the distal duodenal stump to prevent mutual fistulization, a situation that would bypass the antireflux procedure. In addition, maintenance of as long a proximal duodenal stump as possible has been recommended13 to allow adequate functioning of the local neurohormonal mechanisms.32,33
Footnotes
Reprints: Jean-Marie Collard, MD, PhD, Unit of Upper Gastrointestinal Surgery, Saint Luc Academic Hospital, Hippocrate Avenue 10, B-1200, Brussels, Belgium. E-mail: collard@chir.ucl.ac.be.
REFERENCES
- 1.Bechi P, Pucciani F, Baldini F, et al. Long-term ambulatory enterogastric reflux monitoring: validation of a new fiberoptic technique. Dig Dis Sci. 1993;38:1297–1306. [DOI] [PubMed] [Google Scholar]
- 2.Byrne JP, Romagnoli R, Bechi P, et al. Duodenogastric reflux of bile: a range of normal values in healthy controls using the Bilitec 2000. Physiol Meas. 1999;20:149–158. [DOI] [PubMed] [Google Scholar]
- 3.Romagnoli R, Collard J-M, Serra A-M, et al. Is the DGR Bilitec® profile different in GERD patients with and without Barrett's esophagus? In: Giuli R, Scarpignato C, Collard J-M, et al. Eds. The Duodenogastroesophageal reflux. Paris: John Libbey, 2006;445–449. [Google Scholar]
- 4.Mabrut JY, Romagnoli R, Collard J-M, et al. Familial adenomatous polyposis predisposes to pathological exposure of the stomach to bilirubin. Surgery. In press. [DOI] [PubMed]
- 5.Fuchs KH, Maroske J, Fein M, et al. Variability in the composition of physiologic duodenogastric reflux. J Gastrointest Surg. 1999;3:389–396. [DOI] [PubMed] [Google Scholar]
- 6.Gowen GF. Spontaneous enterogastric reflux gastritis and esophagitis. Ann Surg. 1985;201:170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warshaw AL. Bile gastritis without previous gastric surgery: contributing role of cholecystectomy. Am J Surg. 1979;137:527–531. [DOI] [PubMed] [Google Scholar]
- 8.Ritchie WP. Alkaline reflux gastritis: an objective assessment of its diagnosis and treatment. Ann Surg. 1980;192:288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermans D, Buts JP, Collard JM, et al. Primary duodenogastric reflux in children and adolescents. Eur J Pediatr. 2003;162:598–602. [DOI] [PubMed] [Google Scholar]
- 10.Romagnoli R, Collard JM, Bechi P, et al. Gastric symptoms and duodenogastric reflux in patients referred for gastroesophageal reflux symptoms and endoscopic esophagitis. Surgery. 1999;125:480–486. [PubMed] [Google Scholar]
- 11.Meshkinpour H, Elashoff J, Steward H, et al. Effect of cholestyramine on the symptoms of reflux gastritis: a randomized, double-blind, crossover study. Gastroenterology. 1977;73:441–443. [PubMed] [Google Scholar]
- 12.Buch KL, Weinstein WM, Hill TA, et al. Sucralfate therapy in patients with symptoms of alkaline reflux gastritis: a randomized, double-blind study. Am J Med. 1985;79:49–54. [DOI] [PubMed] [Google Scholar]
- 13.Demeester TR, Fuchs KH, Ball CS, et al. Experimental and clinical results with proximal end-to-end duodenojejunostomy for pathologic duodenogastric reflux. Ann Surg. 1987;206:414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinder RA. Duodenal switch: a new form of pancreatico-biliary diversion. Surg Clin North Am. 1992;72:487–499. [DOI] [PubMed] [Google Scholar]
- 15.Fountos A, Chrysos E, Tsiaoussis J, et al. Duodenogastric reflux after biliary surgery: scintigraphic quantification and improvement with erythromycin. Aust NZ J Surg. 2003;73:400–403. [DOI] [PubMed] [Google Scholar]
- 16.Gutschow C, Collard JM, Romagnoli R, et al. Bile exposure of the denervated stomach as an esophageal substitute. Ann Thorac Surg. 2001;71:1786–1791. [DOI] [PubMed] [Google Scholar]
- 17.Romagnoli R, Collard J-M, Bechi P, et al. What is the in vivo reliability of the Bilitec® sensor for the detection of duodenal reflux through the foregut? In: Giuli R, Scarpignato C, Collard J-M, et al. Eds. The Duodenogastroesophageal reflux. Paris: John Libbey, 2006;98–104. [Google Scholar]
- 18.Dixon MF, O'Connor HJ, Axon ATR, et al. Reflux gastritis: distinct histopathological entity? J Clin Pathol. 1986;39:524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collard J-M, Michel J-M, Romagnoli R. et al. What are the side effects of duodenal switch? In: Giuli R, Scarpignato C, Collard J-M, et al. Eds. The Duodenogastroesophageal reflux. Paris: John Libbey, 2006;414–420. [Google Scholar]
- 20.Klingler PJ, Perdikis G, Wilson P, et al. Indications, technical modalities and results of the duodenal switch operation for pathologic duodenogastric reflux. Hepatogastroenterology. 1999;46:97–102. [PubMed] [Google Scholar]
- 21.Michel JM, Dierieck V, Romagnoli R, et al. Oesophagite par reflux jejuno-oesophagien après gastrectomie totale et anse de Roux en Y. Gastroenterol Clin Biol. 2001;25:811–813. [PubMed] [Google Scholar]
- 22.O’ Connor HJ, Wyatt JI, Dixon MF, et al. Campylobacter like organisms and reflux gastritis. J Clin Pathol. 1986;39:531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathai E, Arora A, Cafferkey M, et al. The effect of bile acids on the growth and adherences of Helicobacter pylori. Aliment Pharmacol Ther. 1991;5:653–658. [DOI] [PubMed] [Google Scholar]
- 24.Han SW, Evans DG, El-Zaatari FA, et al. The interaction of pH, bile and Helicobacter pylori may explain duodenal ulcer. Am J Gastroenterol. 1996;1991:1135–1137. [PubMed] [Google Scholar]
- 25.Perniceni T, Gayet B, Fekete F. Total duodenal diversion in the treatment of complicated peptic oesophagitis. Br J Surg. 1988;75:1108–1111. [DOI] [PubMed] [Google Scholar]
- 26.Bonavina L, Incarbone R, Segalin A, et al. Duodeno-gastro-esophageal reflux after gastric surgery: surgical therapy and outcome in 42 consecutive patients. Hepatogastroenterology. 1999;46:92–96. [PubMed] [Google Scholar]
- 27.Collard JM, Romagnoli R. Roux-en-Y jejunal loop and bile reflux. Am J Surg. 2000;179:298–303. [DOI] [PubMed] [Google Scholar]
- 28.Mabrut J-Y, Collard J-M, Romagnoli R, et al. Oesophageal and gastric bile exposure after gastroduodenal surgery with Henley's interposition or a Roux-en-Y loop. Br J Surg. 2004;91:580–585. [DOI] [PubMed] [Google Scholar]
- 29.Strignano P, Collard J-M, Romagnoli R, et al. Can Barrett's oesophagus develop after total gastrectomy and construction of a long Roux-en-Y jejunal loop? In: Giuli R, Scarpignato C, Collard J-M, et al. Eds. The Duodenogastroesophageal reflux. Paris: John Libbey, 2006;386–391. [Google Scholar]
- 30.Hinder RA, Stein HJ, Bremner CG, et al. Relationship of a satisfactory outcome to normalization of delayed gastric emptying after Nissen fundoplication. Ann Surg. 1989;210:458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welch NT, Yasui A, Kim CB, et al. Effect of duodenal switch procedure on gastric acid production, intragastric pH, gastric emptying, and gastrointestinal hormones. Am J Surg. 1992;163:37–44. [DOI] [PubMed] [Google Scholar]
- 32.Wilson P, Welch NT, Hinder RA, et al. Abnormal plasma gut hormones in pathologic duodenogastric reflux and their response to surgery. Am J Surg. 1993;165:169–177. [DOI] [PubMed] [Google Scholar]
- 33.Takada T, Yasuda H, Shikata JI, et al. Postprandial plasma gastrin and secretin concentrations after a pancreaticoduodenostomy. Ann Surg. 1989;210:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]