Abstract
Purpose:
Anastomotic leaks are among the most dreaded complications after colorectal surgery. However, problems with definitions and the retrospective nature of previous analyses have been major limitations. We sought to use a prospective database to define the true incidence and presentation of anastomotic leakage after intestinal anastomosis.
Methods:
A prospective database of two colorectal surgeons was reviewed over a 10-year period (1995–2004). The incidence of leak by surgical site, timing of diagnosis, method of detection, and treatment was noted. Complications were entered prospectively by a nurse practitioner directly involved in patient care. Standardized criteria for diagnosis were used. A logistic regression model was used to discriminate statistical variation.
Results:
A total of 1223 patients underwent resection and anastomosis during the study period. Mean age was 59.1 years. Leaks occurred in 33 patients (2.7%). Diagnosis was made a mean of 12.7 days postoperatively, including four beyond 30 days (12.1%). There was no difference in leak rate by surgeon (3.6% vs. 2.2%; P = 0.08). The leak rate was similar by surgical site except for a markedly increased leak rate with ileorectal anastomosis (P = 0.001). Twelve leaks were diagnosed clinically versus 21 radiographically. Contrast enema correctly identified only 4 of 10 leaks, whereas CT correctly identified 17 of 19. A total of 14 of 33 (42%) patients had their leak diagnosed only after readmission. Fifteen patients required fecal diversion, whereas 18 could be managed nonoperatively.
Conclusions:
Anastomotic leaks are frequently diagnosed late in the postoperative period and often after initial hospital discharge, highlighting the importance of prospective data entry and adequate follow-up. CT scan is the preferred diagnostic modality when imaging is required. More than half of leaks can be managed without fecal diversion.
A prospective database was reviewed to determine the true incidence of anastomotic leak. A total of 1223 patients underwent resection and anastomosis during the study period. Leaks were frequently diagnosed late in the postoperative period, often after initial discharge (42%).
Anastomotic disruption is perhaps the most dreaded complication after intestinal surgery. Some leaks presents in a dramatic fashion early in the postoperative period, leaving little doubt about the diagnosis. However, many others present in a far more subtle fashion, often relatively late in the postoperative period, and can be difficult to distinguish from other postoperative infectious complications.
It is this latter scenario where definitions are critical and retrospective analyses become rather suspect. In this light, it is not surprising that the reported incidence of anastomotic leakage varies so dramatically in the literature.1–7 The aim of this study was to use a rigorous, prospectively maintained complication database to more accurately define the incidence and presentation of anastomotic leakage after intestinal anastomosis.
METHODS
All patients undergoing laparotomy, bowel resection, and anastomosis by 2 board-certified colon and rectal surgeons at Fletcher Allen Health Care (the teaching hospital of the University of Vermont) from January 1, 1995 to December 30, 2004 were entered into a prospectively maintained database. Patients were followed daily throughout their hospitalization and complications were recorded by a single nurse practitioner who was a member of the surgical team and rounded with housestaff. A follow-up data form was completed at the time of follow-up in the outpatient clinic to record any readmissions or complications after initial hospital discharge. All recorded complications were reviewed by a panel of gastrointestinal surgeons on a monthly basis to assure appropriate classification and standardization of definitions.
After completion of the study period, the datasheets of all patients who underwent a laparotomy, resection and intestinal anastomosis (CPT codes 44120, 44125, 44140, 44145, 44150, 44153, 44160, 44626, 45119) were scrutinized for complications. The hospital records and clinical charts were retrospectively reviewed for any patients in this group who were listed as having developed an anastomotic leak; to ensure completeness, the charts of those patients who were noted to have had a postoperative enterocutaneous fistula or abscess were also reviewed.
Patients who underwent simple stoma closure or had their anastomosis protected by a proximal diversion were excluded from analysis. Patients who were transferred from outlying hospitals with a leak, abscess or fistula were also excluded unless they redeveloped the complication after surgery at our institution. All postoperative fistulas communicating with the surgical anastomosis were reclassified as a leak. Postoperative abscesses were reclassified as a leak if there was extravasation of enteric contrast on an imaging study, there was significant perianastomotic air, or communication with the anastomosis was noted after radiologic drainage.
Demographics (age, gender), operating surgeon, and anastomotic category (enteroenteric, colocolic, ileocolic, colorectal, ileorectal, ileoanal, or coloanal) were recorded. The postoperative day the leak was diagnosed was noted along with the primary method of diagnosis (clinical or radiologic). The results of any radiologic studies were recorded as was patient outcome (ie, requirement for fecal diversion, death).
Differences in the leak rate by anastomotic location and surgeon were assessed using a logistic regression model and then compared using Fisher exact test. A two-tailed Student t test was used to compare the presentation of those patients who required fecal diversion to those who did not. Fisher exact test was used to compare the positive predictive value of computed tomography scanning (CT) to contrast enema.
RESULTS
A total of 1223 patients underwent an intestinal resection and anastomosis without fecal diversion during the study period. Seventy-four patients (6.1%) had at least one additional anastomosis performed. Thirty-three patients (2.7%) developed an anastomotic leak, including 17 males and 16 females. The mean age of patients with a leak was 59.1 years (range, 27–88 years). Outpatient follow-up complication data were available in 96% of discharged patients.
The incidence of clinical leakage by location of anastomosis and surgeon is displayed in Table 1. There was no difference in the leak rate by surgeon (3.6% vs. 2.2%, P = 0.08). Although the leak rate by an anastomotic location was generally quite similar (Fig. 1), there was a dramatic increase in the anastomotic leak rate for ileorectal anastomosis (23.3%, P = 0.001).
TABLE 1. Incidence of Clinical Leaks by Type of Anastomosis
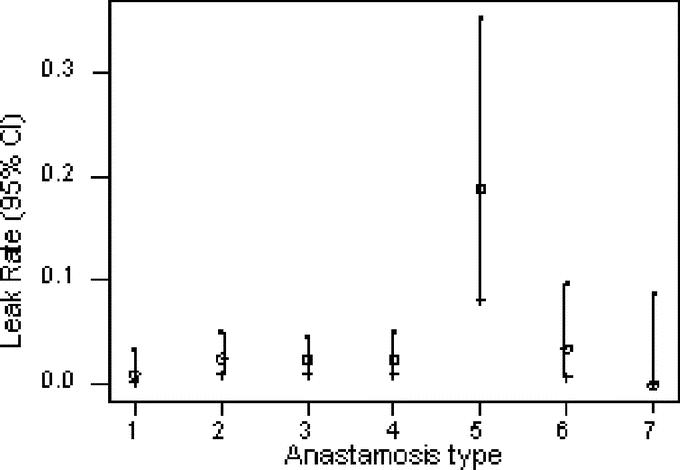
FIGURE 1. Leak rate by anastomotic type (see Table 1 for key).
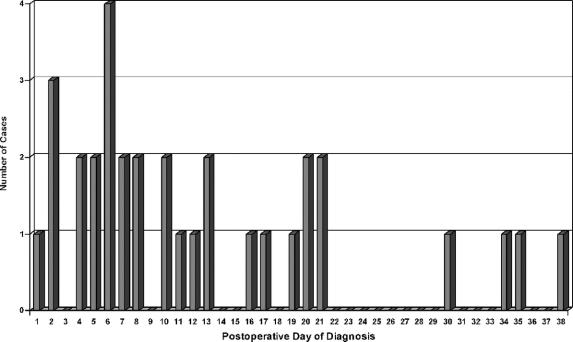
Overall, leaks were diagnosed at a mean of 12.7 days postoperatively (Fig. 2). Twelve of the 33 leaks were diagnosed clinically at a mean of 7.0 days postoperatively (range, 1–19 days). The remaining 21 were diagnosed radiologically at a mean of 16.0 days postoperatively. Contrast enema was obtained in 10 cases; in 4 cases, the leak was observed but in 6 cases, the test was falsely negative. Four of these 6 patients with false-negative contrast enemas had the diagnosis made on CT scan and 2 on clinical grounds.
FIGURE 2. Leak by day of diagnosis.
CT scans were obtained in 19 cases. The leak was correctly diagnosed in 17 instances (89.5%), but 2 scans were falsely negative. Both of these patients went on to have positive contrast enemas. The positive predictive value of CT scan was superior to contrast enema (89.5% vs. 40%, P = 0.009), but these tests were often applied in somewhat different clinical circumstances in this observational study.
Fifteen of 33 patients (45.5%) required fecal diversion; these patients were diagnosed at a mean of 9.6 days postoperatively (range, 1–38 days). Eighteen patients were able to be managed nonoperatively (typically with radiologic drainage and antibiotics); these patients were diagnosed at a mean of 15.2 days postoperatively (range, 2–35 days). Two patients died (5.7%), both of whom had been treated with fecal diversion. Interestingly, 14 of 33 patients (42%) were diagnosed upon readmission to the hospital, and 4 of 33 (12%) had the leak diagnosed 30 days or more after their initial surgery.
DISCUSSION
Surgeons are all too familiar with the potentially devastating consequences of an anastomotic leak. Patients classically develop agonizing abdominal pain, tachycardia, high fevers, and a rigid abdomen, often accompanied by hemodynamic instability. In these cases, urgent return to the operating room for peritoneal washout and fecal diversion is generally required; prolonged stays in the intensive care unit and death are not uncommon. The mortality rate for an anastomotic leak in the literature typically is in the 10% to 15% range.8–11 Further, anastomotic leakage has been associated with increased local recurrence and diminished survival after colorectal cancer surgery.12,13
However, a large number of patients ultimately found to have an anastomotic leak develop a more insidious presentation, often with low-grade fever, prolonged ileus, or failure to thrive.14 In these patients, making the diagnosis may be much more difficult as the clinical course is often similar to other postoperative infectious complications. Radiologic imaging is usually required; even then, the diagnosis may be elusive or at least uncertain.
We think that this latter group of patients often escapes detection when retrospective analyses are performed, thereby grossly underestimating the true incidence of anastomotic leakage after intestinal anastomosis. These patients are often discharged from the hospital without the correct diagnosis in the present environment of cost containment as their nonspecific symptoms (ie, poor appetite, failure to thrive) are not enough to “justify” continued hospitalization (ie, “he'll do better at home”). Forty-two percent of the patients in this series with a leak had been sent home from the hospital; they may not have been identified from a traditional retrospective audit, as most studies use hospital discharge as a study endpoint.
Distinguishing an “anastomotic leak” from a postoperative abscess, especially retrospectively, can be very difficult. Unless concomitant review is undertaken of patients classified as having a postoperative abscess, some leaks will be missed. In many cases in our database, we were able to show that a postoperative abscess was caused by a small anastomotic leak.
Although the literature is replete with studies that specify a rate of anastomotic leakage, it is seldom possible to know what constitutes a “leak.” Bruce et al performed a systematic review of studies measuring the incidence of anastomotic leaks after gastrointestinal surgery; in the 97 studies reviewed, there were a total of 56 separate definitions of anastomotic leak.15 A leak may be defined by the need for reoperation, clinical findings, or radiologic criteria, making comparisons between studies difficult or impossible. Further, there is typically a “cutoff” at 30 days postoperatively and/or hospital discharge for diagnosis, which will fail to capture many leaks, as our study clearly shows.
We think that our large prospective database, maintained by an independent nurse practitioner with concomitant review by a surgical panel, provides a far more accurate representation of the true incidence and presentation of an anastomotic leak. Compared with other series, we likely included a number of patients with small contained leaks, which tend to present later in the clinical course and can often be treated without fecal diversion.16 Our low mortality rate (5.7%) likely represents a much higher representation of small leaks, although improvements in critical care may also have played a role.
CT scanning appears to be far more helpful than contrast enema in the radiologic diagnosis of a leak. Contrast enemas failed to identify the leak 60% of the time. However, in the 2 cases where the CT scan was negative and a leak was suspected, the contrast enema successfully diagnosed the leak suggesting a complimentary nature of these diagnostic tests. Although the numbers are small, CT scan does appear to be the radiologic procedure of choice to diagnose an anastomotic leak after intestinal surgery when clinical findings alone are insufficient.
Although our leak rate (2.7%) compares favorably to the published literature, we were surprised and disturbed by the strikingly higher rate of anastomotic leak after ileorectal anastomosis. Even though the total number of patients in this subgroup was relatively small (30), the increase was highly significant. All 7 leaks occurred in patients who had an anastomosis constructed with a circular stapler using the double-stapled technique; 5 of the patients had subtotal colectomy for multiple synchronous neoplasms and 2 for Crohn's disease. The reason for our poor performance in this subgroup is uncertain, although subtotal colectomy has previously been associated with an increased leak rate.17 We wonder if the terminal ileum in some patients may be too narrow and/or thin walled for the atraumatic placement of the anvil of the circular stapler and plan to change our anastomotic technique in this subgroup.
We were also impressed by the marked similarity in leak rates among the other locations, which ranged from 0.9% to 3.5%. Higher leak rates are typically reported for low pelvic anastomoses or anastomoses to the anal canal.18–23 The leak rates for colorectal and coloanal anastomosis were 2.5% and 0%, respectively, in this series. However, it is important to note that patients who had a proximal diverting stoma were excluded from analysis in this series. Nonetheless, our results suggest that a low leak rate can be achieved in properly selected patients.
Although we think that our database and methodology offer important advantages over previous studies looking at an anastomotic leakage, we still could have missed a small number of leaks. Patients who develop an anastomotic leak treated at another institution may have escaped our detection. However, University of Vermont/Fletcher Allen Healthcare is the only tertiary care hospital serving a large geographic area; it seems unlikely that these complications were treated at outlying community hospitals. Further, since our database included outpatient follow-up, we had an additional opportunity to capture complications treated in other settings. Another confounding factor is differentiating between “simple” postoperative abscesses and an anastomotic leak in selected instances. Although we tried to use rigorous clinical and radiologic criteria, there are some cases in which the distinction cannot be made with absolute certainty.
CONCLUSION
We think that prospective data collection is required to more accurately determine the true incidence and presentation of anastomotic leakage. Many of these leaks are diagnosed late in the postoperative period, commonly after discharge from the hospital. Increased awareness of these more subtle leaks may allow for more timely diagnosis and treatment. Further, a prospective database with ongoing peer review allows for meaningful comparison of outcomes as definitions can be standardized and opportunities for improvement may be identified and targeted.
Footnotes
Reprints: Neil Hyman, MD, Dept. of Surgery, Fletcher 464, University of Vermont College of Medicine, 89 Beaumont Ave., Burlington, VT 05405. E-mail: Neil.Hyman@vtmednet.org.
REFERENCES
- 1.Golub R, Golub RW, Cantu R, et al. A multivariate analysis of factors contributing to leakage of intestinal anastomosis. J Am Coll Surg. 1997;184:364–372. [PubMed] [Google Scholar]
- 2.Detry RJ, Karteuser A, Delriviere L, et al. Use of the circular stapler in 1000 consecutive colorectal anastomoses: experience of one surgical team. Surgery. 1995;117:140–145. [DOI] [PubMed] [Google Scholar]
- 3.Mileski WJ, Joehl RJ, Rege RV, et al. Treatment of anastomotic leakage following low anterior colon resection. Arch Surg. 1988;123:968–971. [DOI] [PubMed] [Google Scholar]
- 4.Hansen O, Schwenk W, Hucke HP, et al. Colorectal stapled anastomoses: experiences and results. Dis Colon Rectum. 1996;39:30–36. [DOI] [PubMed] [Google Scholar]
- 5.Jex RK, Van Hcerden JA, Wolff BG, et al. Gastrointestinal anastomoses: factors affecting early complications. Ann Surg. 1992;206:138–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Max E, Sweeney WB, Bailey HR, et al. Results of 1,000 single-layer continuous polypropylene intestinal anastomoses. Am J Surg. 1991;162:461–467. [DOI] [PubMed] [Google Scholar]
- 7.Nesbakken A, Nygaard K, Lunde OC. Outcome and late functional results after anastomotic leakage following mesorectal excision for rectal cancer. Br J Surg. 2001;88:400–404. [DOI] [PubMed] [Google Scholar]
- 8.Docherty JG, McGregor JR, Akyol AM, et al. Comparison of manually constructed and stapled anastomoses in colorectal surgery: West of Scotland and Highland Anastomosis Study Group. Ann Surg. 1995;221:176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fingerhut A, Elhadad A, Hay JM, et al. Infraperitoneal colorectal anastomosis: hand-sewn versus circular staples. A controlled clinical trial: French Associations for Surgical Research. Surgery. 1994;116:484–490. [PubMed] [Google Scholar]
- 10.Fingerhut A, Hay JM, Elhadad A, et al. Supraperitoneal colorectal anastomosis: hand-sewn versus circular staples. A controlled clinical trial: French Associations for Surgical Research. Surgery. 1995;118:479–485. [DOI] [PubMed] [Google Scholar]
- 11.Bokey EL, Chapuis PH, Fung C, et al. Postoperative morbidity and mortality following resection of the colon and rectum for cancer. Dis Colon Rectum. 1995;38:480–487. [DOI] [PubMed] [Google Scholar]
- 12.Walker KG, Bell SW, Rickard MJ, et al. Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg. 2004;240:255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McArdle CS, McMillan DC, Hole DJ. Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br J Surg. 2005;92:1150–1154. [DOI] [PubMed] [Google Scholar]
- 14.Pickleman J, Watson W, Cunningham J, et al. The failed gastrointestinal anastomosis: an inevitable catastrophe? J Am Coll Surg. 1999;188:473–482. [DOI] [PubMed] [Google Scholar]
- 15.Bruce J, Krukowski ZH, Al-Khairy G, et al. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg. 2001;88:1157–1168. [DOI] [PubMed] [Google Scholar]
- 16.Alves A, Panis Y, Pocard M, et al. Management of anastomotic leakage after nondiverted large bowel resection. J Am Coll Surg. 1999;189:554–559. [DOI] [PubMed] [Google Scholar]
- 17.Alves A, Panis Y, Trancart D, et al. Factors associated with clinically significant anastomotic leakage after large bowel resection: multivariate analysis of 707 patients. World J Surg. 2002;26:499–502. [DOI] [PubMed] [Google Scholar]
- 18.Vignali A, Fazio VW, Lavery IC. Factors associated with the occurrence of leaks in stapled rectal anastomoses: a review of 1014 patients. J Am Coll Surg. 1997;185:105–113. [DOI] [PubMed] [Google Scholar]
- 19.van Geldare D, Fa-Si-Oen P, Noach LA, et al. Complications after colorectal surgery without mechanical bowel preparation. J Am Coll Surg. 2002;194:40–47. [DOI] [PubMed] [Google Scholar]
- 20.Eckman C, Kujath P, Schiedeck THK, et al. Anastomotic leakage following low anterior resection: results of a standardized diagnostic and therapeutic approach. Int J Colorectal Dis. 2004;19:128–133. [DOI] [PubMed] [Google Scholar]
- 21.Griffen FD, Knight CD, Whitaker JM, et al. The double stapling technique for low anterior resection: results, modifications, and observations. Ann Surg. 1990;211:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuh Yeh C, Changchien CR, Wang JY, et al. Pelvic drainage and other risk factors for leakage after elective anterior resection in rectal cancer patients. Ann Surg. 2005;241:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karanjia ND, Corder AP, Bearn P, et al. Leakage from stapled low anastomosis after total mesorectal excision for carcinoma of the rectum. Br J Surg. 1994;81:1224–1226. [DOI] [PubMed] [Google Scholar]