Abstract
Objective:
To evaluate surgical strategies for neuroendocrine pancreatic tumors (NEPT) in the light of the new WHO classification from 2004 and to draw conclusions for future surgical concepts.
Background:
The extent of surgical resection in primary and recurrent NEPT is unclear.
Methods:
Between 1987 and 2004, 62 patients with sporadic NEPT were treated at our institution and sections from biopsy and resection specimen were histopathologically reclassified. Clinical presentation, surgery, metastases, and pattern of recurrence were related to survival.
Results:
Fifteen well-differentiated tumors (WDT, 24%), 39 low-grade carcinomas (LGC, 63%), and 8 high-grade carcinomas (HGC, 13%) were identified. Median observation time was 30.5 months; 48 of 62 patients (78%) were surgically resected, and in 45 patients R0/R1 status was achieved. Overall 2- and 5-year survival in the latter group was 80% and 64%, respectively. Retrospective WHO classification revealed that organ-preserving segmental resections had been performed in 10 LGC and 1 HGC. These patients showed equal outcome as radically resected counterparts (n = 19). Liver and other organ metastases were present in 19 of 62 patients (31%), and resection was accomplished in 7 of 19 patients, which conferred better overall survival (P = 0.026, log-rank test); 21 of 45 R0/R1-resected patients (47%) suffered from recurrence, and reoperation was accomplished in 9 patients, which resulted in better overall survival (P = 0.066).
Conclusion:
Organ-preserving resections offer sufficient local control in LGC; therefore, radical resections do not seem to be justified. On the other hand, radical resection is indicated even in metastasized patients or in case of loco-regional recurrence. The silent and slow course of the disease facilitates long-term surgical control.
The new WHO classification for neuroendocrine pancreatic tumors is applied to a patients’ collective in an institutional study. Among 62 subjects, both organ-sparing and radical resections were successfully performed in malignant tumors. Reoperations seem to prolong survival in local and organ recurrence.
Pancreatic neuroendocrine neoplasms are rare, benign or malignant epithelial tumors and can be broadly classified as functional or nonfunctional. Functional tumors display a clinical syndrome because of excessive endocrine function. The assessment of the patient's signs and symptoms, various imaging modalities, preoperative biopsy, and laboratory diagnostics should ideally recognize the neuroendocrine character of the tumor and supply necessary information about curativity, tumor extent, surgical feasibility, and consecutively the extent of resection.1–7 The new classification of neuroendocrine tumors of the pancreas as proposed by Capella et al,8 and modified later on by Kloeppel et al,9 provides a valuable tool for postoperative prognosis and management, which very lately have been refined by new proposed parameters.9,19 Reclassification of the tumors now clearly draws a cutoff line between benign and malignant lesions and features 2 types of carcinoma, low-grade carcinoma (LGC) and high-grade carcinoma (HGC). However, from a clinician's point of view, the question of how to best treat a patient arises preoperatively.10
Endosonography (EUS) and fine needle aspiration (FNA) are invasive diagnostic techniques that may help verify the histopathologic features of the tumor preoperatively.11 Even laparoscopy has been proposed for preoperative staging.12 However, still the neuroendocrine nature of the lesion sometimes remains obscure. Because of this uncertainty, very commonly, the surgical strategy is determined by size and site of the tumor. New attempts to clearly separate the surgical policy for neuroendocrine pancreatic disease and for ductal pancreatic adenocarcinoma have been accomplished and vary between different authors.13 Complications after organ-preserving surgery often deter from minimal resection in clearly benign lesions and lead to the more classic right- or left-sided resections. To avoid unnecessary organ loss, however, a “tailored” parenchyma-sparing pancreatic resection should be generally preferred. Concerning malignant neuroendocrine lesions, data about the extent of resection and the benefit of radical and repeated surgery on survival are missing.14 Particularly, it must be assumed that therapeutic guidelines that apply to exocrine cancer of the pancreas are inadequate for malignant neuroendocrine cancer. In the presence of advanced disease, no clear data exist about the benefit of a curative or palliative surgical approach.15,16 Particularly with hepatic metastases, the decision to resect primary tumor metastases or refer the patient to chemotherapy is paramount.17,18 In the light of this new classification, our institutional experience is aimed at critically reviewing outcome of our patients after surgical attempts of cure and palliation and particularly focuses on the pattern of tumor relapse and on the question how recurrences can be readdressed surgically.
PATIENTS AND METHODS
Assessment
Between 1987 and 2004, 62 consecutive patients with sporadic neuroendocrine pancreatic tumors (NEPTs) were operated on at our institution. Patients with documented multiple endocrine neoplasia type I (MEN I) or von Hippel-Lindau disease were not included into the study. Patient data were entered into a computerized database, and follow-up was recorded for each patient. All 62 patients fulfilled the following criteria: they had primary and further operations at our hospital and their postoperative follow-up was longer than 12 months. Median follow-up for all patients was 30.5 months. All data were analyzed with respect to characteristics of the primary tumor, clinical symptoms, type of surgical resection, and resection quality. Quality of resection was determined according to the R-classification by the International Union Against Cancer (R0 = no residual tumor, R1 = all identifiable tumor removed, R2 = tumor left macroscopically in situ). All histologic slides were reclassified according to the new WHO classification for NET of the pancreas9 by 2 pathologists (U.R., S.P.). Functioning tumors were defined by positive hormone immunohistochemistry in the tumor tissue and presence of clinical symptoms or signs associated with systemic secretion of the hormone as proposed by Capella et al.8 Ki-67 (MIB-1) immunohistochemistry was available in 21 tumor sections. Neoadjuvant, adjuvant, or definitive chemotherapy was administered in 36 of 73 patients (49%) when recommended by our interdisciplinary tumor board. Primary end point was the overall survival (OS) and the disease-free survival (DFS). Local recurrence and distant metastases were analyzed as separate outcomes. A secondary endpoint was the disease-specific survival (DSS).
Operative Procedure
Surgical strategy was based on anatomic and oncologic demands. For circumscript tumors, a limited approach was chosen and organ-sparing surgery was performed adopting individually tailored solutions. Right-sided tumors were resected by a duodenum-preserving resection of the pancreatic head, whereas left-sided lesions were treated by a left-sided resection of the tail (often spleen-preserving). Segmental pancreatic body resections were performed for central tumors. In contrast, on suspicion of malignant growth in CT or MRI imaging, extended en bloc resection of the affected part of the pancreas and a radical systematic lymph node dissection were chosen. Where necessary, a total pancreatectomy was performed. When the liver was affected, preoperative liver volume and arterial blood supply were evaluated by angio-CT scan and the affected part was resected on primary operation when feasible. Multistage procedures included concomitant and delayed liver operations after resection of the primary tumor and in some instances right or left portal vein embolization. When the tumor was found to be unresectable, liver biopsy, CT-guided biopsy, explorative laparotomy, palliative gastroenterostomy, or bilio-digestive anastomosis was performed. Organ-preserving surgery included enucleation (excision of affected parenchyma with minimal resection margin) and segmental resections (duodenum-preserving resection of the pancreatic head, segmental resections within the pancreatic body and spleen-preserving resections of the pancreatic tail) with only regional lymphadenectomy. En bloc resections were always oncologic and included a radical systematic lymphadenectomy. Depending on the location of the primary tumor, Whipple's operation or a pylorus-preserving pancreaticoduodenectomy, distal splenopancreatectomy and total pancreatectomy, eventually extended to confining organs, were performed. Hepatic and intrapancreatic recurrence was approached surgically when feasible.
Statistical Analysis
OS, DSS, and DFS with regard to local and distant organ recurrence were assessed. All types of survival and absence of local recurrence and distant metastases were calculated adopting the Kaplan-Meier method with 95% confidence intervals. The log-rank test allowed for comparison of the curves. Candidate variables like WHO classification grades, gender, age, tumor location, tumor size, depth, infiltration, resection technique, and number of operations were explored using the χ2 and Fisher exact test tests. Two-tailed tests were considered significant at a P value <0.5. Analysis was performed using the SPSS statistical software package (version 11.0, SPSS Inc., Chicago, IL).
RESULTS
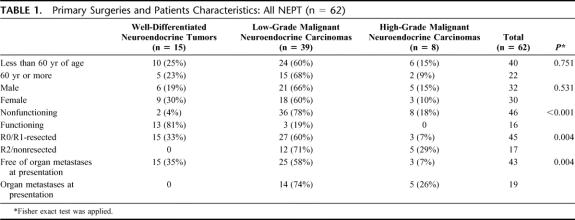
Functioning status of the tumors and characteristics including the WHO classification,9 age, gender, and size are summarized in Table 1.
TABLE 1. Primary Surgeries and Patients Characteristics: All NEPT (n = 62)
Age, Sex, and Clinical Presentation
Median age was 55 years. Distribution of sexes was equal as 30 female and 32 male patients were enrolled. Tumors were nonfunctioning in 46 of 62 (74%) patients and were clearly associated with adverse tumor stage (P < 0.001, Fisher exact test). In functioning tumors (16 of 62, 26%), specific and nonspecific symptoms were reported and in 3 of the 16 functioning tumors only elevated hormone serum levels were found (glucagon, serotonin). Hypoglycemia, diarrhea, and flush occurred in 10 of 16 (62%), 2 of 16 (13%), and 1 of 16 (6%) of the hormone-producing tumors, respectively. Abdominal pain occurred in 27% of all tumors and was more common in nonfunctioning tumors (37%). Weight loss and jaundice were less common in hormone-producing tumors (both 6%) compared with nonfunctioning ones (17% and 13%, respectively). In 17 of 62 patients (27%), tumors were completely asymptomatic.
Histopathology and Site
All tumors were neuroendocrine pancreatic tumors. Tumor size, mitotic count, differentiation grade, angioinvasion, and immunohistochemical staining of hormones, precursor amines, and peptides were evaluated to classify tumors according to the new WHO classification.9 Fifteen well-differentiated tumors (24%, 3 microadenoma and 12 macroadenoma), 39 low-grade malignant carcinomas (63%), and 8 high-grade malignant carcinomas (13%) were identified (Table 1). Twenty-nine tumors were localized in the head of the pancreas (47%), 18 in the body (29%), and 15 in the tail (24%). No distinct anatomic distribution pattern was observed for the 16 functioning tumors. Nonfunctioning status (46 of 62, 74%) was highly associated with bigger size (P < 0.001, Fisher exact test), and a clear dependence of malignancy on loss of endocrine cell function was observed (P < 0.001, χ2 test). There were 21 immunohistochemical Ki-67 data available. No correlation with tumor stage according to the new classification was observed. One of 4 well-differentiated NEPTs showed expression in more than 10% of cells, 8 of 13 (62%) low-grade NECs showed expression in more than 10% of cells, and 2 of 4 high-grade NECs showed expression in more than 10% of cells. (χ2 test: P = 0.4). Organ metastases to the liver and lung were more often seen in nonfunctioning (18 of 62, 39%) compared with functioning tumors (1 of 16, 6%, P = 0.014).
Resections for Primary Tumors With and Without Concomitant Metastases
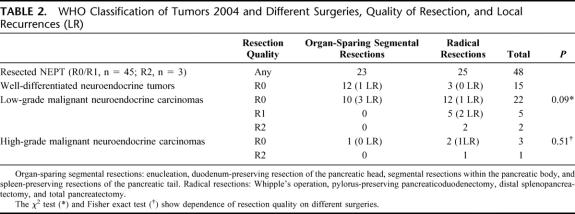
Primary tumors were resected in 48 of 62 patients (77%). R0/R1 resections were performed in 45 of 62 patients (73%). We performed 5 total pancreatectomies (8%), 6 Whipple operations (10%), 2 pylorus-preserving pancreaticoduodenectomies (3%), 8 duodenum-preserving pancreatectomies (13%), 4 duodenum-preserving segmental head and body resections (7%), 2 segmental body resections (3%), 3 spleen-preserving tail resections (5%), 13 distal splenopancreatectomies (21%), and 5 enucleations (8%). In 14 patients (23%), no resection of the tumor was performed. For statistical analysis, surgical procedures were grouped into oncologic en bloc resections (as commonly accomplished for exocrine pancreatic cancer, eg, Whipple's procedure or modular anterograde distal splenopancreatectomy) and organ-preserving resections confined to pancreatic segments. These 2 groups featured different accuracy in lymph node dissection: radical lymph node dissection of the peripancreatic, portal, hepatoduodenal, celiac, superior mesenteric, pancreaticoduodenal, and interaortocaval lymph nodes was effected only in oncologic resections. In contrast, segmental resections were limited to the organ and in these instances regional peripancreatic lymphadenectomy was carried out. Segmental resections were carried out for presumably benign lesions. Very interestingly, the retrospective reclassification and reevaluation of tumor sections revealed carcinoma in 11 subjects (10 LGCs, 1 HGC) treated by organ-sparing segmental resection (Table 2). The present study will focus on survival analysis of these particular cases later on and compare their outcome with those who received oncologic resections.
TABLE 2. WHO Classification of Tumors 2004 and Different Surgeries, Quality of Resection, and Local Recurrences (LR)
R2 status or no resection was accomplished in 17 of 62 (27%) patients mostly due to distant tumor load (15 of 17 patients) and rarely because of locally advanced disease (2 of 17). Fifteen of 16 (94%) functioning tumors could be R0/R1-resected and only in 1 of 16 an R2 situation was accomplished. Nonfunctioning tumors (n = 46) were more often nonresectable (14 of 46, 30%, P = 0.012, Fisher exact test) and 25 R0, 5 R1, and 2 R2 resections were accomplished.
Exploration and Bypass Surgery
Fourteen patients were not resected. Gastroenteric bypass surgery or bilio-digestive anastomosis was performed for gastrointestinal obstruction and in case of jaundice in 7 of 14 patients. Seven of 14 received percutaneous or transgastric biopsy. In these instances, a nonsurgical oncologic therapy was started. Chemotherapy included gemcitabine, mitomycin, streptozotocin, and 5-fluorouracil/doxorubicin, depending on the year of diagnosis and the prevailing oncologic guidelines. For attenuation of their carcinoid syndrome some patients received somatostatin analogues.
Metastases at Primary Presentation
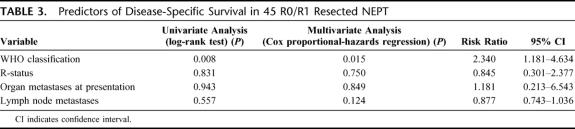
At primary operation, metastases to distant organs including liver were present in 19 of 62 patients (31%). Of these, liver metastases were present in 16 of 62 patients (26%) and partly occurred together with non-liver localizations such as lymph nodes, lungs, peritoneum, stomach, and spleen. Diffuse bilobar hepatic spread, a compromised liver function, or diffuse pulmonary or peritoneal metastases were contraindicative for resection. However, concomitant resection of primary tumors and distant organ metastases was performed in 7 of 19 patients. Resection status was R0 in 3 of 7, R1 in 1 of 7, and R2 in 3 of 7 patients. Considering liver metastases alone (n = 4), concomitant resection (primary tumor and liver metastases) was successfully carried out in all cases (R0 in 3 of 4, R1 in 1 of 4). In the other localizations (n = 3) of distant disease, no R0/R1 status could be achieved. Resectable liver metastases were localized in up to 4 segments and could be resected by one extended right hemihepatectomy and segmental liver resections in 3 subjects. These segmental resections comprised at least 3 segments in 2 patients and 1 solitary in 1 patient. Survival analysis of those primary R0/R1/R2 resections (7 of 19) against nonresected patients (12 of 19) showed a distinct benefit on OS (Fig. 3, P=0.026, log-rank test). A radical lymphadenectomy was performed in oncologic en bloc resections (25 of 48) and showed lymph node metastases in 48% of cases. Among segmental resections (23 of 48), lymphadenectomy of peritumoral nodes revealed lymph node involvement in 9%. Lymph nodes were affected in 2 of 11 carcinoma patients treated by segmental resections. Positive lymph nodes in either resection group did not significantly predict outcome (P = 0.62, log-rank test, data not shown).
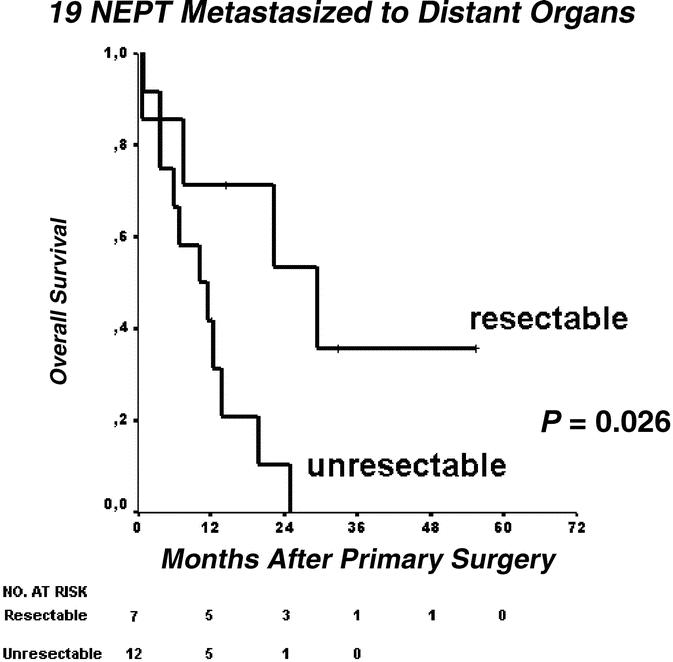
FIGURE 3. Overall survival of metastasized neuroendocrine pancreatic tumors (LGC and HGC) with organ metastases at presentation stratified according to resectability.
Local Recurrence
Among 45 R0/R1-resected tumors, local recurrence was encountered in 1 of 15 well-differentiated tumors (WDTs), 6 of 27 LGCs, and 1 of 3 HGCs (Table 2). Organ-sparing resection technique by itself was not associated with a higher rate of local recurrence in our study cohort as it was observed in 4 of 23 segmental resections and in 4 of 25 radical resections. Recurrence was mainly located in the head of the pancreas (46.7%). Redo surgery was effected in the single WDT patient who suffered local relapse 24 months after primary surgery and no further relapse was observed. Among LGCs and HGCs, 4 of 7 locally recurrent tumors were readdressed surgically. Their onset was seen 2.9, 14, 51, and 85 months after primary resection. In 3 of 7 tumors, no reresection was performed due to nonresectable concomitant distant tumor masses.
Distant Organ Recurrence
Among the R0/R1-resected patients (n = 45), 15 patients suffered distant organ recurrence (33%). Of these, 5 had been segmentally R0-resected and 10 had undergone radical R0/R1 resections (3 R1, 7 R0). The underlying tumors were 12 LGCs and 3 HGCs. Among the 41 patients free of distant metastases at primary presentation, 14 (34%) developed liver metastases after a median observation period of 38 months and 1 developed lung metastases. Among 4 patients who received concomitant primary tumor and liver resection for metastatic involvement, 1 developed metachronic hepatic filiae after 4.1 months. No significantly shorter metastases-free survival was observed in those who already had displayed liver involvement at primary presentation and had been resected (P = 0.76, data not shown).
Surgery for Recurrence
Recurrence was partly amenable by repeated resection. Overall, 18 of 45 R0/R1-resected patients (40%) suffered from recurrence, mainly in the liver (15 of 45 patients). In 5 of 15 patients, combined liver recurrence and local recurrence was encountered, whereas only distant metachronous disease was observed in 10 of 15 patients and 3 patients suffered only local recurrence. We performed 5 of 8 reresections for local relapse and 6 of 15 liver resections for metachronous liver disease. In these instances, either a solitary metastasis had occurred (n = 2) or less than 4 segments were affected (n = 4). Three of 13 patients were operated more than twice for repeated liver recurrence.
Follow-up
A median observation time of 30.5 months (95% confidence interval [CI], 18–43 months) and 55 months (95% CI, 28–83 months) was reached for all patients and the R0/R1-resected patients, respectively.
Overall Survival
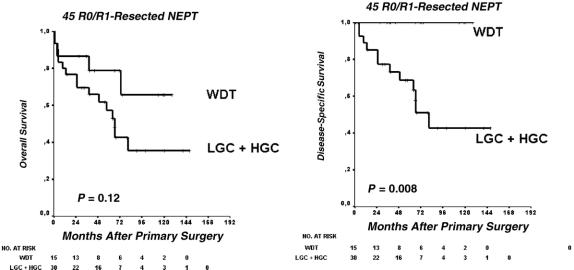
Among all 62 patients, the OS rate was 64% and 49%, and the DSS rate was 73% and 60% at 2 and 5 years, respectively. The calculated median OS was 57 months (95% CI, 25–90 months). OS was favorable in surgically curable disease (Fig. 1, P < 0.001, log-rank test). There were 25 of 35 (71%) tumor-related deaths and 10 of 35 (29%) nontumor deaths. OS and DSS of R0/R1-resected patients (n = 45) are shown in Figure 2. These include patients with concomitant hepatic and pulmonary resections who achieved R0/R1 status (see “Metastases at primary presentation”). Among patients primarily metastasized and concomitantly resected for organ metastases (n = 19), survival was much better in subjects amenable to surgery than those who were not (30 months; 95% CI, 6–53 months, vs. median 10 months; 95% CI, 2–18 months, P = 0.026 in the log-rank test, Fig. 3).
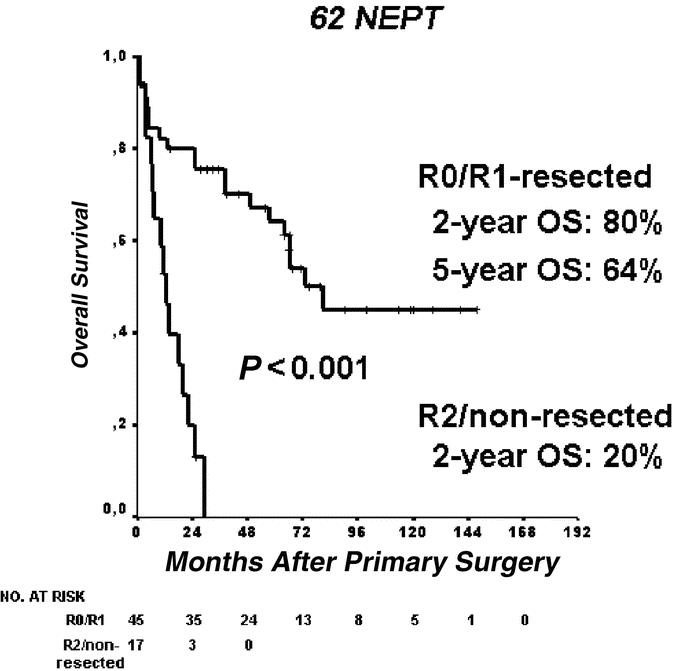
FIGURE 1. Overall survival of the total patients’ collective. Log-rank test.
FIGURE 2. Overall (A) and disease-specific (B) survival after potentially curative R0/R1 resections. WDT, well-differentiated tumor; LGC, low-grade malignant carcinoma; HGC, high-grade malignant carcinoma. Log-rank test.
OS did not differ significantly in the 2 groups (median not reached in the WDT group vs. 66 months; 95% CI, 54–79 months, P = 0.12, Fig. 2A) probably because of the long course of the tumor and upcoming nononcologic death events. DSS was excellent for WDT (100%, Fig. 2B). LGC showed cumulative 2-year and 5-year survival rates of 66% and 48%, respectively. One of 8 patients from the HGC group survived 2 years (38.2 months follow-up time). Eleven patients were initially misdiagnosed as nonmalignant WDT and treated by nononcologic segmental resections. They now are reclassified as LGC (n = 10) and HGC (n = 1). These patients display similar survival compared with their radically resected counterparts. Overall survival was regarded as relevant for comparison to rule out bias due to increased morbidity after radical resection. It did not differ between the radical and organ-sparing resection groups (median, 81 months; 95% CI, 8–154 months, vs. 64 months; 95% CI, 46–82 months, P = 0.90, Fig. 4).
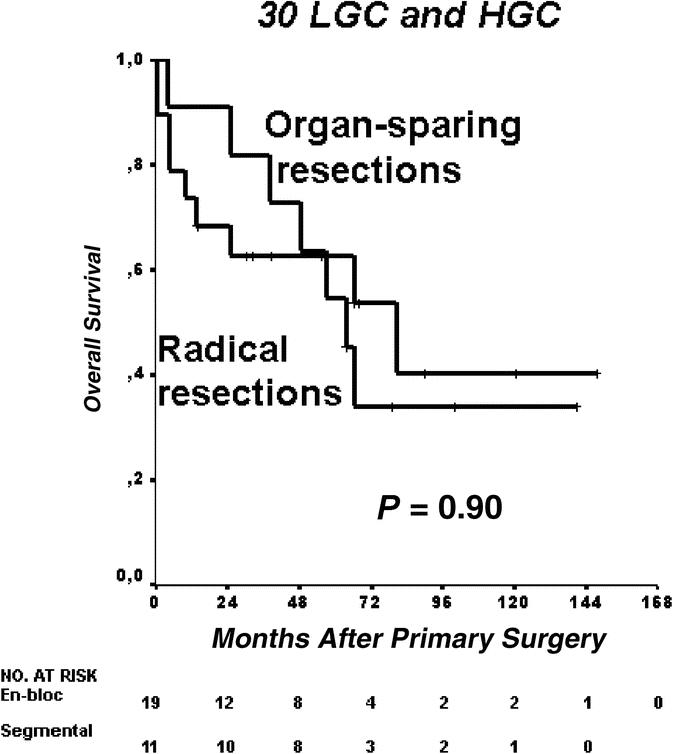
FIGURE 4. Type of resection and overall survival in patients with neuroendocrine carcinomas (LGC and HGC, n = 35).
DFS
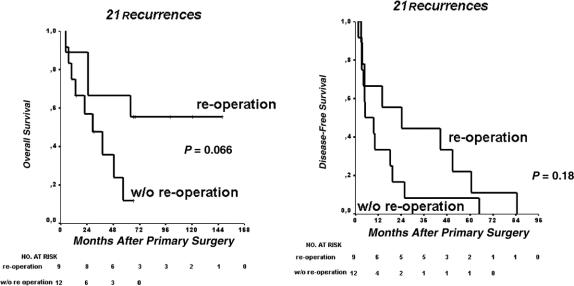
The calculated cumulative 2-year and 5-year DFS for all R0/R1/R2-resected patients was 75% and 57%, respectively. Twenty-one of 48 patients developed recurrence after a median of 65 months (95% CI, 26–104 months). Redo surgery in 9 of 21 patients improved survival, although this was not statistically significant (Fig. 5A). The median OS for those reresected was 95 months (95% CI, 56–134 months) versus 29 months (95% CI, 8–51 months). DFS was longer in patients who were reresected, indicating slower progression of the tumor in these instances with a median of 24 months (95% CI, 0.1–55 months) versus 5 months (95% CI, 0.1–13 months; P = 0.18, Fig. 5B).
FIGURE 5. Influence of reoperation on 25 patients with recurrence: overall (A) and disease-free (B) survival.
Multivariate Analysis
Factors potentially influencing the clinical behavior of the tumors were analyzed by multivariate analysis (Cox regression model) (Table 3). For OS, mainly the WHO classification reflected prognosis in both univariate and multivariate analysis. Whereas univariate analysis showed positive tendencies for R-status, lymph node metastases, and organ metastases at presentation, the only parameter that remained significant in multivariate analysis was the stage according to the new WHO classification.
TABLE 3. Predictors of Disease-Specific Survival in 45 R0/R1 Resected NEPT
DISCUSSION
The present institutional study reclassified 62 cases of neuroendocrine pancreatic neoplasms according to the criteria proposed in the very recent WHO classification 2004. It is obvious that neuroendocrine pancreatic cancer is much more favorable than exocrine cancer in terms of outcome and should therefore be addressed differently. There is still controversy about the radicality of surgery for neuroendocrine pancreatic neoplasms. From a surgeon's point of view, the therapeutic benefit of organ-preserving surgical procedures in circumscript lesions and radical and repeated surgery for advanced neuroendocrine carcinoma is still unclear and should be reevaluated. Because of the rarity of this entity, information about long-term and repeated surgical management of this disease is scarce.
Surgical policy is, first of all, reflected by the decision to operate at primary presentation. In a large surgical series, 64% of patients underwent radical operations for mostly nonfunctioning malignant tumors.19 Our experience is compatible with these findings as 47 of 62 (76%) of our sporadic tumors clearly fulfilled the malignancy criteria. Resectability was found generally in 45 of 62 cases (73%) and among malignant tumors (LGC and HGC), 30 of 47 (64%) were resectable. Our survival data clearly show that resected patients do much better than their nonresected counterparts and, remarkably, in this group R0/R1-resected patients with distant metastases at primary presentation (n = 4, 9%) are also included. According to our observation and to similar excellent survival results for patients with liver resections for metastatic neuroendocrine tumors from the literature,17 we conclude that the decision to operate should be courageously taken whenever the remaining liver volume is not impeding surgery. In a large surgical study including 163 patients, resection of primary tumors and liver metastases conferred much better survival (P = 0.033),20 and our institutional analysis yields similar results (P = 0.026).
There has been a great need for clarification in classifying NEPT. According to our data, the new WHO classification from 2004 well reflects the oncologic dignity of NEPT in terms of outcome. The histopathologic criteria contained in the classification supply a clear distinction between benign and malignant neuroendocrine neoplasms. Our cohort reliably “behaves” as the classification predicts and this is of particular interest, since a radical approach was not applied to all malignancies. As a particular circumstance, the reclassification revealed 11 malignant tumors that had been treated by organ-sparing segmental resection without wide resection margin, and these subjects did not do worse than their radically resected counterparts. Their reclassification as carcinoma now apparently reveals no impact of surgical radicality on survival in NEPT. To our perception, a second crucial point after deciding whether or not to operate is the extent of resection. Little is known about “gold standard” techniques of resection in sporadic NEPT. This does not apply to hereditary conditions of NEPT, with clear recommendations as how to resect pancreaticoduodenal disease.15,21 On the basis of our observations about outcome in LGC, we would like to suggest a modified surgical policy for sporadic NEPT: For benign and small low-grade malignancies, a segmental resection of the pancreas might be sufficient. We are well aware that only at a larger number of patients and ideally in randomized studies, the oncologic value of both segmental and radical resections will be more clearly defined in the future.13,15 On the other hand, at presentation of recurrence, reresection should be generally considered. Both local and distant recurrences are, in principle, a case for the surgeon. It should be noted that, by excluding hereditary conditions, local recurrence is a rare event in our patient cohort. Among R0/R1-resected patients, we observed 7 of 39 recurrences in both benign (n = 1) and low-grade malignant tumors (n = 6). In high-grade malignancies, 1 of 3 tumors recurred. In total, we were able to “cure” local recurrence in 5 patients. Local recurrence was not readdressed surgically when bulky distant tumor masses impeded redo surgery. We cannot conclude that local recurrence is more likely to occur after segmental resections, as the P value in the χ2 test shows no significance on the 5% level. However, 3 of 10 local recurrences in the organ-sparing segmental group versus 1 of 12 local recurrences in the radical resection group might show a need for further clarification. We think that larger trials might clarify the risk of local recurrence after organ-sparing resections.
The most affected distant organ was the liver, and concomitant liver resections in primary surgeries were successfully accomplished in 3 of 4 patients (R0 status). Our data show that resectability of the primary tumor and metastases is feasible and this is confirming previous data.20 On recurrence, liver resections were carried out in 9 patients. This resulted in better OS, although not statistically significant. The DFS curve of our 21 recurrent tumors shows that late relapsing tumors are more likely to be readdressed surgically. Redo surgery reflected the time course of recurrence. According to our experience, a tardy nature of the tumor is a better condition for reresection and has been beneficial for our patients.
Generally, redo pancreatic resections and metastasectomies should be attempted. Our findings are compatible with those from other authors who advocate an aggressive and repeated surgical policy for hereditary neuroendocrine pancreatic neoplasms.17 The silent and slow course of the disease often allows for excellent tumor control, even in the liver.15,17,18,22 This suggests that a slow progression of the disease is a key determinant of surgical success in these patients.
A very large series of 13,715 carcinoid tumors from 3 consecutive cancer registries of the National Cancer Institute revealed that, in 138 pancreatic neuroendocrine neoplasms with a very long follow-up, the actuarial 5-year OS was 37.5%. Another high-volume surgical experience showed that the median OS for NEPT was 41 months.20 In the same study, half of the patients developed metachronous liver disease during observation, which is slightly higher than our institutional rate of 37%. We are well aware that, at a median observation period of 30.5 months in our study group, our data are to be considered preliminary. However, we would like to suggest that our radical surgical policy might possibly account for our excellent calculated survival rate of 49% at 5 years. As mentioned, we observed a lower incidence of liver recurrence than the surgical study mentioned above. Studies from other referral centers have shown slightly worse OS data, and differences in surgical management are not mentioned.23
Finally, multivariate analysis helped delineate the importance of different risk factors for survival. For DSS, the WHO classification 2004 remained the strongest independent risk factor.
Generally, as has been stated,9 new attempts have to be made to define the natural course of a tumor interrupted by surgery to predict further outcome. Still, apart from the WHO classification 2004 that describes classic pathologic features, little molecular determinants are known preoperatively that can predict the dignity of the tumor.
Some key observations may be made in the present study: First, organ-sparing and repeated surgery is possibly sufficient for local tumor control including LGC. Second, in recurrent and nonlocalized neuroendocrine pancreatic cancer, aggressive surgery should be performed in an attempt to gain tumor control of loco-regional recurrence and organ metastases. Third, at present, only the postoperative clinicopathologic classification as proposed by the WHO 2004 gives a good estimation of prognosis. Better preoperative parameters for risk assessment have yet to be discovered to spare unnecessary resections to patients.
ACKNOWLEDGMENTS
The authors thank Michael Bubenheim, PhD, for advice in statistical analysis as well as Guel Cataldegirmen, MD, Claus Schneider, MD, and Oliver Mann, MD for reviewing the patients’ charts and for assistance in follow-up.
Footnotes
Reprints: Jakob R. Izbicki, MD, FACS, Department of General, Visceral and Thoracic Surgery, University Medical Center of Hamburg-Eppendorf, University of Hamburg, Martinistrasse 52, 20246 Germany. E-mail: schurr@uke.uni-hamburg.de.
REFERENCES
- 1.Eriksson B, Arnberg H, Oberg K, et al. Chromogranins: new sensitive markers for neuroendocrine tumors. Acta Oncol. 1989;28:325–329. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson B, Oberg K, Skogseid B. Neuroendocrine pancreatic tumors: clinical findings in a prospective study of 84 patients. Acta Oncol. 1989;28:373–377. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson B, Lilja A, Ahlstrom H, et al. Positron-emission tomography as a radiological technique in neuroendocrine gastrointestinal tumors. Ann NY Acad Sci. 1994;733:446–452. [DOI] [PubMed] [Google Scholar]
- 4.Hall JT, Wallace S, Carrasco CH, et al. Gastrointestinal and pancreatic endocrine tumours. Baillieres Clin Endocrinol Metab. 1989;3:121–152. [DOI] [PubMed] [Google Scholar]
- 5.Hawes RH, Zaidi S. Endoscopic ultrasonography of the pancreas. Gastrointest Endosc Clin North Am. 1995;5:61–80. [PubMed] [Google Scholar]
- 6.Kann P, Bittinger F, Engelbach M, et al. Endosonography of insulin-secreting and clinically non-functioning neuroendocrine tumors of the pancreas: criteria for benignancy and malignancy. Eur J Med Res. 2001;6:385–390. [PubMed] [Google Scholar]
- 7.Kumbasar B, Kamel IR, Tekes A, et al. Imaging of neuroendocrine tumors: accuracy of helical CT versus SRS. Abdom Imaging. 2004;29:696–702. [DOI] [PubMed] [Google Scholar]
- 8.Capella C, Heitz PU, Hofler H, et al. Revised classification of neuroendocrine tumours of the lung, pancreas and gut. Virchows Arch. 1995;425:547–560. [DOI] [PubMed] [Google Scholar]
- 9.Kloppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann NY Acad Sci. 2004;1014:13–27. [DOI] [PubMed] [Google Scholar]
- 10.Banfield A, Green S, Ramage JK. Neuroendocrine tumour management: a team approach. Hosp Med. 2005;66:37–42. [DOI] [PubMed] [Google Scholar]
- 11.Anderson MA, Carpenter S, Thompson NW, et al. Endoscopic ultrasound is highly accurate and directs management in patients with neuroendocrine tumors of the pancreas. Am J Gastroenterol. 2000;95:2271–2277. [DOI] [PubMed] [Google Scholar]
- 12.Hochwald SN, Weiser MR, Colleoni R, et al. Laparoscopy predicts metastatic disease and spares laparotomy in selected patients with pancreatic nonfunctioning islet cell tumors. Ann Surg Oncol. 2001;8:249–253. [DOI] [PubMed] [Google Scholar]
- 13.Dralle H, Krohn SL, Karges W, et al. Surgery of resectable nonfunctioning neuroendocrine pancreatic tumors. World J Surg. 2004;28:1248–1260. [DOI] [PubMed] [Google Scholar]
- 14.Chu QD, Hill HC, Douglass HO Jr, et al. Predictive factors associated with long-term survival in patients with neuroendocrine tumors of the pancreas. Ann Surg Oncol. 2002;9:855–862. [DOI] [PubMed] [Google Scholar]
- 15.Hausman MS Jr, Thompson NW, Gauger PG, et al. The surgical management of MEN-1 pancreatoduodenal neuroendocrine disease. Surgery. 2004;136:1205–1211. [DOI] [PubMed] [Google Scholar]
- 16.Skogseid B. Nonsurgical treatment of advanced malignant neuroendocrine pancreatic tumors and midgut carcinoids. World J Surg. 2001;25:700–703. [DOI] [PubMed] [Google Scholar]
- 17.Norton JA, Warren RS, Kelly MG, et al. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery. 2003;134:1057–1063. [DOI] [PubMed] [Google Scholar]
- 18.Norton JA, Kivlen M, Li M, et al. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg. 2003;138:859–866. [DOI] [PubMed] [Google Scholar]
- 19.Hochwald SN, Zee S, Conlon KC, et al. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20:2633–2642. [DOI] [PubMed] [Google Scholar]
- 20.Solorzano CC, Lee JE, Pisters PW, et al. Nonfunctioning islet cell carcinoma of the pancreas: survival results in a contemporary series of 163 patients. Surgery. 2001;130:1078–1085. [DOI] [PubMed] [Google Scholar]
- 21.Norton JA. Intra-operative procedures to localize endocrine tumours of the pancreas and duodenum. Ital J Gastroenterol Hepatol. 1999;31(suppl 2):195–197. [PubMed] [Google Scholar]
- 22.Fritscher-Ravens A, Izbicki JR, Sriram PV, et al. Endosonography-guided, fine-needle aspiration cytology extending the indication for organ-preserving pancreatic surgery. Am J Gastroenterol. 2000;95:2255–2260. [DOI] [PubMed] [Google Scholar]
- 23.Pape UF, Bohmig M, Berndt U, et al. Survival and clinical outcome of patients with neuroendocrine tumors of the gastroenteropancreatic tract in a German referral center. Ann NY Acad Sci. 2004;1014:222–233. [DOI] [PubMed] [Google Scholar]