Abstract
Objective:
Comparison of effectiveness between the pylorus-preserving pancreaticoduodenectomy (“pylorus-preserving Whipple” [PPW]) and the classic Whipple (CW) procedure.
Methods:
A systematic literature search (Medline, Embase, Cochrane Library, Biosis, Science Citation Index, Ovid Journals) was performed to identify all eligible articles. Randomized controlled trials (RCTs) comparing PPW versus CW for periampullary and pancreatic carcinoma were eligible for inclusion. The methodologic quality of included studies was evaluated independently by 2 authors. Quantitative data on perioperative parameters (blood loss, transfusion, operation time, and length of hospital stay), mortality, morbidity, and survival were extracted from included studies for meta-analysis. Pooled estimates of overall treatment effect were calculated using a random effects model.
Results:
In total, 1235 abstracts were retrieved and checked for eligibility and 6 RCTs finally included. The critical appraisal revealed vast heterogeneity with respect to methodologic quality and outcome parameters. The comparison of overall in-hospital mortality (odds ratio, 0.49; 95% CI, 0.17 to 1.40; P = 0.18), morbidity (odds ratio 0.89; 95% CI, 0.48 to 1.62; P = 0.69), and survival (hazard ratio, 0.74; 95% CI, 0.52 to 1.07; P = 0.11) showed no significant difference. However, operating time (weighted mean difference, −68.26 minutes; 95% CI, −105.70 to −30.83; P = 0.0004), and intraoperative blood loss (weighted mean difference, −766 mL; 95% CI, −965.26 to −566.74; P = 0.00001) were significantly reduced in the PPW group.
Conclusion:
Hence, in the absence of relevant differences in mortality, morbidity, and survival, the PPW seems to be as effective as the CW. Given obvious clinical and methodological interstudy heterogeneity, efforts should be intensified in the future to perform high quality RCTs of complex surgical interventions on the basis of well defined outcome parameters.
A systematic review and meta-analysis comparing pylorus-preserving pancreaticoduodenectomy versus classic Whipple procedure showed no difference in overall morbidity, mortality, and survival. Significant interstudy heterogeneity underscores the need to intensify efforts to standardize clinical research in pancreatic surgery.
Pancreatic cancer is the fourth leading cause of cancer death in men and the fifth in women, accounting for 4.8% and 5.5% of cancer deaths in men and women, respectively.1,2 As shown in large case series,3 the aggressive biology of these tumors and the high local recurrence rate in combination with the early metastatic spread lead to 5-year survival rates between 11% and 21% after resection.4
Surgical resection by means of pancreaticoduodenectomy provides the only chance of cure for patients with periampullary and pancreatic carcinoma.4–6 Advances in surgical technique have reduced the operative mortality rate to below 5% in high-volume centers.6–8 Nevertheless, operative morbidity remains high, occasionally approaching 30% to 40%,9–11 most often including pancreatic fistula, intra-abdominal abscesses, sepsis, and delayed gastric emptying (DGE).
Two operation techniques are performed predominantly in the treatment of periampullary and pancreatic head cancer: the classic Whipple operation (CW) developed by Kausch12 and Whipple13 and the pylorus-preserving Whipple procedure (PPW) inaugurated by Watson14 and popularized by Traverso and Longmire.15
The CW operation consists of an en bloc removal of the pancreatic head, the duodenum, the common bile duct, the gall bladder, and the distal portion of the stomach together with the adjacent lymph-nodes.16 This operation can lead to specific complications such as early and late dumping, postoperative weight loss,17 and postoperative reflux.18 Leaving the functioning pylorus at the gastric outlet, the PPW represents a surgical alternative that is being performed by an increasing number of surgeons.
Which is the better technique? This question is still being debated. Some authors suggested possible advantages of the PPW procedure in terms of reduced operation time,19 less blood loss, better access to the biliary anastomosis for postoperative endoscopy in patients with recurrent biliary obstruction, improved postoperative weight gain,17 and higher quality of life.20 On the other hand, the reported incidence of early DGE seemed to be higher in the PPW group in previous series.21–23 Moreover, it has not been unequivocally shown that the lesser trauma induced by a PPW, beneficial as it may be to the patient, is yet oncologically adequate.
The inconclusive results of several nonrandomized studies have triggered a number of randomized controlled trials (RCTs). A qualitative appraisal and statistical aggregation of the individual RCTs that will give a more precise estimate of the treatment effect is still lacking, as systematic reviews of high quality primary studies represent, if rigorously performed, the highest level of evidence.24
The primary objective of this study is to analyze the existing evidence regarding the PPW and CW procedures in a systematic review (SR) and to provide a meta-analysis (MA) of perioperative parameters (blood loss, transfusion, operation time), postoperative mortality and morbidity, length of hospital stay, and survival.
METHODS
The rationale and design of this study were prepared according to the described methodology25 and approved by peer-review of the Cochrane Collaboration (Upper Gastrointestinal and Pancreatic Diseases Group, Leeds, UK).
Databases searched included the Cochrane Library Central (Register of Controlled Trials; 2005 Issue 4), Medline (1966 to November Week 3 2005), Premedline (Ovid), Journals Ovid (update November 28, 2005), Embase (1974 to December 2005), Biosis (1989 to December 2005) and the Science Citation Index Database (1945 to December 2005). Also searched were reference lists of retrieved relevant articles for additional trials. Moreover, investigators and experts in the field of pancreatic surgery were contacted to ensure that all relevant studies were identified. The search was not restricted to specific languages or years of publication. The last search was carried out on December 12, 2005.
Search Strategy
The search strategy in Medline (PubMed) was based on the following search terms (Medical Subject Heading terms and text words) with the appropriate combinations:
(Pylorus preserv* OR whipple OR pancreat* resection OR pancreatojejunostom* OR pancreaticojejunostom* OR pancreaticojejunostomy [MeSH] pancreatoduodenectom* OR pancreaticoduodenectom* OR pancreaticoduodenectomy [MeSH] OR duodenopancreatectom* OR pancreatectom* OR pancreatectomy [MeSH])
AND
(Adenocarcinom* panc* OR panc* tumor* OR panc* carc* OR panc* cancer* OR panc* neoplas* OR Pancreatic Neoplasm [MeSH])
AND
(randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double-blind method [mh] OR single-blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR (“clinical trial” [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] NOT (animal [mh] NOT human [mh])).
Study Selection
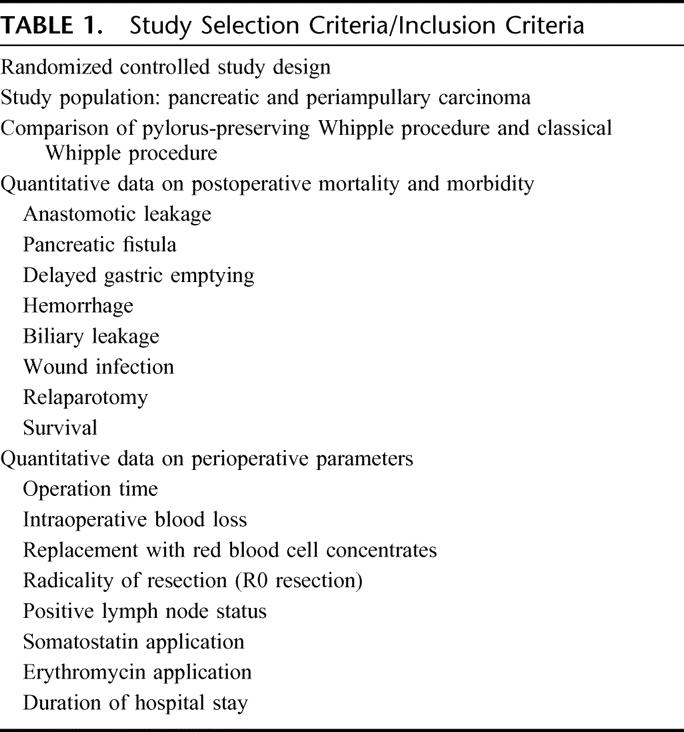
RCTs comparing CW versus PPW for periampullary and pancreatic carcinoma were eligible for this SR. Only RCTs reporting on the following outcomes were selected for data extraction: quantitative data on perioperative parameters (blood loss, transfusion, operation time, length of hospital stay), postoperative mortality and morbidity, and survival.
Two authors (M.K.D. and C.H.) independently identified and screened the search findings for potentially eligible RCTs. Abstracts and full articles were obtained for detailed evaluation and eligible trials were included into the SR. Any disagreements during the selection process were resolved by discussion with a third author (C.M.S.).
Data Extraction and Analysis
According to international recommendations,26,27 the methodologic quality of RCTs was assessed using a standardized form to extract prespecified parameters.28 Thus, the critical appraisal of the extracted data included rating of the randomization procedure, allocation concealment, sample size, consistency of the study population, length and quality of follow-up, rate of patients lost to follow-up, and statistical analysis of individual trials.
Two authors (M.K.D. and C.H.) independently extracted quantitative data on perioperative parameters, postoperative mortality, morbidity, and survival (Table 1). The synchronized extraction results were pooled as estimates of overall treatment effects in a MA and presented as weighted odds ratios (OR) for parameters of mortality and morbidity, weighted mean difference (WMD), a weighted average of the differences in means, for perioperative parameters and the corresponding 95% confidence intervals (CI). Data for the pooled overall survival were generated by extracting events (Kaplan-Meier curves) and calculating logarithmic hazard ratios (±standard error) using the corresponding P value (log-rank test).29 The weight of each study relates to its sample size and study quality. All results were investigated for clinical and statistical heterogeneity. Clinical heterogeneity was defined as the existence of inhomogeneous study population, the variability of interventions, and the insufficient definition of outcome parameters or major variance in perioperative management. Clinical heterogeneity was explained where appropriate and possible. Statistical heterogeneity was explored by inspecting the forest plot and I2 statistic. To account for clinical heterogeneity (varying or missing definitions of outcome parameters), overall estimates were calculated by using the random effects models30 (Review Manager, Version 4.2 for Windows, Cochrane Collaboration, 2003). For this reason, the results of the MA are presented as more conservative estimates compared with an analysis with a fixed effect model in absence of clinical heterogeneity.31
TABLE 1. Study Selection Criteria/Inclusion Criteria
RESULTS
Excluded Studies
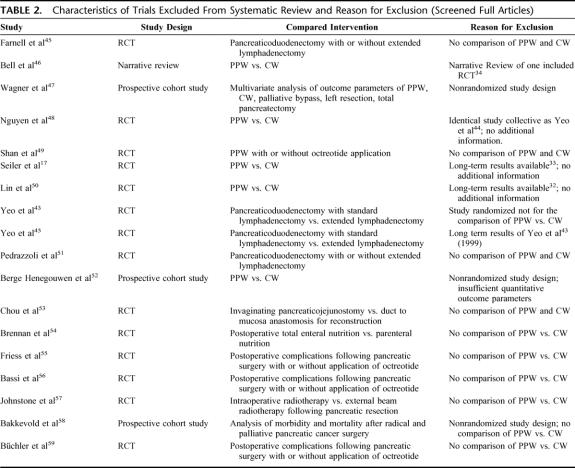
The systematic literature search retrieved 1235 abstracts. During this process of study selection, 1211 screened abstracts had to be excluded. Of the remaining 24 screened full articles, 18 did not fulfill the inclusion criteria. Most of the excluded articles did not cover surgical aspects of the comparison of PPW and CW nor provide quantitative data on the prespecified outcome parameters (Table 2; Fig. 1).
TABLE 2. Characteristics of Trials Excluded From Systematic Review and Reason for Exclusion (Screened Full Articles)
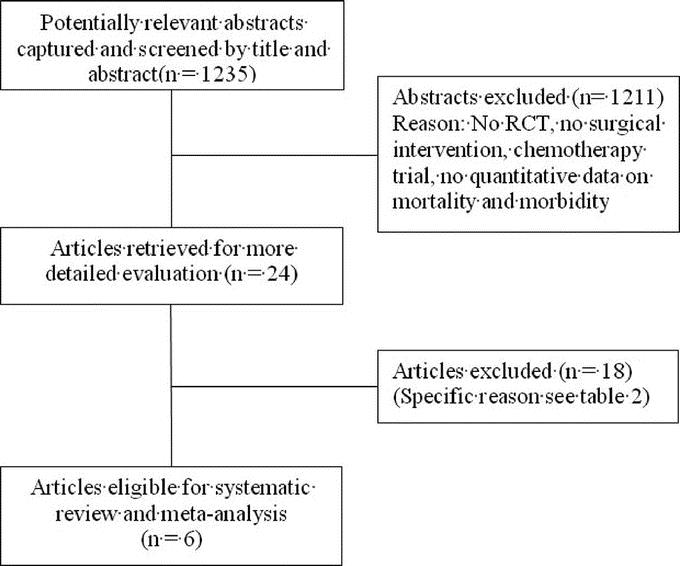
FIGURE 1. Number of abstracts and articles identified and evaluated during the review process. Modified flow chart according to Quorom.28
FIGURE 1. (Continued).
Included Studies
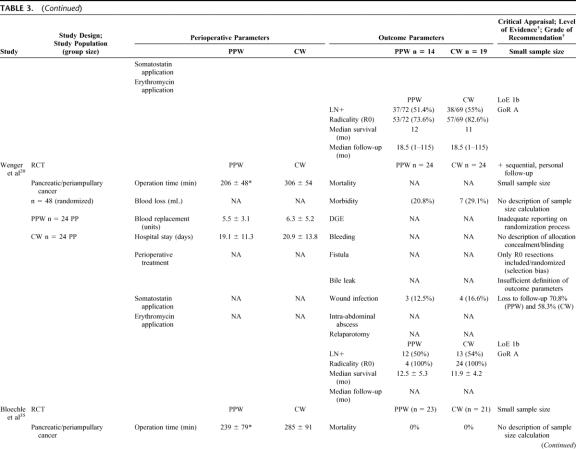
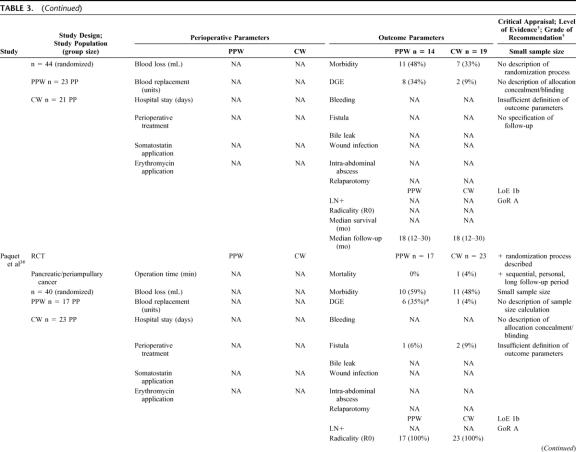
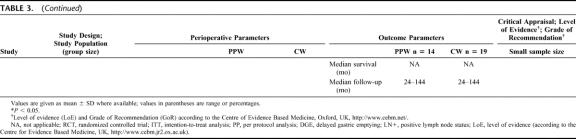
Six RCTs20,32–36 involving 578 randomized patients were eligible. Because of a per-protocol analysis of 5 trials,20,32,33,35,36 quantitative data of 465 analyzed patients comparing the PPW (229 patients) and the CW (236 patients) procedure were found suitable for the SR (Table 3) and MA (Figs. 2, 3).
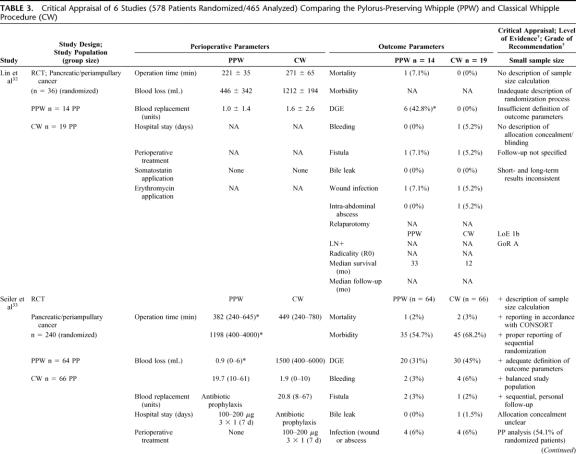
TABLE 3. Critical Appraisal of 6 Studies (578 Patients Randomized/465 Analyzed) Comparing the Pylorus-Preserving Whipple (PPW) and Classical Whipple Procedure (CW)
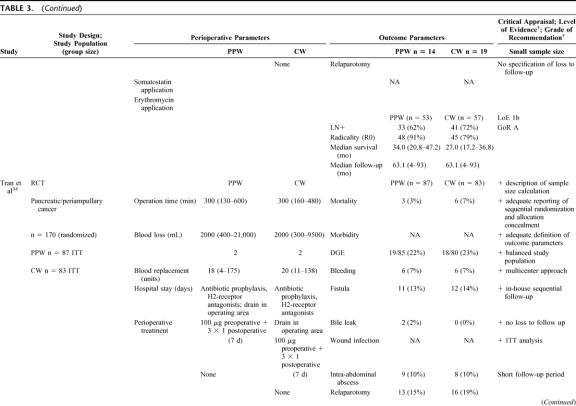
TABLE 3. (Continued)
TABLE 3. (Continued)
TABLE 3. (Continued)
TABLE 3. (Continued)
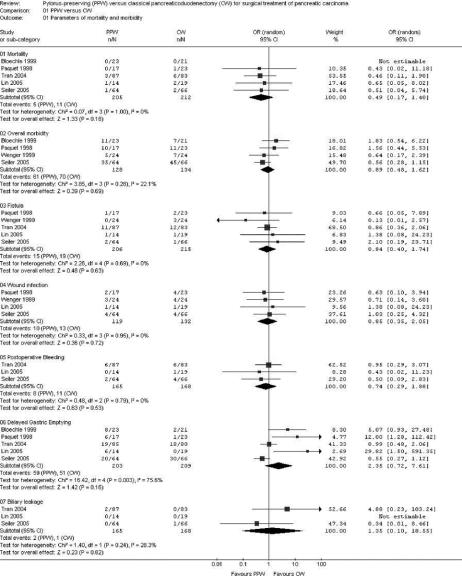
FIGURE 2. Meta-analysis of parameters of mortality and morbidity. Effect estimates (odds ratio; 95% CI). Pooled treatment effect is shown as a diamond that spans the 95% CI. Study quality assessment: allocation concealment adequate (A), unclear (B), inadequate (C), or not used (D).
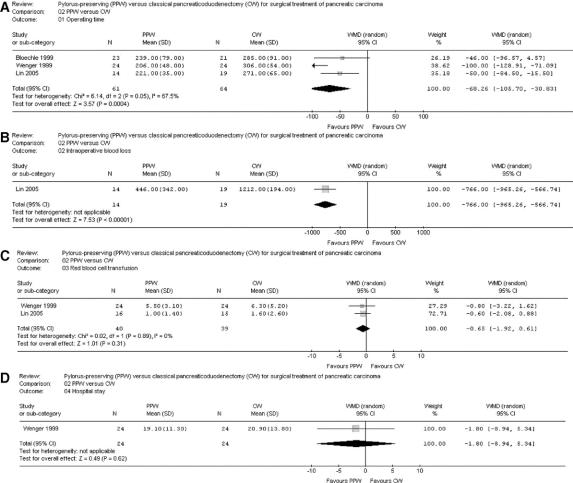
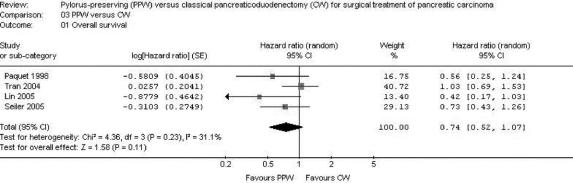
FIGURE 3. Meta-analysis of perioperative factors, length of hospital stay, and overall survival. Effect estimates (odds ratio; 95% CI). Pooled treatment effect is shown as a diamond that spans the 95% CI.
Besides obvious variation in the sample size (range from 3632 to 24033) the evaluation of the study population baseline data revealed adequate consistency: all analyzed patients were included because of resectable pancreatic or periampullary cancer. Moreover, 4 studies20,33,34,36 provided data on resection margin status following PPW or CW, respectively: all studies showed a balanced interstudy and intrastudy distribution of R0 resections (mean R0 resection, 91.1% [PPW]; 90.4% [CW]). In addition, we observed a similar distribution of positive lymph node status in 3 studies (mean positive lymph node status: 54.4% [PPW]; 60.3% [CW]).20,33,34
The methodologic appraisal of the single studies revealed considerable heterogeneity in study design. Only 2 trials, the largest (17034 and 24033 patients, respectively) described the underlying sample size calculation, whereas 4 trials20,32,35,36 did not justify the number of included patients.
The randomization process was described in 3 trials,33,34,36 the 3 others20,32,35 did not specify the process of random allocation adequately. Only 2 trials33,34 described how allocation concealment was maintained and blinded outcome assessment was performed.
The trials by Seiler et al33 and Tran et al34 provided the most comprehensive definitions. Four trials20,32,35,36 did not specify the criteria of their endpoints adequately.
Follow-up quality was evaluated by assessing the follow-up sequence and the length of follow-up. Median follow-up varied from 18 months35 up to 144 months;36 one study32 did not report and specify on the follow-up at all.
Statistical analysis was performed in one trial34 according to the intention-to-treat principle; 5 trials20,32,33,35,36 applied a per-protocol analysis.
Regarding the perioperative management, we observed a nonstandardized administration of somatostatin as to dosage, route, frequency, and length. Two of the 6 RCTs reported the use of octreotide (100–200 μg).33,34 Most of the trials discarded the use of erythromycin33,34 or did not mention its perioperative application.20,32,35,36
Meta-Analysis of Mortality, Morbidity, Perioperative Parameters, and Survival
Six studies20,32–36 provided quantitative data on overall mortality and morbidity in addition to rates of pancreatic fistula, wound infection, postoperative bleeding, DGE, and biliary leakage following the PPW and the CW procedures, respectively.
Mortality and Overall Morbidity
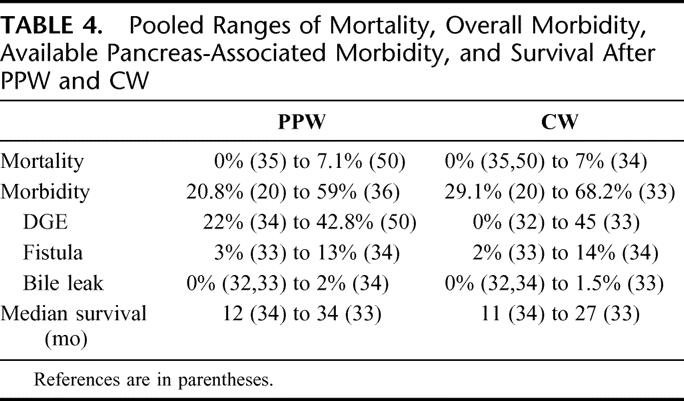
Both mortality and postoperative overall morbidity showed similar ranges in the PPW group (mortality, 0%35 to 7.1%;32 morbidity, 20.8%20 to 59%36) and the CW group (mortality, 0%32,35 to 7%;34 morbidity, 20.8%20 to 68.2%33), respectively.
Four RCTs,20,33,35,36 including 262 analyzed patients, were suitable for analysis of overall postoperative morbidity; no significant difference was noted: 47.6% (PPW) versus 52.2% (CW) (OR, 0.89; 95% CI, 0.48 to 1.62; P = 0.69; I2 test, 22.1% data heterogeneity) (Table 4; Fig. 2).
TABLE 4. Pooled Ranges of Mortality, Overall Morbidity, Available Pancreas-Associated Morbidity, and Survival After PPW and CW
The overall effect estimate of 205 (PPW) versus 212 (CW) patients revealed no difference in mortality (OR, 0.49; 95% CI, 0.17 to 1.40; P = 0.18; I2 test, 0% data heterogeneity).
Perioperative Parameters
MA of perioperative parameters such as blood loss, red blood cell transfusion, operating time, and length of hospital stay of 3 RCTs20,32,35 demonstrated a significant reduction of operating time [min] for the PPW (WMD, −68.26; 95% CI, −105.70 to −30.83; P = 0.0004; I2 test, 67.5% data heterogeneity). Intraoperative blood loss (mL) could be extracted in an analyzable way from only one trial32 and was significantly reduced in the PPW group (WMD, −766.00; 95% CI, −965.26 to −566.74; P < 0.00001). In contrast, the summarized effect estimate of blood replacement (units) indicated similar application of blood products intraoperatively (WMD, −0.65; 95% CI, −1.92 to 0.61; P = 0.31; I2 test, 0% data heterogeneity). Length of hospital stay (days) showed similar results in both groups (WMD, −1.80; 95% CI, −8.94 to 5.34; P = 0.62), with only one article providing adequate data for pooling20 (Fig. 3).
Parameters of Morbidity
Data on pancreas associated morbidity were inconclusive due to broad ranges of rates for DGE, occurrence of pancreatic fistula, and biliary leakage (Table 4). To further differentiate the underlying heterogeneity, MA of these parameters was performed.
Five studies32–36 provided data on the occurrence of DGE. Given statistical heterogeneity (I2 test 75.6%), 3 studies32,35,36 were in favor of CW, one in favor of PPW,33 whereas one study34 presented an equal rate of DGE. Pooling of these individual results revealed 59 patients of 203 (29.0%) in the PPW group compared with 51 of 209 (24.4%) in the CW group (OR, 2.35; 95% CI, 0.72 to 7.61; P = 0.16).
The summarized effect size of 3 RCTs32–34 comparing biliary leakage following PPW and CW showed no difference: 2 of 165 (1.2%) in the PPW group versus 1 of 168 (0.5%) in the CW group (OR, 1.35; 95% CI, 0.10 to 18.55; P= 0.82; I2 test, 28.3%). Three studies32–34 evaluated postoperative bleeding, with 8 of 165 patients (4.8%) in the PPW group and eleven of 168 patients (6.5%) in the CW group, which was statistically nonsignificant (OR, 0.74; 95% CI, 0.29 to 1.88; P = 0.53; I2 test, 0%). Wound infection showed similar levels and occurred in 10 of 119 (8.4%) patients in the PPW group and in 13 of the 132 (9.8%) patients in the CW group (OR, 0.85; 95% CI, 0.35 to 2.05; P = 0.72; I2 test, 0%). The fistula rate also showed no difference between both groups: 15 of 206 patients undergoing a PPW (7.3%) versus 19 of 215 patients in the CW group (8.8%) (OR, 0.84; 95% CI, 0.40 to 1.74; P = 0.63; I2 test, 0%), as shown in the MA of 5 RCTs20,32–34,36 (Fig. 2).
Survival
Four studies32–34,36 provided survival data suitable for MA: long-term results (60 months32,34,36 and 36 months,33 actuarial Kaplan-Meier analysis) from a total of 284 patients (138 PPW; 146 CW) were evaluated comparing the hazard ratios (HR) and the corresponding 95% CI in the random effects model. As in the findings of the individual trials, the summarized effect size indicated no difference in survival following PPW or CW, respectively (HR, 0.74; 95% CI, 0.52 to 1.07; P = 0.11; I2 test, 31.1%) (Fig. 3).
DISCUSSION
The preservation of the pylorus in patients undergoing duodenopancreatectomy for cancer has been a controversial issue for the last decade. Numerous studies have been performed, including several RCTs, but the cumulative knowledge gained from these studies needed to be captured in a quantitative summary of the results to establish whether the pylorus-preserving pancreaticoduodenectomy (PPW) is a better technique than the classic Whipple (CW).
From a curative perspective, this SR with MA provides further evidence that the CW is not better than the PPW procedure. Pooled long-term results of 4 RCTs showed no difference in terms of overall survival (HR, 0.74; P = 0.11). Our findings are in line with a recently published study by Bassi et al37 where survival data were correlated with the type of surgery by multivariate analysis. The results indicated that neither the type of surgery (PPW vs. CW: HR, 0.80; P = 0.078) nor the occurrence of postoperative complications significantly affected the hazard of death, once tumor staging is taken into account (grade, nodal status, and maximum tumor size). As shown by Neoptolemos et al,38 not the resection margin status R0/R1, but the tumor grade and lymph node status were by far the most powerful prognostic factors of survival.
Mortality and cumulative morbidity are not significantly different between both techniques so that they can be said to be in clinical equipoise. As for surgically and clinically relevant parameters of morbidity, we found in particular no significant differences for the occurrence of pancreatic fistula (OR, 0.84; P = 0.63), biliary leakage (OR, 1.35; P = 0.82), and postoperative bleeding (OR, 0.74; P = 0.53).
Concerning DGE, previous reports of nonrandomized cohort studies22,39 stating higher rates of DGE in the PPW procedure could not be confirmed by the results of prior RCTs and our MA: Three studies32,35,36 of the 6 included studies showing higher rates of DGE in the PPW group were also the smallest ones. Given a nonsignificant difference of DGE when considering all included studies (29.1% PPW vs. 24.4% CW: OR, 2.35; P = 0.16), this result indicates that underpowered studies potentially overestimate the benefits of CW on DGE.
Our Cochrane highly sensitive search strategy was approved by an expert in the field (G.A.) but, because of incompleteness, may still be biased. In an attempt to identify all relevant studies, we contacted experts in pancreatic surgery and clinical research to identify ongoing trials. However, no additional study has been brought to our knowledge.
Systematic reviews currently provide the best methodology to summarize the existing evidence, but the quality of such reviews depends on the quality of the primary studies.40,41 In our case, the reviewed studies are partially marred by bias and clinical heterogeneity, which may distort our results.
The fact that the randomization process has been carried out differently in the reviewed studies can be considered as a source of bias, as adequate randomization and allocation concealment generates balanced groups for known, unknown, and unmeasured confounders. Three studies33,34,36 of the 6 included studies provided adequate description of the randomization process, whereas the 3 other studies20,32,35 failed to describe the method of allocation satisfactorily.
Moreover, maintained allocation concealment and blinding of the outcome assessor were specified only in 2 trials.33,34
Also, median follow-up and follow-up sequences varied significantly between the individual studies (median follow-up, 1835 months to 144 months36). This insufficient reporting on inconsistent follow-up sequences is a possible indicator of performance bias.
Furthermore, we found varying or even lacking definitions of surrogate parameters such as pancreatic fistula and DGE. The trials by Seiler et al33 and Tran et al34 provided the most comprehensive definitions. Four trials20,32,35,36 did not specify the criteria of their endpoints at all. The heterogeneous definition of outcome parameters may have caused detection bias.
At the moment, there are no internationally accepted scaled definitions for surgical outcome parameters in pancreatic cancer surgery. Efforts, such as the consensus conference of the international study group for definition of postoperative pancreatic fistula, should be encouraged to reduce interobserver variability.42
Concerning the use of antibiotics, somatostatin, drains, nasogastric tubes, etc, we observed a nonstandardized therapeutic concept or even nonreporting with respect to dosage, route, frequency, and length of administration. Thus, the considerable variation of perioperative management is a further indicator for of interstudy heterogeneity that may influence the external validity of the summarized results.
Another possible source of bias lies in the statistical analysis. Only one trial34 used the intention-to-treat method, whereas the 5 remaining trials20,32,33,35,36 applied the PP analysis. This distorted analysis of patients lost to follow-up or missing information on excluded patients (attrition bias) makes it difficult to decide whether the remaining study population is representative (external validity). Moreover, statistical analysis of obviously underpowered trials32 reduces the explanatory power of these results.
In this context, we should point out that we did not investigate the influence of the neo- and adjuvant treatment, as relevant data were not reported in an interpretable way in the reviewed studies. Therefore, our results may be biased since adjuvant treatment, eg, chemotherapy or chemo-radiation, represents a possible confounder of an impact on survival, mortality and morbidity in a neoadjuvant setting.
The trial by Yeo et al43,44 had to be excluded from our analyses due to the fact that patients were randomized for pancreaticoduodenectomy with standard versus extended lymphadenectomy and not explicitly for PPW versus CW. Although 86% of patients of the “standard” group were subjected to a PPW and all of the radical (extended) group underwent a classic Whipple procedure with retroperitoneal lymphadenectomy, inclusion of these 294 patients might have contorted the results.
Despite the mentioned sources of clinical and methodologic heterogeneity, we still observed adequate balanced groups when we compared interstudy baseline population characteristics: all analyzed patients were included due to suspect pancreatic or periampullary carcinoma. Our SR also revealed a balanced distribution of R0/R1 resections (mean R0 resection, 91.1% [PPW]; 90.4% [CW]) and lymph node status (mean positive lymph node status, 54.4% [PPW]; 60.3% [CW]). The calculated average level of the statistical heterogeneity of all 12 meta-analytic approaches indicated moderate heterogeneity of 22.4% (I2 test). Nevertheless, given obvious sources of clinical and methodologic heterogeneity, we decided to compute the statistics of our MA using the random effects model, which allows for variation in the treatment effects of the individual studies but also provides a more conservative pooled effect estimate.30 Because of multifactorial heterogeneity and only 6 trials included, subgroup analysis seemed not to be feasible.
CONCLUSION
Given these obvious sources of bias (small sample size, lacking definition of outcome parameters, inexplicit randomization, and allocation concealment), the results of our systematic review and meta-analysis should be interpreted with caution. We must recognize that neither PPW nor CW has been showed to be the better technique. To demonstrate a clear superiority of the PPW technique, much larger and rigorously designed studies would be needed than currently available. On the other hand, the present study illustrates an intolerable situation. Six trials of the highest level of evidence addressing the same surgical problem still show major clinically relevant heterogeneity. This highlights the urgent need for the surgical community to develop standardized RCTs for complex interventions. To reach an evidence-based consensus on refined definitions of surgical interventions and their possible outcome parameters, international cooperation is called upon. This systematic review and meta-analysis might serve as a basis for such a development.
Footnotes
Reprints: Markus W. Büchler, MD, Department of Surgery, University of Heidelberg, Im Neuenheimer Feld 110, D-69120 Heidelberg, Germany. E-mail: Markus_Buechler@med.uni-heidelberg.de.
REFERENCES
- 1.Edwards BK, Howe HL, Ries LA, et al. Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94:2766–2792. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. [DOI] [PubMed] [Google Scholar]
- 3.Sperti C, Pasquali C, Piccoli A, et al. Survival after resection for ductal adenocarcinoma of the pancreas. Br J Surg. 1996;83:625–631. [DOI] [PubMed] [Google Scholar]
- 4.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas: 201 patients. Ann Surg. 1995;221:721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lillemoe KD, Yeo CJ, Cameron JL. Pancreatic cancer: state-of-the-art care. CA Cancer J Clin. 2000;50:241–268. [DOI] [PubMed] [Google Scholar]
- 6.Buchler MW, Wagner M, Schmied BM, et al. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. 2003;138:1310–1314. [DOI] [PubMed] [Google Scholar]
- 7.Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy: 118 consecutive resections without an operative mortality. Ann Surg. 1990;211:447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassi C, Falconi M, Salvia R, et al. Management of complications after pancreaticoduodenectomy in a high volume centre: results on 150 consecutive patients. Dig Surg. 2001;18:453–457. [DOI] [PubMed] [Google Scholar]
- 10.Gouma DJ, van Geenen RC, van Gulik TM, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232:786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richter A, Niedergethmann M, Sturm JW, et al. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg. 2003;27:324–329. [DOI] [PubMed] [Google Scholar]
- 12.Kausch W. Das Carcinom der Papilla duodeni und seine radikale Entfernung. Beitr klin Chir. 1912;78:439–486. [Google Scholar]
- 13.Whipple AO. Treatment of carcinoma of the ampulla of Vater. Ann Surg. 1935;102:763–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson K. Carcinoma of the ampulla of Vater: successful radical resection. Br J Surg. 1944;31:368–373. [Google Scholar]
- 15.Traverso LW, Longmire WP Jr. Preservation of the pylorus in pancreaticoduodenectomy a follow-up evaluation. Ann Surg. 1980;192:306–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trede M, Carter DC. Surgery of the Pancreas. Edinburgh: Churchill Livingstone, 1993. [Google Scholar]
- 17.Seiler CA, Wagner M, Sadowski C, et al. Randomized prospective trial of pylorus-preserving vs. Classic duodenopancreatectomy (Whipple procedure): initial clinical results. J Gastrointest Surg. 2000;4:443–452. [DOI] [PubMed] [Google Scholar]
- 18.Williamson RC, Bliouras N, Cooper MJ, et al. Gastric emptying and enterogastric reflux after conservative and conventional pancreatoduodenectomy. Surgery. 1993;114:82–86. [PubMed] [Google Scholar]
- 19.Horstmann O, Markus PM, Ghadimi MB, et al. Pylorus preservation has no impact on delayed gastric emptying after pancreatic head resection. Pancreas. 2004;28:69–74. [DOI] [PubMed] [Google Scholar]
- 20.Wenger FA, Jacobi CA, Haubold K, et al. Gastrointestinal quality of life after duodenopancreatectomy in pancreatic carcinoma: preliminary results of a prospective randomized study: pancreatoduodenectomy or pylorus-preserving pancreatoduodenectomy. Chirurg. 1999;70:1454–1459. [DOI] [PubMed] [Google Scholar]
- 21.Warshaw AL, Torchiana DL. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy. Surg Gynecol Obstet. 1985;160:1–4. [PubMed] [Google Scholar]
- 22.Roder JD, Stein HJ, Huttl W, et al. Pylorus-preserving versus standard pancreatico-duodenectomy: an analysis of 110 pancreatic and periampullary carcinomas. Br J Surg. 1992;79:152–155. [DOI] [PubMed] [Google Scholar]
- 23.Park YC, Kim SW, Jang JY, et al. Factors influencing delayed gastric emptying after pylorus-preserving pancreatoduodenectomy. J Am Coll Surg. 2003;196:859–865. [DOI] [PubMed] [Google Scholar]
- 24.Bhandari M, Devereaux PJ, Montori V, et al. Users’ guide to the surgical literature: how to use a systematic literature review and meta-analysis. Can J Surg. 2004;47:60–67. [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins J, Green S (Eds). Cochrane Handbook of Systematic Reviews of Interventions, 4. 2.5 [updated May 2005]. Chichester, UK: John Wiley & Sons, 2005. [Google Scholar]
- 26.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 27.Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–1900. [DOI] [PubMed] [Google Scholar]
- 29.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. [DOI] [PubMed] [Google Scholar]
- 30.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 31.DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med. 1987;6:341–350. [DOI] [PubMed] [Google Scholar]
- 32.Lin PW, Shan YS, Lin YJ, et al. Pancreaticoduodenectomy for pancreatic head cancer: PPPD versus Whipple procedure. Hepatogastroenterology. 2005;52:1601–1604. [PubMed] [Google Scholar]
- 33.Seiler CA, Wagner M, Bachmann T, et al. Randomized clinical trial of pylorus-preserving duodenopancreatectomy versus classical Whipple resection-long term results. Br J Surg. 2005;92:547–556. [DOI] [PubMed] [Google Scholar]
- 34.Tran KT, Smeenk HG, van Eijck CH, et al. Pylorus preserving pancreaticoduodenectomy versus standard Whipple procedure: a prospective, randomized, multicenter analysis of 170 patients with pancreatic and periampullary tumors. Ann Surg. 2004;240:738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bloechle C, Broering DC, Latuske C, et al. Prospective randomized study to evaluate quality of life after partial pancreatoduodenectomy according to Whipple versus pylorus preserving pancreatoduodenectomy according to Longmire-Traverso for periampullary carcinoma. Dtsch Gesellschaft Chir. 1999;(suppl 1):661–664. [Google Scholar]
- 36.Paquet K-J. Vergleich der partiellen Duodenopankreatektomie (Whipple-Operation) mit der pyloruserhaltenden Zephaloduodenopankreatektomie: eine prospektive kontrollierte, randomisierte Langzeitstudie. Chir Gastroenterol. 1998;14:54–58. [Google Scholar]
- 37.Bassi C, Stocken DD, Olah A, et al. Influence of surgical resection and post-operative complications on survival following adjuvant treatment for pancreatic cancer in the ESPAC-1 randomized controlled trial. Dig Surg. 2005;22:353–363. [DOI] [PubMed] [Google Scholar]
- 38.Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234:758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosca F, Giulianotti PC, Balestracci T, et al. Long-term survival in pancreatic cancer: pylorus-preserving versus Whipple pancreatoduodenectomy. Surgery. 1997;122:553–566. [DOI] [PubMed] [Google Scholar]
- 40.Egger M, Smith GD. Meta-analysis: potentials and promise. BMJ. 1997;315:1371–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulrow CD. Rationale for systematic reviews. BMJ. 1994;309:597–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. [DOI] [PubMed] [Google Scholar]
- 43.Yeo CJ, Cameron JL, Sohn TA, et al. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: comparison of morbidity and mortality and short-term outcome. Ann Surg. 1999;229:613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farnell MB, Pearson RK, Sarr MG, et al. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005;138:618–628. [DOI] [PubMed] [Google Scholar]
- 46.Bell RH Jr. Pancreaticoduodenectomy with or without pylorus preservation have similar outcomes. Cancer Treat Rev. 2005;31:328–331. [DOI] [PubMed] [Google Scholar]
- 47.Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–594. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen TC, Sohn TA, Cameron JL, et al. Standard vs. radical pancreaticoduodenectomy for periampullary adenocarcinoma: a prospective, randomized trial evaluating quality of life in pancreaticoduodenectomy survivors. J Gastrointest Surg. 2003;7:1–9. [DOI] [PubMed] [Google Scholar]
- 49.Shan YS, Sy ED, Lin PW. Role of somatostatin in the prevention of pancreatic stump-related morbidity following elective pancreaticoduodenectomy in high-risk patients and elimination of surgeon-related factors: prospective, randomized, controlled trial. World J Surg. 2003;27:709–714. [DOI] [PubMed] [Google Scholar]
- 50.Lin PW, Lin YJ. Prospective randomized comparison between pylorus-preserving and standard pancreaticoduodenectomy. Br J Surg. 1999;86:603–607. [DOI] [PubMed] [Google Scholar]
- 51.Pedrazzoli S, DiCarlo V, Dionigi R, et al. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg. 1998;228:508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berge Henegouwen MI, Moojen TM, van Gulik TM, et al. Postoperative weight gain after standard Whipple's procedure versus pylorus-preserving pancreatoduodenectomy: the influence of tumour status. Br J Surg. 1998;85:922–926. [DOI] [PubMed] [Google Scholar]
- 53.Chou FF, Sheen-Chen SM, Chen YS, et al. Postoperative morbidity and mortality of pancreaticoduodenectomy for periampullary cancer. Eur J Surg. 1996;162:477–481. [PubMed] [Google Scholar]
- 54.Brennan MF, Pisters PW, Posner M, et al. A prospective randomized trial of total parenteral nutrition after major pancreatic resection for malignancy. Ann Surg. 1994;220:436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friess H, Klempa I, Hermanek P, et al. Prophylaxis of complications after pancreatic surgery: results of a multicenter trial in Germany. Digestion. 1994;55(suppl 1):35–40. [DOI] [PubMed] [Google Scholar]
- 56.Bassi C, Falconi M, Lombardi D, et al. Prophylaxis of complications after pancreatic surgery: results of a multicenter trial in Italy: Italian Study Group. Digestion. 1994;55(suppl 1):41–47. [DOI] [PubMed] [Google Scholar]
- 57.Johnstone PA, Sindelar WF. Lymph node involvement and pancreatic resection: correlation with prognosis and local disease control in a clinical trial. Pancreas. 1993;8:535–539. [DOI] [PubMed] [Google Scholar]
- 58.Bakkevold KE, Kambestad B. Morbidity and mortality after radical and palliative pancreatic cancer surgery: risk factors influencing the short-term results. Ann Surg. 1993;217:356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buchler M, Friess H. Prevention of postoperative complications following pancreatic surgery. Digestion. 1993;54(suppl 1):41–46. [DOI] [PubMed] [Google Scholar]