Abstract
Objectives:
The aims of this study were to determine if statins reduce adhesion formation in vivo and to identify the mechanism of action in vitro.
Background:
Intraperitoneal adhesions develop in up to 95% of patients following laparotomy. Adhesions are reduced by mechanisms that up-regulate fibrinolysis within the peritoneum. Statins promote fibrinolysis in the cardiovascular system and may play a role in the prevention of adhesions.
Methods:
Adhesions were induced in rats (n = 102) using our previously described ischemic button model. Rats received vehicle (controls), lovastatin (30 mg/kg), or atorvastatin (30 mg/kg) as a single intraperitoneal dose at the time of laparotomy. Animals were killed and adhesions were quantified at day 7. Peritoneal fluid and tissue were collected at day 1 to measure tissue plasminogen activator (tPA) and plasminogen activator inhibitor-1 (PAI-1) by real-time PCR and ELISA. To assess the effects of statins on wound healing, burst pressures were measured in anastomoses of the colon. The effects of lovastatin on tPA and PAI-1 production were measured in vitro in human mesothelial cells (HMC) in the presence or absence of mevalonate (MVA), geranylgeranyl-pyrophosphate (GGPP) and farnesyl-pyrophosphate (FPP), all intermediates in the cholesterol pathway downstream of HMG-CoA. The effect of a Rho protein inhibitor, exoenzyme C3 transferase, on tPA production was also determined.
Results:
Lovastatin and atorvastatin reduced adhesion formation by 26% and 58%, respectively (P < 0.05), without affecting anastomotic burst pressure. At 24 hours, tPA mRNA levels in peritoneal tissue and tPA activity in peritoneal fluid from lovastatin-treated animals were increased by 57% and 379%, respectively (P < 0.05), while PAI-1 levels were unchanged. HMC incubated with either lovastatin or atorvastatin showed concentration-dependent increases in tPA production and decreases in PAI-1 production (P < 0.05). These lovastatin-induced changes in tPA and PAI-1 production were significantly reversed by the addition of MVA, GGPP, and FPP. The Rho protein inhibitor increased tPA production and rescued tPA production from the inhibitory effect of GGPP.
Conclusion:
These data suggest that statins administered within the peritoneum can up-regulate local fibrinolysis, while the in vitro studies show that this effect may be mediated, in part, by intermediates of the cholesterol biosynthetic pathway that regulate Rho protein signaling.
Adhesion formation occurs commonly after abdominal surgery. This occurs in part due to impaired fibrinolysis within the peritoneum, which is primarily governed by the balance between tPA and its inhibitor, PAI-1. Our rat model of adhesions demonstrates that the intraperitoneal administration of statins reduces adhesion formation by up-regulating a profibrinolytic environment.
Postoperative adhesions, formed after abdominal surgery, pose a significant clinical and financial problem. Up to 95% of patients develop adhesions after surgery,1–4 the majority of which have increased risk of long-term sequelae, including small bowel obstruction (SBO), chronic pelvic pain, infertility, and difficult reoperative surgery.3,5–7
In addition to meticulous surgical technique, several approaches have been used in the prevention of adhesions including: anti-inflammatory agents, fibrinolytic agents, antibiotics, and synthetic solid barriers.3,8 However, none of these methods has proven to be uniformly efficacious under all surgical conditions. Ultimately, a more profound understanding of the mechanisms of normal peritoneal healing and the molecular and cellular components involved in the formation of adhesions will expedite a safe and efficient strategy of adhesion prevention.
Adhesion formation begins with trauma to the peritoneal surfaces. This local injury results in the production of an inflammatory exudate, sometimes connecting 2 adjacent visceral structures creating fibrin bands. Under normal conditions, these bands are resolved by fibrinolysis. However, under ischemic or inflammatory conditions, the peritoneal fibrinolytic system is suppressed and these bands are infiltrated with inflammatory cells and fibroblasts to organize into dense adhesions. Fibrin is principally degraded by plasmin, a protease converted from plasminogen by 2 plasminogen activators (PA), tissue (tPA), and urokinase (uPA). tPA, which is produced primarily by mesothelial cells and inactivated by plasminogen activator inhibitor-1 (PAI-1), is the primary PA in the peritoneum.3,5,8–10
HMG-CoA reductase inhibitors (statins) inhibit the rate-limiting enzyme in the production of cholesterol. This class of drugs has a well-studied lipid-lowering benefit; however, recent studies have elucidated additional effects of statins beyond their impact on serum cholesterol levels. They have been shown to have potent anti-inflammatory, antioxidant, and pro-fibrinolytic properties,11–15 all of which may play a role in the prevention of adhesion formation. Using in vitro experiments, Haslinger et al15,16 provided strong evidence that simvastatin is a potent stimulator of fibrinolytic activity in human mesothelial cells both under normal and inflammatory conditions. Despite these findings, there are no in vivo studies demonstrating that statins reduce adhesion formation.
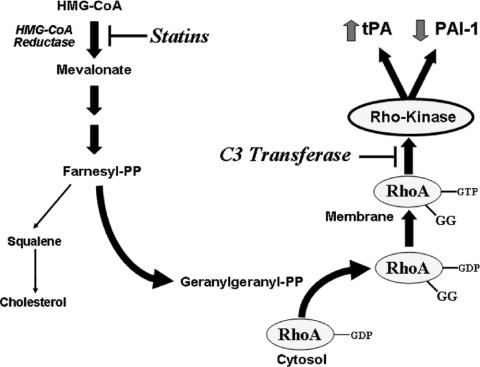
The effects of statins on the fibrinolytic pathway are mainly mediated by the inhibition of the Rho pathway (Fig. 1), which has also been implicated in critical relays in signal transduction, regulating various cellular processes such as cell growth, differentiation, and survival.17 The aims of this study were to determine whether statins could reduce postoperative adhesion formation in vivo and to elucidate the mechanism of action in vitro.
FIGURE 1. Effects of the inhibition of HMG-CoA reductase by statins. By blocking cholesterol biosynthesis, HMG-CoA reductase inhibitors or statins also decrease the synthesis of key isoprenoid intermediates such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP). GGPP mediates the isoprenylation or geranylgeranylation of Rho proteins, a critical step in the translocation of Rho proteins from the cytoplasm to the plasma membrane where they initiate downstream signaling via Rho-Kinase. The activation of Rho proteins by GGPP is an important signaling event in fibrinolysis by modulating tissue plasminogen activator (tPA) and plasminogen activator inhibitor-1 (PAI-1) levels. Exoenzyme C3 transferase (C3 transferase) selectively blocks RhoA/B/C signaling. Adapted from Budzyn et al.23
MATERIALS AND METHODS
Materials
All chemicals were obtained from Sigma unless otherwise noted.
In Vivo Studies
Animals
Male Wistar rats 150 to 175 g (Charles River Breeding Laboratories) were used for all experiments. The animals were housed at a constant room temperature, with 12-hour light and dark cycles, and were provided standard rodent chow (Purina, catalog no. 5001) and water ad libitum. The Institutional Animal Care and Use Committee at Boston University School of Medicine approved these studies. All animal care and procedures were performed in accordance with the recommendations outlined in the NIH Guide for the Care and Use of Laboratory Animals.
Determination of the Effect of Statins on Peritoneal Adhesion Formation
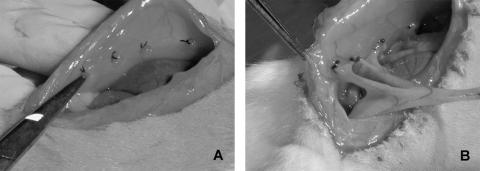
Laparotomies via a midline incision were performed under isoflurane anesthesia in 102 male Wistar rats and 3 or 4 ischemic buttons were created approximately 1 cm apart on each peritoneal sidewall by discretely ligating small areas of peritoneum with 4-0 silk sutures as we have previously described18 (Fig. 2). To assess the effect of statins on adhesion formation, 54 animals were randomized to receive a single intraoperative peritoneal bolus (1 mL total volume) of lovastatin (30 mg/kg), atorvastatin (30 mg/kg), or the vehicle control (DMSO), which remained within the peritoneum. To assess the therapeutic window for administration, 20 animals were also randomized to receive delayed administration of lovastatin (30 mg/kg), atorvastatin (30 mg/kg), or vehicle control via intraperitoneal injections 6 hours or 24 hours after closure of the laparotomy. After 24 hours and 7 days, the animals were killed and the number of adhesions to each ischemic button was quantified.19 Each animal received a percent adhesion score based on the number of ischemic buttons with attached adhesions.
FIGURE 2. A, Creation of ischemic buttons to peritoneal sidewall as previously discribed (see reference 19). B, Adhesions to buttons at postoperative day 7. Reprinted from J Surg Res, 108 (1), Reed KL, Fruin AB, Bishop-Bartolomei KK, et al. Neurokinin-1 receptor and substance P messenger RNA levels increase during intraabdominal adhesion formation, pp 165–172, Copyright (2002) with permission from Elsevier.
Determination of tPA Activity in Peritoneal Fluid
Since our previous studies18 showed that tPA mRNA levels rose significantly by 24 hours after the administration of a neurokinin-1 receptor antagonist, we chose the 24-hour time point to measure tPA activity in peritoneal fluid and mRNA expression in peritoneal tissue. The peritoneal fluid of operative and nonoperative animals was determined using a kinetic assay. Briefly, 50 μL of each sample was mixed in 96-well plates with an assay buffer containing: 2.11 mL of plasminogen (Athens Research and Technology, Georgia), 2.11 mL of S-2251 (DiaPharma, Columbus, OH), a chromogenic substrate for plasmin, 6.33 mL of dilution buffer (50 mmol/L Tris, pH 8.3), and 5.275 mL of Milli-Q water for up to 8 hours. The change in absorbance per second at 405 nm was then measured using a spectrophotometer (SpectroMax 250, Molecular Devices).
RNA Isolation and Quantitative PCR
Prior to death, peritoneal tissue directly adjacent to and including the ischemic button was collected from animals in each treatment group and stored at −80°C until use. Peritoneal tissues were collected from nonoperated animals as well. Total RNA was isolated from this tissue with the SV Total RNA Isolation System (Promega). Quantitative real-time PCR was performed and analyzed with the ABI Prism 7000 Sequence Detection System (Applied Biosystems). The following primer sets were used to amplify tPA and PAI-1: tPA, 5′-CAGCAGGCCCTGTATTTCTC-3′ (sense) and 5′-TGCTCCATGTGCCTCTGTAG-3′ (antisense); PAI-1 5′-ATCAACGACTGGGTGGAGAG-3′ (sense) and 5′-TGAAATAGAGGGCGTTCACC-3′ (antisense). Actin, with the following primer sequences, was used as a housekeeping gene: 5′-CGAGGCCCAGAGCAAGAGAG-3′ (sense) and 5′-CGGTTGGCCTTAGGGTTCAG-3′ (antisense).
Anastomotic Strength
To assess whether statins have an impact on wound healing, a well-established model of anastomotic healing was used.20 Sixteen male Wistar rats (200–250 g) were anesthetized using isoflurane and a lower midline incision was performed. Segments of colon approximately 2 cm distal to the cecum were identified and completely transected, following which continuity was restored by an end-to-end anastomosis using a continuous 6-0 polypropylene suture. Prior to closure of the abdomen, lovastatin (30 mg/kg) or vehicle control (DMSO) was administered within the peritoneum. The animals were carefully monitored postoperatively for 7 days for signs of bowel obstruction. On postoperative day 7, the animals were killed and anastomotic segments approximately 4 cm in length (with the anastomosis in the middle), were carefully resected, including surrounding tissue and adhesions. The specimens were washed in saline and the stool was removed from the lumen. The anastomotic bursting pressure was measured as previously described.20,21 Briefly, one end of the bowel segment was cannulated with a short segment of 8-Fr intravenous tubing and secured with 0-silk suture. The other end was occluded with an atraumatic bowel clamp, and the entire segment was submerged in water while being insufflated with a constant flow of air (1–2 mL/s) through bifurcated tubing with a digital manometer (Precision Digital, Natick, MA) attached to one end and a 60-mL syringe to the other. The burst pressure was defined as the pressure at which the bowel segment leaked air.
In Vitro Studies
Mesothelial Cell Culture
A mesothelial cell culture system was established to determine the mechanisms by which statins and intermediates of the cholesterol biosynthetic pathway regulate tPA and PAI-1 production. LP-9 cells, an untransformed human mesothelial cell (HMC) line, were obtained from the Coreill Institute for Medical Research (Camden, NJ). All mesothelial cells were cultured (5% CO2, 95% air at 37°C) in HAM's F-12/Medium 199 with l-glutamine (1:1) (Cellgro) supplemented with 15% fetal bovine serum, 0.05 μg/mL hydrocortisone, 10 ng/mL epidermal growth factor, 10,000 U/mL penicillin G, 25 μg/mL amphotericin B, and 10,000 μg/mL streptomycin. Medium was replaced twice weekly and experiments were carried out on cells from the third to seventh passage. Conditioned media was obtained by incubating cells at 37°C for up to 48 hours in 6-well plates with 1 mL of media per well containing the appropriate concentration of lovastatin (Calbiochem) (activated as previously described),22 atorvastatin (Pfizer), mevalonate (100 μmol/L) (MVA), farnesyl pyrophosphate (10 μmol/L) (FPP), geranylgeranyl pyrophosphate (10 μmol/L) (GGPP), vehicle (DMSO, final concentration 0.125% [vol/vol] in serum free media), or the Rho protein inhibitor, exoenzyme C3 transferase (8.33 μg/mL) (Cytoskeleton). Samples of conditioned media were centrifuged to remove cells and/or debris and frozen at −80°C until use.
Throughout all the experiments, the treated cells maintained their normal morphology compared with untreated controls and there was no evidence of cell rounding or excessive vacuolation to suggest that the cells were undergoing apoptosis. In addition, the cells continued to produce copious amounts of tPA, further indicating that these cells were functioning normally and not metabolically challenged by the treatment.
Determination of tPA and PAI-1 Production
tPA and PAI-1 antigens in conditioned media were measured with Biopool TintElize ELISA kits according to the manufacturer's instructions (DiaPharma, Columbus, OH).
Statistical Analysis
Data are expressed as mean ± SEM and were analyzed by one-way ANOVA using SigmaStat statistical software (SPSS, Chicago, IL). When significant (P value <0.05), the difference between specific means was determined using the Student-Newman-Keuls test.
RESULTS
Intraperitoneal Administration of Lovastatin and Atorvastatin Decreases Adhesion Formation
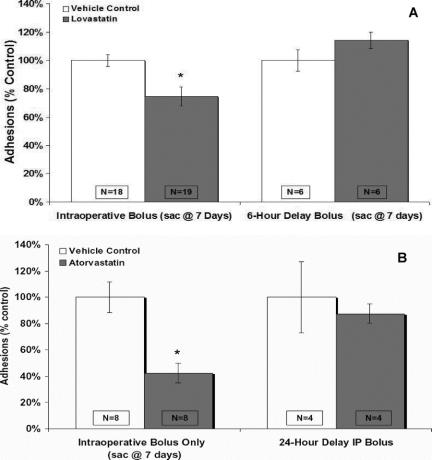
Twenty-four hours and 7 days after the initial laparotomy, adhesions in animals administered an intraoperative bolus of lovastatin (30 mg/kg) were significantly (P < 0.05) decreased by 32% and 26%, respectively, compared with animals administered vehicle control (Fig. 3A). Similarly, 7 days after the initial laparotomy, adhesions to the ischemic buttons in animals administered a single intraoperative bolus of atorvastatin (30 mg/kg) were significantly (P < 0.05) decreased by 57% compared with animals administered vehicle control (DMSO) (Fig. 3B). Interestingly, if the intraperitoneal administration of atorvastatin or lovastatin was delayed by 6 hours or 24 hours, respectively, there was no reduction in adhesion formation (Fig. 3). This suggests that there may be a “therapeutic window” for the administration of the HMG-CoA reductase inhibitor at least within the first 6 hours of peritoneal insult to prevent adhesion formation. Twelve male Wistar rats were also given either an oral dose of atorvastatin (30 mg/kg) or vehicle control daily for 7 days prior to and after laparotomy, and we found that there was no statistically significant difference in the number of adhesions formed. This implies that the statins have an important localized effect on mesothelial function and oral administration may not raise peritoneal concentrations to therapeutic levels.
FIGURE 3. The intraperitoneal administration of lovastatin (30 mg/kg) and atorvastatin (30 mg/kg) in rats. Lovastatin (A) and atorvastatin (B) administered as a single peritoneal bolus at the time of laparotomy significantly decreased peritoneal adhesion formation compared with controls. Delay in administration resulted in no reduction in adhesion formation. Results are expressed as a percent control ± % SEM. *P < 0.05 compared with vehicle controls.
Lovastatin Administration Increases tPA Expression and Activity in the Peritoneum
At 24 hours, peritoneal tissue immediately surrounding the ischemic buttons (∼1 cm) was collected in each treatment group and nonoperative controls, which were animals that did not undergo an initial laparotomy for creation of ischemic buttons, but were instead killed after the administration of anesthesia. Nonoperated controls were used instead of sham-operated controls for comparative purposes since our previous work has shown that the midline laparotomy alone causes enough trauma to induce marked biochemical alterations in the peritoneum that may compromise tPA levels.19 Subsequently, RNA was isolated and quantitative PCR demonstrated that there was a significant (P < 0.05) increase in tPA mRNA expression in the animals administered lovastatin (30 mg/kg) compared with both operative and nonoperative controls, by 57% and 346% respectively (Fig. 4A). While PAI-1 mRNA expression was significantly (P < 0.05) increased compared with nonoperative control animals, there was no significant difference in PAI-1 mRNA expression between operative groups (Fig. 4B). tPA activity in the peritoneal fluid isolated from animals administered an intraperitoneal bolus of lovastatin and killed at 24 hours was 379% higher than operative controls and 430% higher than nonoperative controls (Fig. 5).
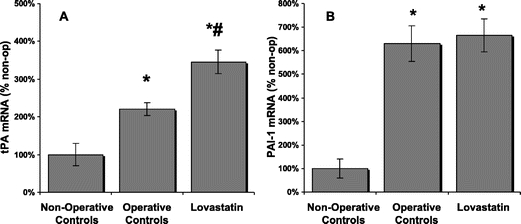
FIGURE 4. tPA and PAI-1 mRNA expression in peritoneal tissue. Lovastatin-treated animals (n = 8) showed a significant increase in tPA mRNA expression (A) compared with operative (*) (n = 6) and nonoperative controls (#) (n = 3). While PAI-1 mRNA expression (B) was significantly increased compared with nonoperative controls; there was no difference between treatment groups. Results are expressed as a percent of nonoperative controls ± % SEM. *P < 0.05 compared with nonoperative controls. #P < 0.05 compared with operative controls.
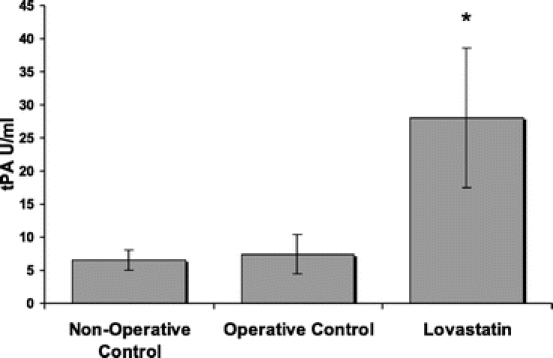
FIGURE 5. tPA activity in peritoneal fluid. Following the intraperitoneal administration of lovastatin (n = 9), there was a significant increase in tPA activity in the peritoneal fluid. Results shown as mean ± SEM. *P < 0.05 compared with operative controls (n = 10) and nonoperative controls (n = 4).
Lovastatin Administration Does Not Impair Anastomotic Healing
On postoperative day 7, lovastatin-treated and control animals were killed and the previously created colonic anastomoses were excised with care taken not to cause iatrogenic enterotomies. These 3- to 4-cm segments of colon (with the anastomoses in the middle) were then insufflated with air until perforation and the burst pressures were measured. We found that the colonic segments of the lovastatin-treated group had higher burst pressures (376 ± 13.1 mm Hg) compared with controls (283.6 ± 50.6 mm Hg) (P < 0.05). There was no gross evidence of anastomotic dehiscence in either group at the time of death, and there was one animal inthe control group that died due to intestinal obstruction. In control animals, 50% of the colonic ruptures occurred at the anastomosis and the remainder occurred adjacent to the anastomosis. In lovastatin-treated animals, 43% of ruptures occurred at the anastomosis.
Statins Increase tPA and Decrease PAI-1 Production In Vitro
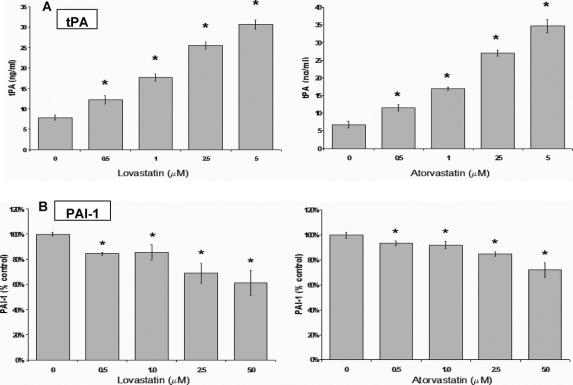
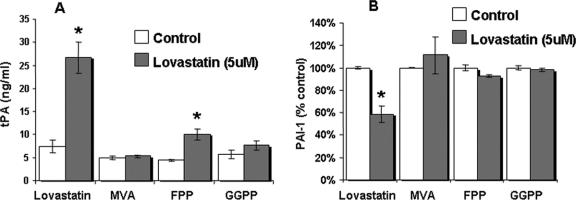
LP-9 cells incubated with lovastatin or atorvastatin for 48 hours showed a concentration-dependent increase in tPA production (Fig. 6A) with more than a 4-fold difference at the highest concentration (5 μmol/L) compared with control. Lovastatin induced a significant (P < 0.05) increase in tPA production starting at 0.5 μmol/L (12.2 ± 0.9 ng/mL), reaching even greater effect at 5 μmol/L (30.7 ± 0.9 ng/mL) versus control (7.8 ± 1.0 ng/mL). Conversely, LP-9 cells treated with lovastatin for 48 hours showed a significant (P < 0.05) concentration-dependent decrease in PAI-1 production with a 40% decrease at the highest concentration (5 μmol/L) compared with control (Fig. 6B). Overall, there was an 8-fold increase in the tPA/PAI-1 ratio at the highest concentration, reflecting a profound increase in the overall fibrinolytic potential of HMC in response to lovastatin. The third generation statin, atorvastatin, produced an even more pronounced result, increasing tPA production by almost 6-fold at 5 μmol/L (34.7 ng/mL ± 2.6) and decreasing PAI-1 production by 29% at the same concentration compared with control (Fig. 6, right panels).
FIGURE 6. Concentration-dependent effect of lovastatin and atorvastatin on (A) tPA and (B) PAI-1 production in LP-9 cells. Results for tPA shown are mean ± SEM. PAI-1 results expressed as a percent of control ± % SEM. *P < 0.05 compared with control (n = 4).
Intermediates of the Cholesterol Synthesis Pathway Reverse the Effects of Lovastatin on HMC
To identify the underlying molecular basis for the effect of lovastatin on tPA and PAI-1 production in HMC, we investigated the role of downstream intermediates in the cholesterol synthesis pathway (Fig. 1) on fibrinolysis in a cellculture model. HMC incubated with mevalonate (100 μmol/L), the product of the HMG-CoA reductase reaction and the precursor for the synthesis of the isoprenoids (FPP and GGPP), completely inhibited the effect of lovastatin (5μmol/L) on tPA (Fig. 7A) and PAI-1 (Fig. 7B) production compared with controls.
FIGURE 7. Intermediates of the cholesterol biosynthetic pathway inhibit fibrinolysis. Mevalonate (100 μmol/L) (MVA), geranylgeranyl pyrophosphate (10 μmol/L) (GGPP), and farnesyl pyrophosphate (10 μmol/L) (FPP) significantly reverse tPA (A) and PAI-1 (B) production in LP-9 cells treated with lovastatin (5 μmol/L). tPA results shown are mean ± SEM. PAI-1 results expressed as a percent of control ± % SEM. *P < 0.05 compared with control (n = 3). HMC were incubated in the presence ( ) or absence (□) of lovastatin 5 μmol/L.
) or absence (□) of lovastatin 5 μmol/L.
As previously mentioned, isoprenoids are integral in the post-translational modification of specific proteins playing a role in diverse signaling events. Incubation of HMC with GGPP (10 μmol/L) and FPP (10 μmol/L) also significantly abolished the effect of lovastatin (5 μmol/L) on both tPA (Fig. 7A) and PAI-1 production (Fig. 7B).
A Rho Protein Inhibitor Modulates tPA Production
It has been previously reported that the activation and translocation of the Rho protein to the cell membrane from the cytoplasm may be dependent on geranylgeranylation;23 therefore, to identify the effect of the Rho protein on fibrinolysis, HMCs were incubated with a Rho protein inhibitor, exoenzyme C3 transferase (8.33 μg/mL). tPA production was significantly increased by 260% compared with control and rose to levels comparable to those seen with lovastatin (5 μmol/L) administration (Fig. 8). Furthermore, the addition of exoenzyme C3 transferase was able to increase tPA production by 213% in the presence of GGPP, which was previously shown to impair fibrinolytic potential in HMC incubated with lovastatin.
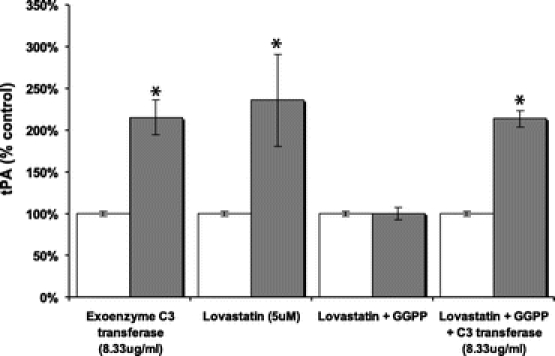
FIGURE 8. The Rho protein inhibitor, C3 transferase, significantly increases tPA Production and reverses the inhibition of GGPP (10 μmol/L) on tPA production in HMC. Results expressed as a percent of control ± % SEM. *P < 0.05 compared with control (n = 3). □ untreated,  treated as noted.
treated as noted.
DISCUSSION
The data presented in this study demonstrate that the intraperitoneal administration of the HMG-CoA reductase inhibitors lovastatin and atorvastatin, at least within 6 hours of initial peritoneal insult, can reduce intra-abdominal adhesion formation, in part, by up-regulating fibrinolysis in a rat model. Furthermore, this effect may be mediated through the inhibition of Rho protein, as is evident from in vitro data that demonstrate that a Rho inhibitor both increases tPA production to levels similar to those achieved with lovastatin and, additionally, is able to rescue this profibrinolytic state from the inhibitory effect of the downstream intermediates of the cholesterol pathway.
Postoperative adhesions remain a significant problem for patients and surgeons following abdominal and, especially, colorectal surgery. It has been recently reported that the presence of adhesions is among the strongest risk factors leading to bowel resection in patients with SBO.24 While there have been studies elucidating the pathogenesis of adhesion formation and some progress in the prevention of adhesions, there is still convincing literature such as the Surgical and Clinical Adhesions Research study,25 among others, that has demonstrated that the overall incidence of adhesion-related readmissions has not diminished despite improvements in surgical technique, the use of adhesion-prevention products,4 or the advent of minimally invasive surgery. Fevang et al26 concluded that adhesions could produce long-term morbidity with considerable risk up to 20 years after laparotomy, carrying a cumulative risk for adhesive small bowel obstruction of approximately 29% following one previous abdominal surgery. Therefore, a more profound understanding of the molecular and cellular components involved in the formation of adhesions will propel a safe and efficient strategy of adhesion prevention.
As discussed previously, the genesis of adhesion formation is trauma to the peritoneal surface, resulting in the initiation of an inflammatory response. Subsequently, there is production of a fibrin-rich inflammatory exudate, which, if not resolved by fibrinolysis, organizes to form dense adhesions. Decreased peritoneal fibrinolytic capacity due to an imbalance of tPA and PAI-1 has been shown to play a pivotal role in the formation of postoperative adhesions. However, studies that have directly instilled tPA within the peritoneum have shown a significant risk of both hemorrhagic and wound healing complications.3,27,28 Consequently, compounds that optimize the fibrinolytic potential within the peritoneum by increasing tPA and decreasing PAI-1 without causing significant hemorrhagic complication or impairing wound healing may be of importance in strategies for adhesion prevention. This study demonstrates that statins are effective in reducing intra-abdominal adhesions by promoting a profibrinolytic environment in the peritoneum without imposing peritoneal hemorrhage or impairing anastomotic healing. Furthermore, our data, showing that statins administered 6 hours or more after surgery do not inhibit adhesion formation, suggest that the cascade of events leading to newly forming adhesions must be modulated within hours to prevent adhesion formation. This is a critical consideration in designing strategies to prevent adhesions.
In addition to their anti-inflammatory and antioxidant capabilities, both of which are certain to be important in adhesion prevention, statins have been shown to be potent modulators of fibrinolysis under normal and inflammatory conditions.16 While this effect has been well studied within the vascular compartment, there has been limited focus in the literature on the mesothelium. By promoting fibrinolysis, this study demonstrates that statins can play a role in the reduction of adhesion formation in a rat model. In vivo, lovastatin significantly increased tPA mRNA expression in peritoneal tissue and tPA activity in peritoneal fluid. In HMC in vitro, lovastatin and atorvastatin both induced a profound increase in tPA production and simultaneously reduced PAI-1. This effect could be negated in vitro by the addition of cholesterol intermediates, mevalonate, FPP, and GGPP, indicating a regulatory mechanism possibly involving intervention in the mevalonate-GGPP pathway.
It is well established that the small GTPase Rho (particularly its Rho A isoform) can regulate numerous cellular functions, primarily through its downstream effector Rho-kinase23 (Fig. 1). The RhoA, RhoB, and RhoC isoforms belong to the Ras superfamily of GTPases that typically cycle between an inactive cytosolic GDP-bound form and an active membrane GTP-bound form. GTP-bound Rho recognizes and interacts with its effector proteins to initiate a downstream response.29 In blocking cholesterol biosynthesis, statins also prevent the formation of isoprenoid intermediates, including GGPP, which is required for the geranylgeranylation of RhoA. The isoprenylation of Rho is a prerequisite for Rho activation and mediates its interaction with the plasma membrane (Fig. 1). By preventing this membrane interaction, statins can rapidly inactivate RhoA, leading to increased tPA expression and activity in peritoneal mesothelial cells. Thus, as our in vivo data suggest, even a single dose of a statin is efficacious in reducing adhesion formation in a rat model. As our in vitro data demonstrate, selectively inhibiting the geranylgeranylated-activated Rho protein further downstream with C3 transferase reproduces the effect of lovastatin on mesothelial cell tPA levels and blocks its reversal by GGPP (Fig. 8). This is another indication that statins mediate peritoneal fibrinolytic activity via the Rho-Rho kinase pathway.
CONCLUSION
Our data provide direct evidence from both in vivo and in vitro studies that statins reduce intra-abdominal adhesion formation by modulating the peritoneal fibrinolytic environment. Whether statins prove to be therapeutically efficacious in adhesion prevention in humans remains to be investigated. However, this study has identified a pathway, the Rho/Rho kinase signaling pathway, which appears to be intimately involved in the regulation of the peritoneal fibrinolytic pathway. This signaling pathway offers many potential therapeutic approaches and consideration should perhaps be given to widening the application of existing drugs known to modulate Rho-Rho kinase signaling.
Symbol.

Footnotes
Reprints: James M. Becker, MD, Department of Surgery, Boston University School of Medicine, 88 East Newton Street/C500, Boston, MA 02118. E-mail: james.becker@bmc.org.
REFERENCES
- 1.Menzies D. Peritoneal adhesions: incidence, cause, and prevention. Surg Annu. 1992;24:27–45. [PubMed] [Google Scholar]
- 2.Parker MC, Wilson MS, Menzies D, et al. Colorectal surgery: the risk and burden of adhesion-related complications. Colorectal Dis. 2004;6:506–511. [DOI] [PubMed] [Google Scholar]
- 3.Liakakos T, Thomakos N, Fine PM, et al. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg. 2001;18:260–273. [DOI] [PubMed] [Google Scholar]
- 4.Becker JM, Dayton MT, Fazio VW, et al. Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane: a prospective, randomized, double-blind multicenter study. J Am Coll Surg. 1996;183:297–306. [PubMed] [Google Scholar]
- 5.Cheong YC, Laird SM, Li TC, et al. Peritoneal healing and adhesion formation/reformation. Hum Reprod Update. 2001;7:556–566. [DOI] [PubMed] [Google Scholar]
- 6.Parker MC, Ellis H, Moran BJ, et al. Postoperative adhesions: ten-year follow-up of 12,584 patients undergoing lower abdominal surgery. Dis Colon Rectum. 2001;44:822–829; discussion 829–830. [DOI] [PubMed]
- 7.Miller G, Boman J, Shrier I, et al. Natural history of patients with adhesive small bowel obstruction. Br J Surg. 2000;87:1240–1247. [DOI] [PubMed] [Google Scholar]
- 8.DiZerega GS. Peritoneal Surgery. New York: Springer, 2000. [Google Scholar]
- 9.diZerega GS, Campeau JD. Peritoneal repair and post-surgical adhesion formation. Hum Reprod Update. 2001;7:547–555. [DOI] [PubMed] [Google Scholar]
- 10.Falk K, Bjorquist P, Stromqvist M, et al. Reduction of experimental adhesion formation by inhibition of plasminogen activator inhibitor type 1. Br J Surg. 2001;88:286–289. [DOI] [PubMed] [Google Scholar]
- 11.Bruni F, Pasqui AL, Pastorelli M, et al. Effect of atorvastatin on different fibrinolysis mechanisms in hypercholesterolemic subjects. Int J Cardiol. 2004;95:269–274. [DOI] [PubMed] [Google Scholar]
- 12.Rosenson RS. Statins in atherosclerosis: lipid-lowering agents with antioxidant capabilities. Atherosclerosis. 2004;173:1–12. [DOI] [PubMed] [Google Scholar]
- 13.Schonbeck U, Libby P. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation. 2004;109(suppl 1):II18–II26. [DOI] [PubMed] [Google Scholar]
- 14.Wolfrum S, Jensen KS, Liao JK. Endothelium-dependent effects of statins. Arterioscler Thromb Vasc Biol. 2003;23:729–736. [DOI] [PubMed] [Google Scholar]
- 15.Haslinger B, Goedde MF, Toet KH, et al. Simvastatin increases fibrinolytic activity in human peritoneal mesothelial cells independent of cholesterol lowering. Kidney Int. 2002;62:1611–1619. [DOI] [PubMed] [Google Scholar]
- 16.Haslinger B, Kleemann R, Toet KH, et al. Simvastatin suppresses tissue factor expression and increases fibrinolytic activity in tumor necrosis factor-alpha-activated human peritoneal mesothelial cells. Kidney Int. 2003;63:2065–2074. [DOI] [PubMed] [Google Scholar]
- 17.Ghittoni R, Patrussi L, Pirozzi K, et al. Simvastatin inhibits T-cell activation by selectively impairing the function of Ras superfamily GTPases. FASEB J. 2005;19:605–607. [DOI] [PubMed] [Google Scholar]
- 18.Reed KL, Fruin AB, Gower AC, et al. A neurokinin 1 receptor antagonist decreases postoperative peritoneal adhesion formation and increases peritoneal fibrinolytic activity. Proc Natl Acad Sci USA. 2004;101:9115–9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed KL, Fruin AB, Bishop-Bartolomei KK, et al. Neurokinin-1 receptor and substance P messenger RNA levels increase during intraabdominal adhesion formation. J Surg Res. 2002;108:165–172. [DOI] [PubMed] [Google Scholar]
- 20.Hadaegh A, Burns J, Burgess L, et al. Effects of hyaluronic acid/carboxymethylcellulose gel on bowel anastomoses in the New Zealand white rabbit. J Gastrointest Surg. 1997;1:569–578. [DOI] [PubMed] [Google Scholar]
- 21.de Hingh IH, de Man BM, Lomme RM, et al. Colonic anastomotic strength and matrix metalloproteinase activity in an experimental model of bacterial peritonitis. Br J Surg. 2003;90:981–988. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Moesner P, Kovach NL, et al. Integrin-dependent leukocyte adhesion involves geranylgeranylated protein(s). J Biol Chem. 1999;274:33334–33340. [DOI] [PubMed] [Google Scholar]
- 23.Budzyn K, Marley PD, Sobey CG. Targeting Rho and Rho-kinase in the treatment of cardiovascular disease. Trends Pharmacol Sci. 2006;27:97–104. [DOI] [PubMed] [Google Scholar]
- 24.Bickell NA, Federman AD, Aufses AH Jr. Influence of time on risk of bowel resection in complete small bowel obstruction. J Am Coll Surg. 2005;201:847–854. [DOI] [PubMed] [Google Scholar]
- 25.Wilson MS, Ellis H, Menzies D, et al. A review of the management of small bowel obstruction: members of the Surgical and Clinical Adhesions Research Study (SCAR). Ann R Coll Surg Engl. 1999;81:320–328. [PMC free article] [PubMed] [Google Scholar]
- 26.Fevang BT, Fevang J, Lie SA, et al. Long-term prognosis after operation for adhesive small bowel obstruction. Ann Surg. 2004;240:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diamond MP, Decherney AH. Pathogenesis of adhesion formation/reformation: application to reproductive pelvic surgery. Microsurgery. 1987;8:103–107. [DOI] [PubMed] [Google Scholar]
- 28.DiZerega GS, Rodgers KE. The Peritoneum. New York: Springer-Verlag, 1992. [Google Scholar]
- 29.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. [DOI] [PubMed] [Google Scholar]