Abstract
Objectives:
To determine whether emergency colectomy reduces mortality in patients with fulminant Clostridium difficile-associated disease (CDAD), and to identify subgroups of patients more likely to benefit from the procedure.
Summary Background Data:
Many hospitals in Quebec, Canada, have noted since 2003 a dramatic increase in CDAD incidence and in the proportion of cases severe enough to require intensive care unit (ICU) admission. The decision to perform an emergency colectomy remains largely empirical.
Methods:
Retrospective observational cohort study of 165 cases of CDAD that required ICU admission or prolongation of ICU stay between January 2003 and June 2005 in 2 tertiary care hospitals of Quebec. Multivariate analysis was performed through logistic regression; adjusted odds ratios (AOR) and their 95% confidence intervals (CI) were calculated. The primary outcome was mortality within 30 days of ICU admission.
Results:
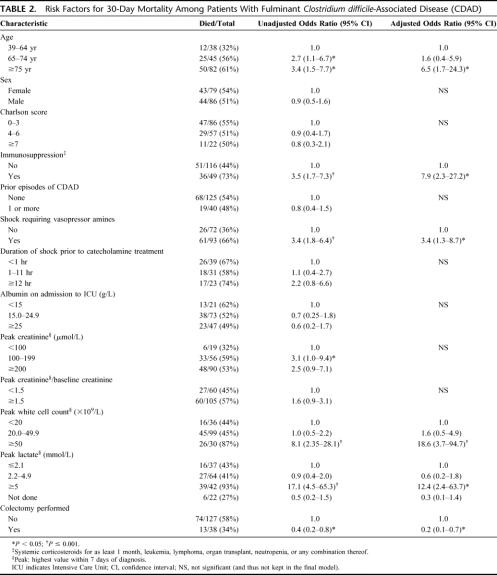
Eighty-seven (53%) cases resulted in death within 30 days of ICU admission, almost half (38 of 87, 44%) within 48 hours of ICU admission. The independent predictors of 30-day mortality were: leukocytosis ≥50 × 109/L (AOR, 18.6; 95% CI, 3.7–94.7), lactate ≥5 mmol/L (AOR, 12.4; 95% CI, 2.4–63.7), age ≥75 years (AOR, 6.5; 95% CI, 1.7–24.3), immunosuppression (AOR, 7.9; 95% CI, 2.3–27.2) and shock requiring vasopressors (AOR, 3.4; 95% CI, 1.3–8.7). After adjustment for these confounders, patients who had an emergency colectomy were less likely to die (AOR, 0.22; 95% CI, 0.07–0.67, P = 0.008) than those treated medically. Colectomy seemed more beneficial in patients aged 65 years or more, in those immunocompetent, those with a leukocytosis ≥20 × 109/L or lactate between 2.2 and 4.9 mmol/L.
Conclusion:
Emergency colectomy reduces mortality in some patients with fulminant CDAD.
In the province of Quebec, Canada, an epidemic of hypervirulent Clostridium difficile colitis was associated with a sharp increase in mortality. Here the most severe cases admitted to the intensive care units of 2 tertiary care hospitals are reviewed retrospectively. High leukocytosis, elevated blood lactates, age ≥75 years, immunosuppression, and shock requiring vasopressors were associated with a higher 30-day mortality; after adjustment for these confounders, emergency colectomy significantly reduced mortality.
Several medical centers in the United States, Canada, and Europe have recently noted an increase in the incidence and severity of Clostridium difficile-associated disease (CDAD). In Pittsburgh, 10% of patients with CDAD diagnosed in 2000 to 2001 required an emergency colectomy.1 Enhanced severity of CDAD has also been reported in several other American states.2 The largest epidemic occurred in the province of Quebec, Canada, where, during the 2004 to 2005 winter, 30 hospitals reported rates over 15 per 10,000 patient-days, at least 5 times higher than historical rates.3–5 In Sherbrooke, Quebec, the incidence of CDAD among individuals aged 65 years or more increased 10-fold between 1991 and 2003, and a recent study showed that the excess mortality that could be attributed to nosocomial CDAD in 2003 to 2004 was 16.7%.6,7 This coincided with the emergence of a hypervirulent toxinotype III NAP1/027 strain of C. difficile, which produces levels of toxins A and B that are 16 to 23 times higher than historical strains, most of which were toxinotype 0.8 The same hypervirulent strain has also been found in the United Kingdom, the Netherlands, and Belgium.9–12
It used to be uncommon for patients with CDAD to require admission into an intensive care unit (ICU), but many hospitals in Quebec have noted a dramatic increase in the number of such cases since 2003.13 In that context, intensive care physicians have been frequently seeking a surgical opinion for the most severe cases, and many patients have undergone an emergency colectomy. Such a decision is largely empirical given the absence of evidence supporting a surgical approach. To improve the surgical decision-making process for patients critically ill with CDAD, we undertook a retrospective study of all patients with CDAD who, between January 1, 2003 and June 30, 2005, required admission into an ICU in 2 tertiary care hospitals of Quebec.
METHODS
Hôpital Maisonneuve-Rosemont (HMR) is a 597-bed hospital located in Montreal, while Centre Hospitalier Universitaire de Sherbrooke (CHUS) is a 683-bed hospital providing care to inhabitants of southeast Quebec. Adult cases of CDAD were identified through laboratory reports and summaries of discharge diagnoses. The same C. difficile toxin B cytotoxin assay was used in both hospitals, on Vero or MRC-5 cells, with readings at 24 and 48 hours and neutralization with C. difficile antitoxin (HMR: TechLab, Blacksburg, VA; CHUS: Bartels, Issaquah, WA). Cases of CDAD were defined as patients meeting at least one of the following criteria: 1) a positive C. difficile cytotoxin assay; 2) endoscopic evidence of pseudomembranous colitis; and 3) histopathologic evidence of pseudomembranous colitis on either an endoscopic biopsy, a specimen obtained during colectomy or autopsy. Lists of cases were compared with records of ICU admission at HMR, while at CHUS a computerized medical records system allows tracking of each room in which a hospitalized patient spent time. Records of patients with both a diagnosis of CDAD and an ICU stay were reviewed to exclude those for whom the ICU stay was clearly unrelated to CDAD. We included cases for which the primary reason for ICU admission had been CDAD as well as patients admitted to ICU for other reasons who then developed CDAD severe enough that it was felt an ICU admission would have been required for the management of their CDAD had they not been there already. CDAD was considered healthcare-associated if the patient was hospitalized when the initial diagnosis of CDAD was made, or had been hospitalized in the preceding 2 months, or was on hemodialysis.
Medical records were reviewed to collect socio-demographic information, past medical histories (from discharge diagnoses of current and all previous hospital admissions, to calculate the Charlson comorbidity index, a measure of overall burden of diseases based on the presence or absence of 19 mostly chronic comorbidities)14 and to extract clinical, diagnostic, and therapeutic data. The outcome was all-cause mortality occurring within 30 days of ICU admission. Data were analyzed with Stata 8.0. Proportions were compared with the χ2 test or, when numbers were small, with Fisher exact test. Unconditional logistic regression was used for multivariate analysis. Models were built up sequentially, starting with the variable most strongly associated with the outcome and continuing until no other variable reached significance. When the final model was reached, each variable was dropped in turn to assess its effect. Different models were compared using the likelihood ratio test, keeping in the final one variables significant at the P = 0.05 level.
RESULTS
A total of 165 cases of CDAD were included in the study, corresponding to 161 patients (4 patients contributed 2 distinct episodes). For 154 cases (93%), CDAD had been health care associated. In 40 (24%) patients, the episode that required ICU admission corresponded to a relapse of CDAD occurring within 3 months of a prior episode (21 a first relapse, 12 a second relapse, 6 a third relapse, and 1 a fifth relapse), while 125 were admitted to ICU at the time of the initial diagnosis of CDAD. Fifty-five (33%) patients were admitted to ICU on the same day they were admitted to hospital, 34 (21%) between 1 and 3 days and 76 (46%) more than 3 days after hospital admission.
Eighty-six (52%) patients were male. Age ranged between 39 and 93 years (median, 75 years; interquartile range [IQR], 65–79 years). Their median Charlson score was 3 (IQR, 2–5), and 49 patients (30%) patients had some form of immunosuppression (systemic corticosteroids for as least 1 month, leukemia, lymphoma, organ transplant, neutropenia, or any combination thereof). For 144 (87%) patients, it was possible to retrieve data on the inciting antibiotics given in the preceding 2 months. Many patients had received more than one antimicrobial; the most commonly used were fluoroquinolones in 86 (60%), third-generation cephalosporins in 39 (27%), macrolides in 32 (22%), β-lactam/β-lactamase inhibitors in 30 (21%), and first-generation cephalosporins in 28 (19%). Only 8 (6%) had received clindamycin.
In 152 (92%) cases, the diagnosis had been confirmed by a positive C. difficile toxin assay. Among the 13 toxin-negative patients, the diagnosis had been confirmed by endoscopy in 8, surgery in 3, and autopsy in 2. Plain x-rays of the abdomen, obtained in 149 patients, were normal in 54 (36%) patients; 50 (34%) had signs of colitis without megacolon, 15 (10%) had megacolon, 1 had free air, 2 had intestinal pneumatosis while 27 (18%) had only nonspecific changes. An abdominal ultrasound was obtained in 35 patients and interpreted as normal in 16 (46%). Eighty-five patients had an abdominal CT scan: signs of colitis were present in 66 (78%). An endoscopy was performed on 38 patients, pseudomembranes were visualized in 33 (87%).
Lactate levels were measured in 143 patients, with peaks varying between 0.7 and 23.0 mmol/L (median, 3.1 mmol/L; IQR, 2.1–5.6 mmol/L). The median peak leukocyte count was 30.9 × 109/L (IQR, 20.8–44.1). Among patients with no preexisting renal failure, median peak creatinine was 181 μmol/L (IQR, 122–245), while in those with pre-existing renal failure it was 292 μmol/L (IQR, 196–364). Median albumin level on admission to ICU was 21.0 g/L (IQR, 16–25 g/L). Blood cultures, obtained in 138 patients, revealed a Gram-negative bacteremia in only 6 cases.
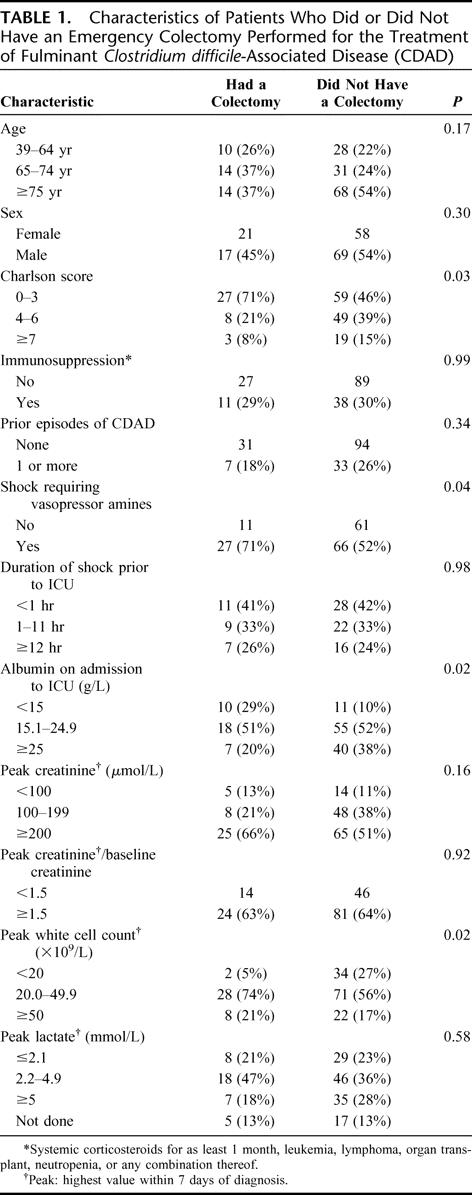
Four patients died before any antimicrobial therapy could be administered, 13 were treated with only metronidazole, 13 with only oral vancomycin, and 135 received combinations of metronidazole and vancomycin, either concomitantly or sequentially. Thirty-eight (23.0%) patients underwent a colectomy, 35 of which were total/subtotal. The main indications for colectomy were shock persisting despite vasopressors (15 patients), megacolon (11 patients), lack of response to medical treatment (10 patients), and perforation (2 patients). Compared with patients treated medically, those who had a colectomy had fewer chronic comorbidities (ie, a lower Charlson score) but higher leukocytosis and were more likely to be in septic shock (Table 1).
TABLE 1. Characteristics of Patients Who Did or Did Not Have an Emergency Colectomy Performed for the Treatment of Fulminant Clostridium difficile-Associated Disease (CDAD)
Eighty-seven (52.7%) cases resulted in death within 30 days of ICU admission; of these deaths, 38 (43.7%) occurred within 48 hours of ICU admission. Table 2 summarizes risk factors for mortality within 30 days of admission to ICU in univariate and multivariate analyses. Fifty-eight percent of patients who were medically treated died, compared with 34% of those who underwent an emergency colectomy (P = 0.02). Factors not associated with mortality included sex, Charlson score, albumin level, duration of shock prior to ICU admission, and whether the current CDAD episode corresponded to an initial episode or a relapse. In multivariate analysis, mortality was significantly higher in patients aged 75 years or more, in the immunosuppressed, those requiring vasopressor amines, those with a lactate level ≥5 mmol/L, or a white cell count ≥50 × 109/L. Adjusting for these confounding factors, colectomy reduced by 78% the odds of death (adjusted odds ratio, 0.22; 95% confidence interval, 0.07–0.67, P = 0.008). No significant interaction was found.
TABLE 2. Risk Factors for 30-Day Mortality Among Patients With Fulminant Clostridium difficile-Associated Disease (CDAD)
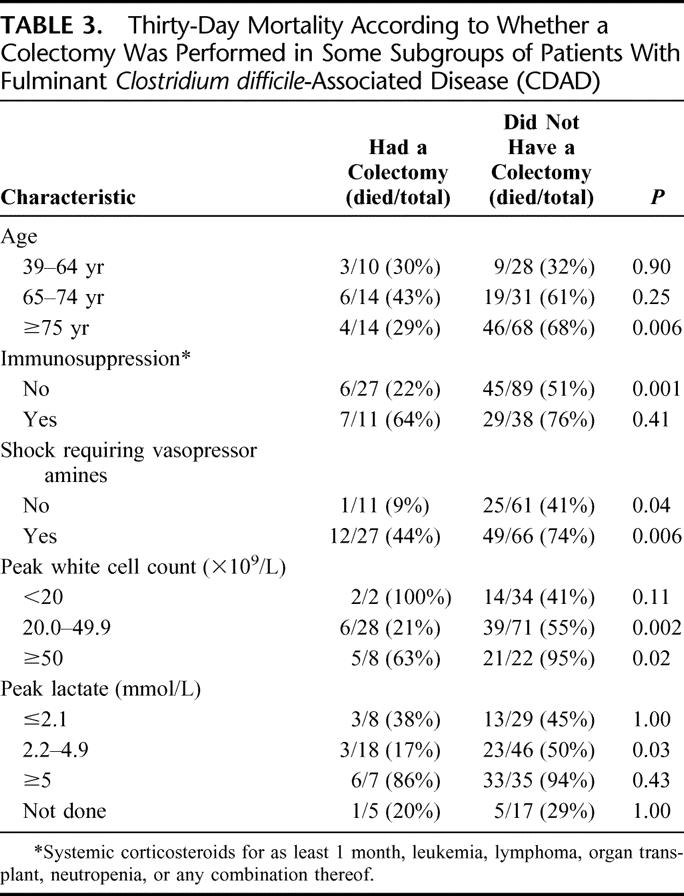
To delineate if some patients might be more likely to benefit from a colectomy, we carried out subgroup analyses (Table 3). Colectomy did not confer a survival benefit in patients younger than 65 years of age, in patients with a peak white cell count <20 × 109/L and those with normal lactate levels. Without a colectomy, 94% to 95% of patients with a white cell count ≥50 × 109/L or with lactate ≥5 mmol/L died within 30 days of admission to ICU.
TABLE 3. Thirty-Day Mortality According to Whether a Colectomy Was Performed in Some Subgroups of Patients With Fulminant Clostridium difficile-Associated Disease (CDAD)
DISCUSSION
Even before the recognition of C. difficile as the cause of pseudomembranous colitis in 1978,15 surgeons had been performing colectomy in patients with severe disease.16 Several authors have suggested that the frequency of colectomy among patients with CDAD may have increased during the last decade. During an epidemic in an Irish hospital in 1995, 3.6% (5 of 138) of patients with CDAD were colectomized.17 In Pittsburgh, 1.1% (27 of 2540) of patients diagnosed with nosocomial CDAD in 1989 to 1999 needed a colectomy,18 while this increased to an impressive 10.3% (26 of 253) of patients in 2000 to 2001 (some of whom had been referred from other facilities specifically because they had severe CDAD).1 For the whole of Quebec, of 3218 patients with nosocomial CDAD diagnosed between August 2004 and March 2005, 32 (1.0%) had a colectomy, but there are no historical data to which this can be compared.5
The postoperative mortality of CDAD patients undergoing an emergency colectomy is very high. A first review of the topic in 1990 enumerated 29 case reports of patients who had surgical management of CDAD: 8 of 23 (35%) of those colectomized for a toxic megacolon died, as did 3 of 6 (50%) patients operated for a perforation.16 Additional case series were published, comprising a total of 54 patients who had a colectomy, 24 (44%) of whom died.17,19–27 Indications for surgical treatment were generally considered to be a toxic megacolon, perforation and/or peritonitis, fulminant disease (shock, multiple organ failure), and failure of medical therapy.17,19 During the 1990s, of 62 CDAD patients colectomized in a Pittsburgh hospital, 57% died.18 In a recent review of CDAD cases from 159 Veterans Administration hospitals, 32 of 67 (48%) patients who had a colectomy died.28
These earlier studies described patients with severe CDAD who had surgery because of a complicated or fulminant course but lacked a comparison group that would allow the life-saving potential of the procedure to be measured. Ours is thus the first study in which all patients with complicated or fulminant CDAD justifying admission to ICU were included, allowing a comparison of the outcomes of those who had a surgical management to those treated conservatively. Our data suggest that an emergency colectomy may reduce mortality in these highly selected and very sick patients.
The major limitation of our study lies in its observational nature. Factors that influenced surgeons' decisions to carry out a colectomy might also be related to the 30-day mortality. To some extent, we avoided such confounding factors by restricting our analysis to patients admitted to an ICU. Clearly, the attending physicians had appreciated that their patient did not have a rapidly fatal comorbidity or very old age such that aggressive management would be inappropriate. We also adjusted, through logistic regression, for the confounding factors that were identified. For instance, patients colectomized were more likely to have a high leukocytosis or to be in septic shock than those treated medically, and as a result the adjusted odds ratio for the effect of colectomy on mortality was lower than the crude odds ratio. Patients colectomized had fewer chronic comorbidities than those who did not undergo surgery, but since the Charlson score was itself not associated with the 30-day mortality, this did not confound the association. Nevertheless, there might have been additional, unmeasured confounders whose effect on the measure of the association between colectomy and a lower mortality could go either way.
This study was conducted during an epidemic caused by the NAP1/027 toxinotype III strain of C. difficile that is a hyperproducer of both toxins A and B.8 In our institutions, between two thirds and four fifths of C. difficile isolates collected in 2003 to 2005 were toxinotype III NAP1/027 strains,8,13 and 6% to 10% of patients with nosocomial CDAD ended up in ICU.7,13 Whether our findings can be extrapolated to centers not yet dealing with this specific strain is unknown.
Among patients with CDAD requiring ICU admission, are some more likely to benefit from an emergency colectomy? The subgroup analyses presented in Table 3 should be interpreted with caution given the small number of patients in some strata, but we could not demonstrate a survival benefit among younger patients, those with normal lactate or a leukocytosis <20 × 109/L. The results also suggest that colectomy should be considered before patients increase their lactate levels ≥5 mmol/L, in which cases the prognosis is dismal with or without surgery.
The recruitment of 165 CDAD cases requiring ICU admission, 38 of whom had an emergency colectomy, in 2 hospitals over a 30-month period underscores the dramatic changes in the incidence and severity of CDAD in Quebec. Presumably because of the severe diarrhea it induces, this strain seems to be more transmissible than others and is spreading rapidly in the United States and parts of Europe.1–2,9–12 Our results suggest that surgeons should consider an emergency colectomy in patients with fulminant disease for whom aggressive care is not contraindicated by other comorbidities.
ACKNOWLEDGMENTS
The authors thank Johanne Harvey, Marie-Andrée Coulombe, and Marie-Eve Alary for their contribution to data collection, and Drs. Serge Dubé and Louis Valiquette for critical review of the manuscript.
Footnotes
Carlos Patino was supported by an unrestricted grant from Astellas Canada Inc.
Reprints: Jacques Pépin, MD, CHUS, 3001, 12eme Avenue Nord, Sherbrooke, Quebec, Canada, J1H 5N4. E-mail: jacques.pepin@usherbrooke.ca.
REFERENCES
- 1.Muto CA, Pokrywka M, Shutt K, et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005;26:273–280. [DOI] [PubMed] [Google Scholar]
- 2.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–2441. [DOI] [PubMed] [Google Scholar]
- 3.Institut National de Santé Publique du Québec. La surveillance des diarrhées associées aux infections à Clostridium difficile: Deuxième rapport tiré du système de surveillance des infections à Clostridium difficile (SSICD) de l'Institut National de Santé Publique du Québec. Available at: http://www.inspq.qc.ca/pdf/publications/370-ResultatsCDifficile-22Aout2004-05Fevrier2005.pdf.
- 4.Institut National de Santé Publique du Québec. Surveillance des diarrhées associées à Clostridium difficile. Bilan du 22 août au 31 mars 2005. Available at: http://www.inspq.qc.ca/pdf/publications/389-SurveillanceCDifficile_Bilan22aout04-31mars05.pdf.
- 5.Alfa M, Du T, Beda G. Survey of incidence of Clostridium difficile infection in Canadian hospitals and diagnostic approaches. J Clin Microb. 1998;36:2076–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pépin J, Valiquette L, Alary ME, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. Can Med Assoc J. 2004;171:466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. Can Med Assoc J. 2005;173:1037–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warny M, Pépin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. [DOI] [PubMed] [Google Scholar]
- 9.Health Protection Agency. Voluntary reporting of Clostridium difficile, England, Wales and Northern Ireland: 2004. CDR Wkly. 2005;15(20):1–3. Available at: http://www.hpa.org.uk/cdr/archives/2005/cdr2005.pdf.
- 10.Health Protection Agency. Outbreak of Clostridium difficile infection in a hospital in south east England. CDR Wkly. 2005;15(24):2–3. Available at: http://www.hpa.org.uk/cdr/archives/2005/cdr2405.pdf.
- 11.Kuijper EJ, Debast SB, van Kregten E, et al. Clostridium difficile ribotype 027, toxinotype III in Nederland. Ned Tijdschr Geneeskd. 2005;149:2087–2089. [PubMed] [Google Scholar]
- 12.Joseph R, Demeyer D, Vanrenterghem D, et al. First isolation of Clostridium difficile PCR ribotype 027, toxinotype III in Belgium. Eurosurveillance. 2005;10. Available at: http://www.eurosurveillance.org/ew/2005/051020.asp. [DOI] [PubMed]
- 13.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–2449. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. [DOI] [PubMed] [Google Scholar]
- 15.Bartlett JG, Chang TW, Gurwith M, et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298:531–534. [DOI] [PubMed] [Google Scholar]
- 16.Morris JB, Zollinger RM, Stellato TA. Role of surgery in antibiotic-induced pseudomembranous enterocolitis. Am J Surg. 1990;160:535–539. [DOI] [PubMed] [Google Scholar]
- 17.Synnott K, Mealy C, Merry C, et al. Timing of surgery for fulminating pseudomembranous colitis. Br J Surg. 1998;85:229–231. [DOI] [PubMed] [Google Scholar]
- 18.Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipsett PA, Samantaray DK, Tam ML, et al. Pseudomembranous colitis: a surgical disease? Surgery. 1994;116:491–496. [PubMed] [Google Scholar]
- 20.Medich DS, Lee KK, Simmons RI, et al. Laparotomy for fulminant pseudomembranous colitis. Arch Surg. 1992;127:847–852. [DOI] [PubMed] [Google Scholar]
- 21.Trudel JL, Deschenes M, Mayrand S, et al. Toxic megacolon complicating pseudomembranous enterocolis. Dis Colon Rectum. 1995;38:1033–1038. [DOI] [PubMed] [Google Scholar]
- 22.Morris LL, Villalba MR, Glover JL. Management of pseudomembranous colitis. Am Surg. 1994;60:548–552. [PubMed] [Google Scholar]
- 23.Viswanath YKS, Griffiths CDM. The role of surgery in pseudomembranous enterocolitis. Postgrad Med J. 1998;74:216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mistry B, Longo W, Solomon H, et al. Clostridium difficile colitis requiring subtotal colectomy in a renal transplant recipient: a case report and review of the literature. Transplant Proc. 1998;30:3914. [DOI] [PubMed] [Google Scholar]
- 25.Klipfel AA, Schein M, Fahoum B, et al. Acute abdomen and Clostridium difficile colitis: still a lethal combination. Dig Surg. 2000;17:160–163. [DOI] [PubMed] [Google Scholar]
- 26.Zahariadis G, Connon JJ, Fong IW. Fulminant Clostridium difficile colitis without diarrhea: lack of emphasis in diagnostic guidelines. Am J Gastroenterol. 2002;97:2929–2930. [DOI] [PubMed] [Google Scholar]
- 27.Grundfest-Broniatowski S, Quader M, Alexander F, et al. Clostridium difficile colitis in the critically ill. Dis Colon Rectum. 1996;39:619–623. [DOI] [PubMed] [Google Scholar]
- 28.Longo WE, Mazuski JE, Virgo KS, et al. Outcome after colectomy for Clostridium difficile colitis. Dis Colon Rectum. 2004;47:1620–1625. [DOI] [PubMed] [Google Scholar]