Abstract
Objective:
To determine the mechanism by which flutamide administration following trauma-hemorrhage (T-H) decreases cytokine production and hepatic injury under those conditions.
Summary Background Data:
Although studies have demonstrated that flutamide administration following T-H improves hepatic and immune functions, the mechanism by which flutamide produces the salutary effects remains unknown.
Methods:
Male Sprague-Dawley rats underwent a 5-cm laparotomy and hemorrhagic shock (40 mm Hg for ∼90 minutes), followed by resuscitation with 4 times the shed blood volume in the form of Ringer's lactate. Flutamide (25 mg/kg body weight, sc) was administered at the middle of resuscitation and animals were killed 2 hours thereafter. To block estrogen receptor (ER), ER antagonist ICI 182,780 was administrated with flutamide.
Results:
Hepatic injury, myeloperoxidase activity, nuclear factor-kappaB (NF-κB) DNA binding activity and protein expression of intercellular adhesion molecule-1, and cytokine-induced neutrophil chemoattractant (CINC-1 and CINC-3) markedly increased following T-H. Hepatic mRNA and plasma IL-6 levels were also elevated following T-H. The alterations in these parameters induced by T-H were significantly attenuated by flutamide administration. The decreased plasma estradiol levels following T-H were restored to sham levels in the flutamide-treated T-H animals. Coadministration of ICI 182,780 prevented those salutary effects of flutamide administration on pro-inflammatory responses and hepatic injury following T-H.
Conclusion:
These findings suggest that the reduction in the production of pro-inflammatory mediators and hepatic injury produced by flutamide administration following T-H is likely due to the down-regulation in hepatic NF-kappaB DNA binding activity. Moreover, the salutary effects of flutamide administration appear to be mediated at least in part via ER-related pathway.
Although flutamide attenuates hepatic injury following trauma-hemorrhage, the mechanisms of its salutary effects are unknown. This study suggests that the flutamide mediates its salutary effects on hepatic function following trauma-hemorrhage via estrogen receptor-dependent down-regulation of hepatic chemokine and cytokine production, nuclear factor-κB DNA binding activity, and neutrophil accumulation.
Hemorrhagic shock results in excessive production of pro-inflammatory mediators, such as cytokines and chemokines, which play a significant role in the development of multiple organ dysfunctions under those conditions.1 Studies have shown that the nuclear factor-kappa B (NF-κB) family is activated in the lung, liver, heart, and lymphocytes following hemorrhagic shock.2–4 Furthermore, the activation of NF-κB induces the expression of pro-inflammatory mediators following hemorrhagic shock and sepsis.5,6 Previous studies have also demonstrated that NF-κB activation regulates gene expression of pro-inflammatory mediators that are associated with neutrophil accumulation in the target organs following hemorrhagic shock. These mediators include intercellular adhesion molecule-1 (ICAM-1),7 cytokine-induced neutrophil chemoattractant-1 (CINC-1),8 and IL-6.2,9
Studies have shown that neutrophils are activated in the early phase of hemorrhagic shock10 and that hepatic injury is associated with an increased neutrophil accumulation in the liver following hemorrhagic shock.11,12 The activated neutrophils appear to infiltrate the injured liver in parallel with increased expression of adhesion molecules on endothelial cells and elevated local chemokine/cytokine levels following hemorrhagic shock. Furthermore, trauma-hemorrhagic shock increases endothelial cell P-selectin and ICAM-1 in the lung and liver.13 Moreover, the levels of the chemokine, CINC-1, are elevated in the lungs after trauma-hemorrhage.14 IL-6 also appears to be an essential component of the inflammatory cascade that is associated with hepatic injury in hemorrhagic shock. Our recent study has shown that IL-6 plays a significant role in the induction of hepatic dysfunction and liver injury following trauma-hemorrhage.15 Moreover, IL-6-deficient mice showed less neutrophil infiltration and organ damage compared with wild-type mice under those conditions.16
Gender-dimorphic immune and organ responsiveness following hemorrhagic shock and sepsis has been reported. In particular, androgens have been shown to be responsible for producing the immunosuppression following trauma-hemorrhage in males. In contrast, female sex steroids exhibit immunoprotective properties following hemorrhagic shock and sepsis.17,18 Furthermore, estrogen administration following hemorrhagic shock has been shown to reduce pro-inflammatory cytokine production and hepatic injury under those conditions.19,20 Gender dimorphism in neutrophil priming and activation following trauma-hemorrhage has also been previously reported.14 In this regard, the increased accumulation of neutrophils in lung in males has been shown to be associated with the elevation in pulmonary chemokine levels.14 Thus, female sex steroids appear to be closely related in the attenuation/prevention of hepatic injury following hemorrhagic shock.
Our previous studies have shown that flutamide, an androgen receptor antagonist, administration improves hepatocellular and immune functions in males following trauma and hemorrhagic shock.21,22 Although flutamide has been shown to produce the above-mentioned salutary effects, the mechanism responsible for the salutary effects of flutamide on decreasing hepatic injury and cytokine production following trauma-hemorrhage remains unclear. Our recent study has shown that flutamide administration following trauma-hemorrhage modulated cardiac estrogen receptor (ER) protein expression in male rats.23 Other studies have demonstrated that flutamide administration increases plasma estrogen levels in male rats.24,25 Therefore, we hypothesized that flutamide administration following trauma-hemorrhage attenuates hepatic injury and cytokine production via an ER-related pathway. To test the hypothesis, we first examined the effects of flutamide administration on hepatic injury by measuring hepatic NF-κB DNA binding activity and chemokine and cytokine production following trauma-hemorrhage. We also used the ER antagonist, ICI 182,780, along with flutamide to determine if the salutary effects of flutamide are abrogated by blockade of ER under those conditions.
METHODS
Animals
Adult male (275–325 g) Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were used in this study. All experiments were performed in adherence with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Experimental Procedures
A nonheparinized rat model of trauma-hemorrhage, as previously described, was used.26 Briefly, male Sprague-Dawley rats were fasted overnight before the experiment but allowed water ad libitum. The rats were anesthetized using isoflurane (Attane, Minrad Inc, Bethlehem, PA) inhalation. A 5-cm midline laparotomy was then performed to induce soft tissue trauma. The abdominal incision was then closed in layers and polyethylene (PE-50, Becton Dickinson & Co, Sparks, MD) catheters were placed in both femoral arteries and the right femoral vein. The catheters were subsequently tunneled through to the dorsal side and the incision sites were closed with sutures. The rats were then placed into a Plexiglas box (21 × 9 × 5 cm) in a prone position and were allowed to awaken, following which they were rapidly bled to a mean arterial pressure (MAP) of 35 to 40 mm Hg within 10 minutes. The time at which the animals could no longer maintain a MAP of 35 to 40 mm Hg without infusing some fluid was defined as maximum bleed-out volume. The rats were maintained at this MAP until 40% of the shed blood was returned in the form of Ringer's lactate. The animals were then resuscitated with 4 times the volume of shed blood with Ringer's lactate over 60 minutes. Following resuscitation, the catheters were removed, the vessels were ligated, and skin incisions closed with sutures. Sham-operated animals underwent the same groin dissection, which included the ligation of the femoral arteries and vein; however, the animals were neither subjected to trauma-hemorrhage nor resuscitated. The animals were returned to their cages and were allowed food and water ad libitum and were killed 2 hours after the end of resuscitation.
In the treatment group, flutamide (25 mg/kg subcutaneously; Sigma, St. Louis, MO) was administered at the middle of resuscitation. In the vehicle-treated group (control group), rats received the same volume of vehicle (propanediol, Sigma) at the middle of resuscitation.23
To block estrogen receptor activity, a high-affinity estrogen receptor antagonist, ICI 182,780 (3 mg/kg intraperitoneally; Tocris Cookson Ltd, Ballwin, MO) was administered along with flutamide at the onset of resuscitation, as described previously.27
Measurement of Hepatic Injury
Two hours after either the completion of resuscitation or sham operation, blood samples with heparin were obtained and plasma was separated by centrifugation, immediately frozen and stored at −80°C until assayed. Hepatic injury was determined by measuring plasma levels of alpha glutathione S-transferase (αGST) using a commercially available EIA kit according to the manufacturer's instructions (Biotrin International Ltd, Dublin, Ireland).
Measurement of Myeloperoxidase (MPO) Activity
MPO activity in homogenates of whole liver was determined as described by Rana et al.28 Briefly, equal weights (100 mg wet weight) of liver were suspended in 1 mL buffer (0.5% hexadecyltrimethylammonium bromide in 50 mmol/L phosphate buffer, pH 6.0) and sonicated at 30 cycles, twice, for 30 seconds on ice. Homogenates were cleared by centrifuging at 12,000 rpm at 4°C, and the supernatants were stored at −80°C. Protein content in the samples was determined (BioRad, Hecules, CA). The samples were incubated with a substrate o-dianisidine hydrochloride. This reaction was carried out in a 96-well plate by adding 290 μL 50 mmol/L phosphate buffer, 3 μL substrate solution (containing 20 mg/mL o-dianisidine hydrochloride), and 3 μL H2O2 (20 mmol/L). Sample (10 μL) was added to each well to start the reaction. Standard MPO (Sigma) was used in parallel to determine MPO activity in the sample. The reaction was stopped by adding 3 μL sodium azide (30%). Light absorbance at 460 nm was read. MPO activity was determined by using the curve obtained from the standard MPO.
Determination of ICAM-1, CINC-1, and CINC-3 Levels
The hepatic ICAM-1, CINC-1, and CINC-3 levels were determined using ELISA kits (R&D, Minneapolis, MN) according to the manufacturer's instructions.14 Briefly, the samples were homogenized in 10 times the volume of PBS (pH 7.4) containing protease inhibitors (Complete Protease Inhibitor Cocktail, Boehringer Mannheim, Germany). The homogenates were centrifuged at 2000 × g for 20 minutes at 4°C and the supernatant was assayed for ICAM-1, CINC-1, and CINC-3 levels.
Measurement of Plasma Levels of IL-6
Plasma levels of IL-6 (Pharmingen, San Diego, CA) were measured using a commercially available ELISA kit according to the manufacturer's instructions.
Measurement of Plasma Levels of Estradiol, Testosterone, and Dihydrotestosterone (DHT)
Blood samples (2 mL) were collected in EDTA-coated test tubes 2 hours after either the completion of resuscitation or sham operation. Plasma was immediately separated by centrifugation, and the plasma samples were stored at −80°C until assayed for sex hormones. The levels of estradiol, testosterone (Cayman Chemical, Ann Arbor, MI) and DHT (Alpco Diagnostics, Windham, NH) were determined by enzyme immunoassay (EIA) kits.
Hepatic mRNA Expression Analysis
Gene expressions of IL-6 and ICAM-1 in the liver were determined by quantitative real-time PCR as described previously.29 Total RNA was isolated from the left lobe of the liver using TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. The cDNA was generated from the total RNA samples by using TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA). Each real-time PCR reaction was performed in a mix of 10 μL reaction mixture containing 20 ng of cDNA, 2 × PCR Master Mix (Applied Biosystems), and each probe and primer set. TaqMan Gene Expression Assays (Applied Biosystems) for IL-6 and ICAM-1 were purchased as probe and primer sets. The reaction mixture was denatured for 1 cycle of 2 minutes at 50°C, 10 minutes at 95°C, and incubated for 40 cycles (denaturing for 15 seconds at 95°C and annealing and extending for 1 minute at 60°C) using ABI Prism 7900HT (Applied Biosystems). All samples were tested in triplicate, and average values were used for quantification. 18S rRNA was used as an endogenous control. The comparative CT method (ΔΔCT, cycle threshold) was used for quantification of gene expression. Analysis was performed using SDS v2.2 software (Applied Biosystems) according to the manufacturer's instruction.
Electrophoretic Mobility Shift Assays (EMSA)
NF-κB DNA binding activities were determined in nuclear extracts of the liver.30 Oligonucleotide probes corresponding either to NF-κB consensus sequences (Santa Cruz Biotechnology, Inc, Santa Cruz, CA) were labeled with γ-32P-ATP (≥6000 Ci/mmol, Amersham, Piscataway, NJ). Nuclear extracts, ≥20,000 cpm of radiolabeled double-stranded target oligonucleotide, poly(dI-dC) and incubation buffer (Promega, Madison, WI) were mixed and incubated. After 30 minutes incubation on RT, each of the samples was loaded onto 6% DNA retardation gel (Novex, Carlsbad, CA) and run at 10 to 15 mA for 90 minutes. Following electrophoresis, band intensities were quantified using autoradiography. Signal densities were evaluated by ChemiImager 5500 software (Alpha Inotech, San Leandro, CA).
Statistical Analysis
Data are presented as mean ± SEM. Statistical differences among groups were determined by one way analysis of variance followed by Fisher's LSD as a post hoc test. Differences were considered significant if P < 0.05.
RESULTS
Alteration in Plasma αGST
In sham-operated animals, no significant differences in plasma αGST levels were found between vehicle- and flutamide-treated groups. Trauma-hemorrhage significantly increased plasma αGST levels. Flutamide treatment attenuated the trauma-hemorrhage-induced increase in plasma αGST; however, the levels remained higher than shams. Although ICI 182,780 coadministration with flutamide did not influence trauma-hemorrhage-induced increase in plasma αGST, its administration prevented the salutary effect of flutamide on the reduction in plasma αGST following trauma-hemorrhage (Fig. 1A).
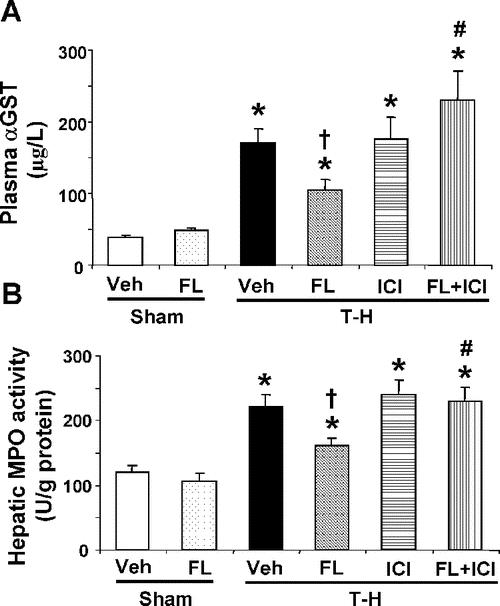
FIGURE 1. Effect of flutamide treatment on plasma αGST (A) and hepatic MPO activity (B) at 2 hours after sham operation or trauma-hemorrhage. Data are presented as mean ± SEM (n = 6 animals/group). *P < 0.05 versus sham. †P < 0.05 versus T-H. #P < 0.05 versus corresponding group without ICI 182,780. T-H, trauma-hemorrhage; Veh, vehicle treatment; FL, flutamide treatment; ICI, ICI 182,780 treatment.
Alteration in Hepatic MPO Activity
As shown in Figure 1B, flutamide administration did not influence hepatic MPO activity in sham groups. The hepatic MPO activity significantly increased following trauma-hemorrhage. However, flutamide administration following trauma-hemorrhage decreased the hepatic MPO activity compared with vehicle-treated trauma-hemorrhage animals. ICI 182,780 coadministration along with flutamide prevented the flutamide-mediated reduction in hepatic MPO following trauma-hemorrhage (Fig. 1A).
Alteration in NF-kappaB DNA Binding Activities
As shown in Figure 2A, NF-κB DNA binding activity was not influenced by flutamide administration in sham animals (lanes 4 and 5) compared with shams receiving vehicle (lanes 1 and 2). Trauma-hemorrhage significantly increased NF-κB DNA binding activity (lanes 6 and 7) compared with sham animals. Flutamide administration following trauma-hemorrhage, however, significantly reduced the elevated NF-κB DNA binding activity and the activity was similar to shams (lanes 8 and 9). DNA binding activity was completely abolished in the presence of specific oligonucleotide cold probe (lane 10). As shown in Figure 2B, NF-κB DNA binding activity was not influenced by ICI 182,780 coadministration in trauma-hemorrhage vehicle-treated animals (lanes 5 and 6). However, the flutamide-mediated reduction in NF-κB DNA binding activity was significantly abolished by ICI 182,780 coadministration (Fig. 2).
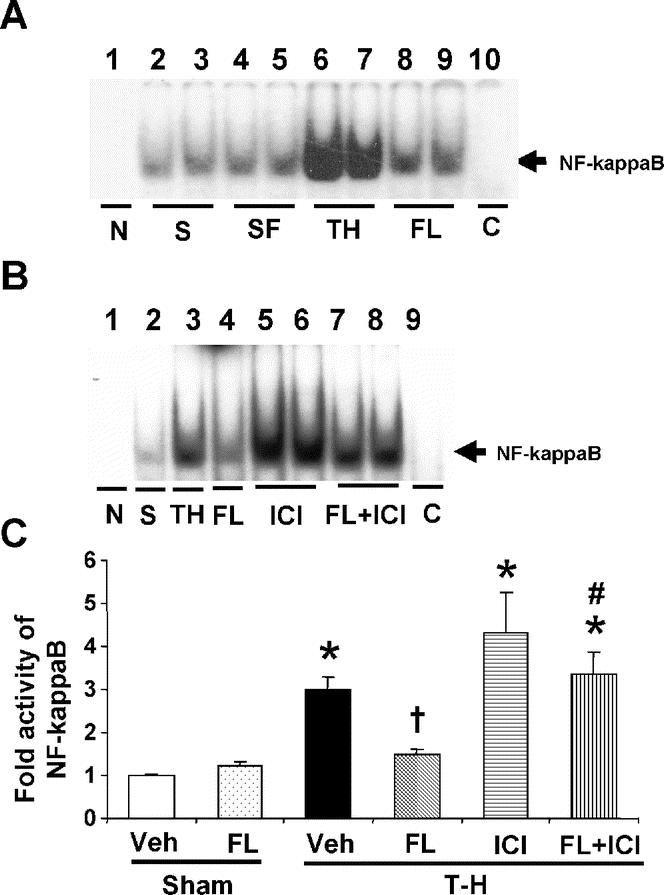
FIGURE 2. Effects of flutamide treatment on NF-kappaB DNA binding activity in the liver at 2 hours after sham operation or trauma-hemorrhage. A, NF-kappaB DNA binding activity. Lane 1, negative (probe only); lanes 2, 3, sham; lanes 4, 5, sham + flutamide; lanes 6, 7, T-H; lanes 8, 9, T-H + flutamide; lane 10, competitive assay using 100 times higher concentration of excessive probe with nuclear extract from T-H group. B, NF-kappaB DNA binding activity. Lane 1, negative (probe only); lane 2, sham; lane 3, T-H; lane 4, T-H + flutamide; lanes 5, 6, T-H + ICI 182,780; lanes 7, 8, T-H + flutamide + ICI 182,780; lane 9, competitive assay using 100 times higher concentration of excessive probe with nuclear extract from T-H group. C, The index of NF-kappaB DNA binding activity in the liver. Data are mean ± SEM (n = 3–5 animals/group). *P < 0.05 versus sham. †P < 0.05 versus T-H. #P < 0.05 versus corresponding group without ICI 182,780. T-H, trauma-hemorrhage; Veh, vehicle treatment; FL, flutamide treatment; ICI, ICI 182,780 treatment; C, competitive assay.
Alteration in Hepatic mRNA and Protein Expression of ICAM-1
There was no significant difference in hepatic mRNA and protein expressions of ICAM-1 between vehicle- and flutamide-treated sham groups. Following trauma-hemorrhage, mRNA and protein expressions of ICAM-1 were increased in the liver. Flutamide administration attenuated the hepatic ICAM-1 expressions to sham levels. Administration of ICI 182,780 along with flutamide prevented the flutamide-induced attenuation in hepatic ICAM-1 expressions (Fig. 3).
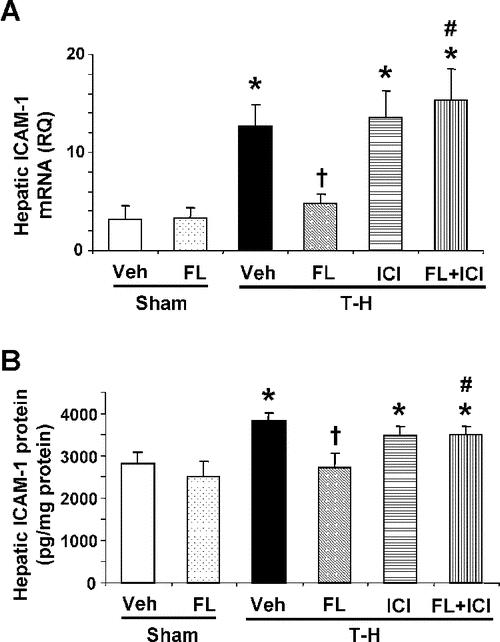
FIGURE 3. Effect of flutamide treatment on hepatic mRNA (A) and protein expression (B) of ICAM-1 at 2 hours after sham operation or trauma-hemorrhage. Data are presented as mean ± SEM (n = 3–5 animals/group for mRNA expression and n = 6 animals/group for protein expression). *P < 0.05 versus sham. †P < 0.05 versus T-H. #P < 0.05 versus corresponding group without ICI 182,780. T-H, trauma-hemorrhage; Veh, vehicle treatment; FL, flutamide treatment; ICI, ICI 182,780 treatment; RQ, relative quantity.
Alteration in Hepatic Protein Expressions of CINC-1 and CINC-3
No significant difference was observed in hepatic protein expressions of CINC-1 and CINC-3 between vehicle- and flutamide-treated sham groups (Fig. 4). Trauma-hemorrhage induced a significant elevation in hepatic CINC-1 and CINC-3 levels (Fig. 4). Administration of flutamide following trauma-hemorrhage reduced the increase in hepatic CINC-1; however, the levels remained significantly higher compared with those in sham animals (Fig. 4A). CINC-3 levels were also improved in trauma-hemorrhage animals treated with flutamide (Fig. 4B). Administration of ER antagonist, ICI 182,780, along with flutamide, prevented the flutamide-induced attenuation in hepatic CINC-1 and CINC-3 elevation (Fig. 4).
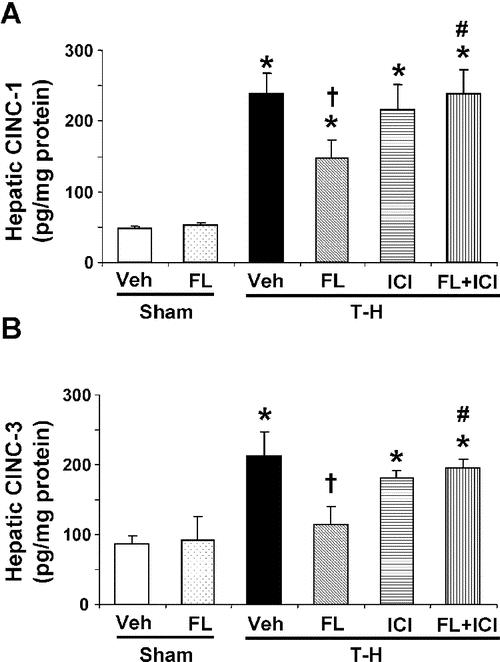
FIGURE 4. Effect of flutamide treatment on hepatic CINC-1 (A) and CINC-3 (B) at 2 hours after sham operation or trauma-hemorrhage. Data are presented as mean ± SEM (n = 6 animals/group). *P < 0.05 versus sham. †P < 0.05 versus T-H. #P < 0.05 versus corresponding group without ICI 182,780. T-H, trauma-hemorrhage; Veh, vehicle treatment; FL, flutamide treatment; ICI, ICI 182,780 treatment.
Alteration in Hepatic mRNA Expression and Plasma Levels of IL-6
Trauma-hemorrhage markedly increased mRNA and plasma levels of IL-6 (Fig. 5). Flutamide administration following trauma-hemorrhage prevented these increases in IL-6 (Fig. 5). However, coadministration of ICI 182,780 prevented the flutamide-mediated decreases in IL-6 production (Fig. 5).
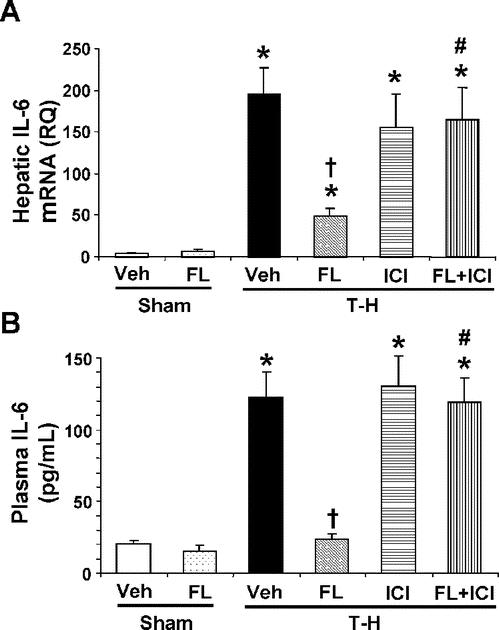
FIGURE 5. Effect of flutamide treatment on hepatic mRNA (A) and plasma levels (B) of IL-6 at 2 hours after sham operation or trauma-hemorrhage. Data are presented as mean ± SEM (n = 6 animals/group). *P < 0.05 versus sham. †P < 0.05 versus T-H. #P < 0.05 versus corresponding group without ICI 182,780. T-H, trauma-hemorrhage; Veh, vehicle treatment; FL, flutamide treatment; ICI, ICI 182,780 treatment.
Alteration in Plasma Levels of Estradiol, Testosterone, and DHT
Flutamide administration in the sham group did not influence plasma testosterone levels (Fig. 6). Trauma-hemorrhage resulted in a significant decrease in plasma levels of testosterone (Fig. 6). Plasma testosterone levels were maintained at sham levels in flutamide-treated trauma-hemorrhage groups. ICI 182,780 did not influence plasma testosterone levels following trauma-hemorrhage (Fig. 6A). There is no significant difference in plasma estradiol levels between sham and sham treated with flutamide groups. Plasma levels of estradiol significantly decreased following trauma-hemorrhage; however, flutamide administration following trauma-hemorrhage restored plasma estradiol levels to sham levels. Moreover, ICI 182,780 coadministration with flutamide prevented this effect of flutamide on plasma estradiol levels following trauma-hemorrhage (Fig. 6B). Plasma DHT levels were the same in the sham group with or without flutamide administration. Although plasma DHT levels were significantly decreased following trauma-hemorrhage, there were no significant differences among any treatment groups under those conditions (Fig. 6C).
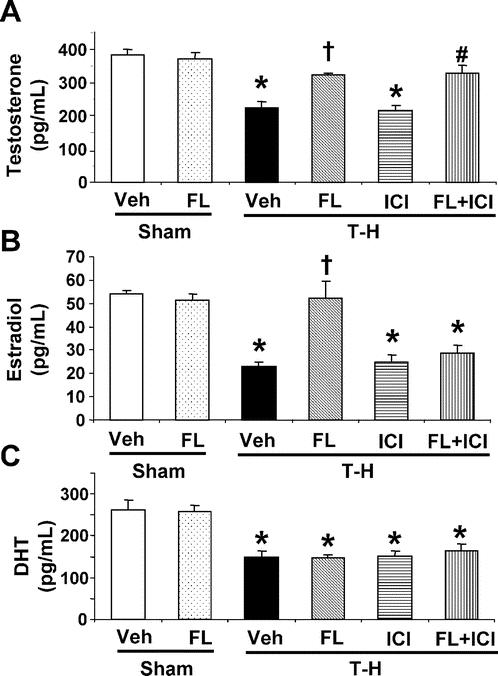
FIGURE 6. Effect of flutamide treatment on plasma testosterone (A), estradiol (B), and DHT (C) at 2 hours after sham operation or trauma-hemorrhage. Data are presented as mean ± SEM (n = 6 animals/group). *P < 0.05 versus sham. †P < 0.05 versus T-H. #P < 0.05 versus T-H + ICI. T-H, trauma-hemorrhage; Veh, vehicle treatment; FL, flutamide treatment; ICI, ICI 182,780 treatment.
DISCUSSION
Our previous studies have shown that flutamide, an androgen receptor antagonist, administration following trauma-hemorrhage had salutary effects on cardiovascular, hepatocellular, and immune functions under those conditions.21,22 The present study indicates that flutamide significantly decreased hepatic injury and plasma IL-6 levels following trauma-hemorrhage. The decrease in hepatic injury was associated with the decrease in MPO activity and neutrophil accumulation. Hepatic neutrophil accumulation was closely related to the alteration in the hepatic protein levels of CINC-1, CINC-3, and ICAM-1. Moreover, the elevated NF-κB DNA binding activity, which is reported to regulate gene expressions of CINC-1, CINC-3, ICAM-1, and IL-6, was attenuated by flutamide administration following trauma-hemorrhage. Furthermore, the salutary effects of flutamide on hepatic injury and cytokine production were prevented by the ER antagonist. In addition, plasma levels of estradiol were restored to sham levels by flutamide administration following trauma-hemorrhage. These findings suggest the mechanism of the salutary effect of flutamide in decreasing hepatic injury and pro-inflammatory cytokine production is likely due to down-regulation in NF-κB DNA binding activity. Furthermore, since administration of ICI 182,790 along with flutamide abolished the salutary effects of flutamide, the results suggest that the salutary effects of flutamide following adverse circulatory conditions appear to be mediated at least in part via ER-related pathway.
The NF-κB family, one of the transcriptional factors, is activated in the lung, liver, and heart following hemorrhagic shock.2,3 The activation of NF-κB plays a pivotal role in inflammation and drives the expression of pro-inflammatory mediators.5,6 Studies have shown that hemorrhagic shock activates NF-κB and induces increased IL-6 gene expression in the liver.2,9 Studies have also reported that CINC-1 and CINC-3 gene expression appears to be related to the activation of NF-κB. A rapid increase in NF-κB binding activity after ischemia-reperfusion injury in the liver preceded the increase in the expression of CINC-1.8 The reduction in hepatic neutrophil recruitment was associated with decreased activation of NF-κB and expression of the CINC-3.31 The role of the NF-κB signaling cascade is also important in ICAM-1 activation.7 The present study clearly shows that flutamide administration following trauma-hemorrhage reduced the hepatic activation of NF-κB under those conditions. Although this down-regulation of activated NF-κB in the liver is likely related to decreases in hepatic neutrophil accumulation and IL-6 production following trauma-hemorrhage, the precise mechanism by which flutamide treatment modulates hepatic NF-κB activation still remains unknown. Recent studies have demonstrated molecular cross-talk between these families of transcription factors in which the ER mediates inhibition of NF-κB activity at several levels.32,33 One study reported that the disruption of NF-κB-mediated transactivation plays a significant role in ER inhibition of IL-6 gene expression.34 Our present study has shown that flutamide administration restored plasma estrogen levels to sham levels following trauma-hemorrhage. Although we have not measured alteration in hepatic estradiol levels and ER protein expression in this study, our recent study has shown that flutamide administration modulated cardiac ER protein expression in male rats following trauma-hemorrhage.23,27 Thus, sustained increase in plasma estrogen levels and/or modulated ER expression after flutamide administration likely contributes to a decrease in hepatic NF-κB activation following trauma-hemorrhage. Additional studies are needed to determine the mechanism by which flutamide suppresses NF-κB activation following trauma-hemorrhage. Nonetheless, since administration of ER antagonist along with flutamide prevented the salutary effects of flutamide on hepatic injury and pro-inflammatory cytokine production, the salutary effects of flutamide administration appear to be mediated at least in part via ER-related pathway.
The present results demonstrate that flutamide administration following trauma-hemorrhage restores the depressed plasma testosterone and estradiol levels, which may have decreased due to the effect of blood loss following trauma-hemorrhage. Plasma testosterone was increased by flutamide administration in male rats. This increase in plasma testosterone levels is likely related to feedback induced by blockade of androgen receptor.35,36 Previous studies have also shown that plasma estrogen levels increased in male rats administered flutamide.24,25 Although we have not measured alteration in hepatic aromatase activity in this study, our recent study has demonstrated that flutamide administration following trauma-hemorrhage increased cardiac aromatase activity in male rats.27 Therefore, the elevated aromatase activity induced by flutamide administration after hemorrhagic shock likely resulted in the elevation of plasma estradiol levels. Interestingly, administration of ICI 182,780 along with flutamide diminished the sustained plasma estradiol levels induced by flutamide, although ICI 182,780 did not influence plasma testosterone levels in both the sham and the trauma-hemorrhage groups. A previous study has reported that ICI 182,780 is not only an ER antagonist but also an inhibitor of cellular aromatase activity.37 Thus, ICI 182,780 likely prevented the salutary effects of flutamide following trauma-hemorrhage not only via ER antagonism but also because it prevented the increase in plasma estradiol levels by flutamide.
The entry of gut-derived inflammatory products into the circulation via mesenteric lymph seems to play a major role in the neutrophil priming and activation.38,39 In this regard, a study has shown that trauma-hemorrhagic shock-induced up-regulation of ICAM-1 expression in the hepatic endothelial cell is blunted by ligation of mesenteric lymph duct.13 A previous study has reported sexual dimorphism in the activation of neutrophils by shock mesenteric lymph. Furthermore, it has been shown that there is a decrease in pathologic neutrophil activation following trauma-hemorrhagic shock in proestrus female rats, in which estrogen levels are highest.40 Our previous study has demonstrated that flutamide administration following treatment restored the reduced blood flow and oxygen delivery and consumption in the intestine under those conditions.41 Although we did not evaluate intestinal alteration in this study, it is possible that flutamide administration also improves gut perfusion and reduces the entry of gut-derived inflammatory products following trauma-hemorrhage.
The long-term use of flutamide has been reported to induce hepatic toxicity.42 Another in vitro study has demonstrated that high concentration of flutamide alters the activation of neutrophils by subsequent phorbol myristate acetate or f-methionyl-leucyl-phenylalanine. Stimulation in neutrophils and minor neutrophil-mediated injury to isolated hepatocytes was observed in the presence of high-dose flutamide.43 However, a clinical prospective study in a large number of patients has shown that the incidence of flutamide-induced liver toxicity is very low.44 Flutamide appears to cause hepatotoxic effects in some patients.45 Nonetheless, our findings suggest that administration of a single dose (25 mg/kg sc) of flutamide in rats as an adjunct to resuscitation following trauma-hemorrhage is effective in attenuating hepatic injury and cytokine production under those conditions. Although the results indicate salutary effects of flutamide at 2 hours after its administration, it remains unclear whether the salutary effects are sustained for longer periods of time after treatment. In this regard, our previous studies have shown that the salutary effects by pharmacologic agents such as estradiol, flutamide, and dehydroepiandrosterone are sustained for prolonged intervals, and they also improve the survival of animals if the improvement in organ functions is observed in the early phase following administration of the pharmacologic agent.17,20,22,41,46
CONCLUSION
Our study indicates that flutamide administration ameliorates hepatic injury and IL-6 production following trauma-hemorrhage. The improvement in hepatic injury following flutamide administration is likely due to a reduction of hepatic neutrophil accumulation associated with down-regulation of CINC-1, CINC-3, and ICAM-1 following trauma-hemorrhage. Furthermore, the suppression in hepatic NF-κB activation by flutamide appears to contribute to the decrease in cytokine production and hepatic expressions of chemokine and adhesion molecule. Flutamide-mediated modulation in hepatic NF-κB activation is likely mediated via ER-related pathway. Since flutamide administration following trauma-hemorrhage decreased hepatic injury and cytokine production, this agent appears to be a novel and useful adjunct for restoring the depressed hepatic function in male animals following adverse circulatory conditions.
ACKNOWLEDGMENTS
The authors thank Mr. Zheng F. Ba and Mr. Philip Sohn for their superb technical assistance during these studies.
Footnotes
Supported by NIH Grant No. R37 GM-39519.
Reprints: Irshad H. Chaudry, PhD, Center for Surgical Research, University of Alabama at Birmingham, 1670 University Boulevard, Volker Hall, Room G094, Birmingham, AL 35294-0019. E-mail: Irshad.Chaudry@ccc.uab.edu.
REFERENCES
- 1.Jarrar D, Chaudry IH, Wang P. Organ dysfunction following hemorrhage and sepsis: mechanisms and therapeutic approaches. Int J Mol Med. 1999;4:575–583. [DOI] [PubMed] [Google Scholar]
- 2.Hierholzer C, Harbrecht B, Menezes JM, et al. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med. 1998;187:917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meldrum DR, Shenkar R, Sheridan BC, et al. Hemorrhage activates myocardial NFkappaB and increases TNF-alpha in the heart. J Mol Cell Cardiol. 1997;29:2849–2854. [DOI] [PubMed] [Google Scholar]
- 4.Samy TS, Ayala A, Catania RA, et al. Trauma-hemorrhage activates signal transduction pathways in mouse splenic T cells. Shock. 1998;9:443–450. [DOI] [PubMed] [Google Scholar]
- 5.Zingarelli B, Sheehan M, Wong HR. Nuclear factor-kappaB as a therapeutic target in critical care medicine. Crit Care Med. 2003;31:S105–S111. [DOI] [PubMed] [Google Scholar]
- 6.Hierholzer C, Billiar TR. Molecular mechanisms in the early phase of hemorrhagic shock. Langenbecks Arch Surg. 2001;386:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876–888. [DOI] [PubMed] [Google Scholar]
- 8.Yamada S, Iida T, Tabata T, et al. Alcoholic fatty liver differentially induces a neutrophil-chemokine and hepatic necrosis after ischemia-reperfusion in rat. Hepatology. 2000;32:278–288. [DOI] [PubMed] [Google Scholar]
- 9.Gaddipati JP, Sundar SV, Calemine J, et al. Differential regulation of cytokines and transcription factors in liver by curcumin following hemorrhage/resuscitation. Shock. 2003;19:150–156. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu T, Tani T, Endo Y, et al. Elevation of plasma peptidoglycan and peripheral blood neutrophil activation during hemorrhagic shock: plasma peptidoglycan reflects bacterial translocation and may affect neutrophil activation. Crit Care Med. 2002;30:77–82. [DOI] [PubMed] [Google Scholar]
- 11.Lehnert M, Arteel GE, Smutney OM, et al. Dependence of liver injury after hemorrhage/resuscitation in mice on NADPH oxidase-derived superoxide. Shock. 2003;19:345–351. [DOI] [PubMed] [Google Scholar]
- 12.Kuebler JF, Yokoyama Y, Jarrar D, et al. Administration of progesterone after trauma and hemorrhagic shock prevents hepatocellular injury. Arch Surg. 2003;138:727–734. [DOI] [PubMed] [Google Scholar]
- 13.Xu DZ, Lu Q, Adams CA, et al. Trauma-hemorrhagic shock-induced up-regulation of endothelial cell adhesion molecules is blunted by mesenteric lymph duct ligation. Crit Care Med. 2004;32:760–765. [DOI] [PubMed] [Google Scholar]
- 14.Toth B, Schwacha MG, Kuebler JF, et al. Gender dimorphism in neutrophil priming and activation following trauma-hemorrhagic shock. Int J Mol Med. 2003;11:357–364. [PubMed] [Google Scholar]
- 15.Toth B, Yokoyama Y, Schwacha MG, et al. Insights into the role of interleukin-6 in the induction of hepatic injury after trauma-hemorrhagic shock. J Appl Physiol. 2004;97:2184–2189. [DOI] [PubMed] [Google Scholar]
- 16.Meng ZH, Dyer K, Billiar TR, et al. Essential role for IL-6 in postresuscitation inflammation in hemorrhagic shock. Am J Physiol Cell Physiol. 2001;280:C343–C351. [DOI] [PubMed] [Google Scholar]
- 17.Angele MK, Schwacha MG, Ayala A, et al. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;4:81–90. [DOI] [PubMed] [Google Scholar]
- 18.Knoferl MW, Angele MK, Diodato MD, et al. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg. 2002;235:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szalay L, Shimizu T, Schwacha MG, et al. Mechanism of salutary effects of estradiol on organ function after trauma-hemorrhage: upregulation of heme oxygenase. Am J Physiol Heart Circ Physiol. 2005;289:H92–H98. [DOI] [PubMed] [Google Scholar]
- 20.Mizushima Y, Wang P, Jarrar D, et al. Estradiol administration after trauma-hemorrhage improves cardiovascular and hepatocellular functions in male animals. Ann Surg. 2000;232:673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Remmers DE, Wang P, Cioffi WG, et al. Testosterone receptor blockade after trauma-hemorrhage improves cardiac and hepatic functions in males. Am J Physiol. 1997;273:H2919–H2925. [DOI] [PubMed] [Google Scholar]
- 22.Wichmann MW, Angele MK, Ayala A, et al. Flutamide: a novel agent for restoring the depressed cell-mediated immunity following soft-tissue trauma and hemorrhagic shock. Shock. 1997;8:242–248. [PubMed] [Google Scholar]
- 23.Yu HP, Yang S, Choudhry MA, et al. Mechanism responsible for the salutary effects of flutamide on cardiac performance after trauma-hemorrhagic shock: upregulation of cardiomyocyte estrogen receptors. Surgery. 2005;138:85–92. [DOI] [PubMed] [Google Scholar]
- 24.O'Connor JC, Frame SR, Ladics GS. Evaluation of a 15-day screening assay using intact male rats for identifying steroid biosynthesis inhibitors and thyroid modulators. Toxicol Sci. 2002;69:79–91. [DOI] [PubMed] [Google Scholar]
- 25.Yamada T, Kunimatsu T, Sako H, et al. Comparative evaluation of a 5-day Hershberger assay utilizing mature male rats and a pubertal male assay for detection of flutamide's antiandrogenic activity. Toxicol Sci. 2000;53:289–296. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu T, Szalay L, Choudhry MA, et al. Mechanism of salutary effects of androstenediol on hepatic function after trauma-hemorrhage: role of endothelial and inducible nitric oxide synthase. Am J Physiol Gastrointest Liver Physiol. 2005;288:G244–G250. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh YC, Yang S, Choudhry MA, et al. Flutamide restores cardiac functions following trauma-hemorrhage via an estrogen-dependent pathway through upregulation of PGC-1. Am J Physiol Heart Circ Physiol. 2006;290:H416–H423. [DOI] [PubMed] [Google Scholar]
- 28.Rana SN, Li X, Chaudry IH, et al. Inhibition of IL-18 reduces myeloperoxidase activity and prevents edema in intestine following alcohol and burn injury. J Leukoc Biol. 2005;77:719–728. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu T, Szalay L, Hsieh YC, et al. A role of PPARγ in androstenediol-mediated salutary effects on cardiac function following trauma-hemorrhage. Ann Surg 2005; in press. [DOI] [PMC free article] [PubMed]
- 30.Jarrar D, Kuebler JF, Rue LW III, et al. Alveolar macrophage activation after trauma-hemorrhage and sepsis is dependent on NF-kappaB and MAPK/ERK mechanisms. Am J Physiol Lung Cell Mol Physiol. 2002;283:L799–L805. [DOI] [PubMed] [Google Scholar]
- 31.Kato A, Gabay C, Okaya T, et al. Specific role of interleukin-1 in hepatic neutrophil recruitment after ischemia/reperfusion. Am J Pathol. 2002;161:1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalaitzidis D, Gilmore TD. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol Metab. 2005;16:46–52. [DOI] [PubMed] [Google Scholar]
- 33.Ray P, Ghosh SK, Zhang DH, et al. Repression of interleukin-6 gene expression by 17 beta-estradiol: inhibition of the DNA-binding activity of the transcription factors NF-IL6 and NF-kappa B by the estrogen receptor. FEBS Lett. 1997;409:79–85. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, Liu K, Bodenner DL. Estrogen receptor inhibits interleukin-6 gene expression by disruption of nuclear factor kappaB transactivation. Cytokine. 2005;31:251–257. [DOI] [PubMed] [Google Scholar]
- 35.Hellman L, Bradlow HL, Freed S, et al. The effect of flutamide on testosterone metabolism and the plasma levels of androgens and gonadotropins. J Clin Endocrinol Metab. 1977;45:1224–1229. [DOI] [PubMed] [Google Scholar]
- 36.Viguier-Martinez MC, Hochereau de Reviers MT, Barenton B, et al. Endocrinological and histological changes induced by flutamide treatment on the hypothalamo-hypophyseal testicular axis of the adult male rat and their incidences on fertility. Acta Endocrinol (Copenh). 1983;104:246–252. [DOI] [PubMed] [Google Scholar]
- 37.Long BJ, Tilghman SL, Yue W, et al. The steroidal antiestrogen ICI 182,780 is an inhibitor of cellular aromatase activity. J Steroid Biochem Mol Biol. 1998;67:293–304. [DOI] [PubMed] [Google Scholar]
- 38.Adams CA Jr, Hauser CJ, Adams JM, et al. Trauma-hemorrhage-induced neutrophil priming is prevented by mesenteric lymph duct ligation. Shock. 2002;18:513–517. [DOI] [PubMed] [Google Scholar]
- 39.Caruso JM, Feketeova E, Dayal SD, et al. Factors in intestinal lymph after shock increase neutrophil adhesion molecule expression and pulmonary leukosequestration. J Trauma. 2003;55:727–733. [DOI] [PubMed] [Google Scholar]
- 40.Adams JM, Adams CA, Xu DZ, et al. Sexual dimorphism in the activation of neutrophils by shock mesenteric lymph. Surg Infect (Larchmt). 2003;4:37–44. [DOI] [PubMed] [Google Scholar]
- 41.Ba ZF, Wang P, Koo DJ, et al. Attenuation of vascular endothelial dysfunction by testosterone receptor blockade after trauma and hemorrhagic shock. Arch Surg. 2001;136:1158–1163. [DOI] [PubMed] [Google Scholar]
- 42.Rosman AS, Frissora-Rodeo C, Marshall AT, et al. Cholestatic hepatitis following flutamide. Dig Dis Sci. 1993;38:1756–1759. [DOI] [PubMed] [Google Scholar]
- 43.Srinivasan R, Buchweitz JP, Ganey PE. Alteration by flutamide of neutrophil response to stimulation: implications for tissue injury. Biochem Pharmacol. 1997;53:1179–1185. [DOI] [PubMed] [Google Scholar]
- 44.Gomez JL, Dupont A, Cusan L, et al. Incidence of liver toxicity associated with the use of flutamide in prostate cancer patients. Am J Med. 1992;92:465–470. [DOI] [PubMed] [Google Scholar]
- 45.Lin AD, Chen KK, Lin AT, et al. Antiandrogen-associated hepatotoxicity in the management of advanced prostate cancer. J Chin Med Assoc. 2003;66:735–740. [PubMed] [Google Scholar]
- 46.Jarrar D, Wang P, Song GY, et al. Inhibition of tyrosine kinase signaling after trauma-hemorrhage: a novel approach for improving organ function and decreasing susceptibility to subsequent sepsis. Ann Surg. 2000;231:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]


