Abstract
Introduction:
Gastrointestinal stromal tumor (GIST) is the most common sarcoma of the intestinal tract. Nearly all tumors have an activating mutation in the KIT or, less often, PDGFRα, gene. Therapy with tyrosine kinase inhibitors benefits over 80% of patients with advanced GIST, but most patients eventually develop drug resistance.
Methods:
Forty patients with metastatic GIST were treated with tyrosine kinase inhibitors and then underwent surgical resection. Based on the growth of their tumors by serial radiologic imaging, patients were categorized at the time of operation as having responsive disease, focal resistance (1 tumor growing), or multifocal resistance (more than 1 tumor growing). Patients were followed for a median of 15 months (range, 6–46 months) after surgery.
Results:
Initially, molecular therapy achieved stable disease or a partial response in all but 1 patient. Surgery was performed after a median of 15 months, and there were no perioperative deaths. After operation, the 20 patients with responsive disease had a 2-year progression-free survival of 61% and 2-year overall survival of 100%. In contrast, the 13 patients with focal resistance progressed after surgery at a median of 12 months and the 2-year overall survival was 36%. There were 7 patients with multifocal resistance and they progressed postoperatively at a median of 3 months and had a 1-year overall survival of 36%.
Conclusion:
Selected patients with metastatic GIST who have responsive disease or focal resistance to tyrosine kinase inhibitor therapy may benefit from elective surgical resection. Surgery for patients with metastatic GIST who have multifocal resistance is generally not indicated, and these patients should be considered for clinical trials of new systemic agents.
Most patients with advanced gastrointestinal stromal tumor (GIST) initially respond to treatment with tyrosine kinase inhibitors but eventually develop drug resistance. We evaluated our results of combining targeted molecular therapy with surgical resection for metastatic GIST.
Gastrointestinal stromal tumor (GIST) is the most common sarcoma of the intestinal tract. GIST most often originates in the stomach or small intestine but can also arise in extraintestinal sites, such as the mesentery and omentum. Patients with primary localized GIST who undergo complete resection of their disease often develop tumor recurrence, and the 5-year survival can be as low as 54%.1 Historically, the outcome of patients with advanced GIST (unresectable primary disease or metastatic disease) has been poor, with a median survival of approximately 1.5 years.1 The response rate to conventional cytotoxic chemotherapy was only 5%.2 In 1998, Hirota et al identified activating mutations in the KIT proto-oncogene in GIST.3 Subsequent reports have found KIT mutations in up to 85% of GIST while another 3% to 5% have a PDGFRα mutation.4–6 Imatinib mesylate (Gleevec, Novartis Pharmaceuticals, Basel, Switzerland) inhibits the KIT and PDGFRα tyrosine kinases and was first applied to GIST in 2000.7 Imatinib achieves a partial response or stable disease in nearly 80% of patients, and remarkably the 2-year survival in advanced GIST is now 75% to 80%.7,8 More recently, sunitinib (Sutent, Pfizer, New York, NY), which inhibits VEGFR in addition to KIT and PDGFRα, has proven efficacious in patients who are intolerant or refractory to imatinib.9
While the majority of patients initially benefit from tyrosine kinase inhibitors, it is now clear that resistance commonly develops. Indeed, the median time to progression on imatinib mesylate is 2 years.8 Previously, we and others have defined the major mechanisms of acquired imatinib resistance in GIST.10–13 To improve the results of targeted therapy in metastatic GIST, we have used a multimodality approach that includes surgery. Here, we report the clinical outcome of 40 patients with metastatic GIST who were treated with tyrosine kinase inhibitors and then underwent surgery.
METHODS
Patients
From 2001 to July 2005, we performed surgery on 40 patients with metastatic GIST who were being treated with tyrosine kinase inhibitors. Pathologic material was examined and the diagnosis was confirmed using standard hematoxylin/eosin staining and CD117 immunohistochemistry on formalin-fixed, paraffin-embedded tissue as previously described.5 Patient data were collected and recorded in a departmental sarcoma database. This study was approved by our Institutional Review Board.
Definitions
Based on the recent growth status of their tumors by serial cross-sectional imaging performed preoperatively, patients were classified at the time of surgery as having responsive disease, focal resistance, or multifocal resistance to tyrosine kinase inhibition. Patients with responsive disease had either a partial response or stable disease just prior to surgery. Patients with focal resistance had radiologic evidence of growth in 1 tumor. Multifocal resistance denotes growth in more than 1 tumor. Disease remaining at the completion of surgery was categorized based on whether there was gross residual tumor. Gross disease was further distinguished as suboptimal debulking when sarcomatosis was present or any residual tumor was larger than 1 cm.
Postoperative complications are graded at our institution on a 1 to 5 scale.14 Grade 1 complications require only supportive care. Grade 2 complications necessitate moderate intervention, such as intravenous medications. Grade 3 complications require invasive surgical or radiologic treatment. Grade 4 complications produce chronic disability and grade 5 complications result in death. There were no grade 4 or 5 complications in the current study.
Statistics
No patient was lost to follow-up and the status of each patient was updated to within 4 weeks of the present analysis. All patients had a minimum follow up of 6 months. All times are reported in months. Actuarial progression-free survival and overall survival were calculated from the time of surgery for metastatic GIST. Kaplan-Meier and log rank analyses were performed using SPSS statistical software (SPSS, Chicago, IL). A P value <0.05 was considered significant.
RESULTS
Patients and Medical Therapy
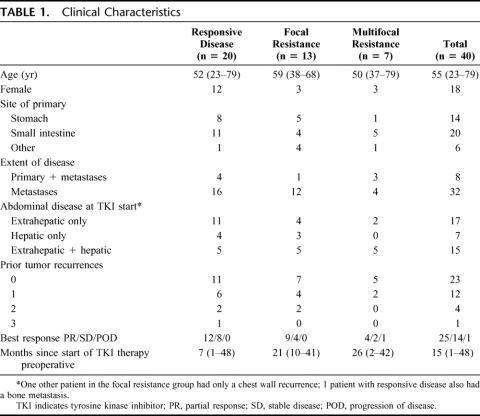
We performed surgery in 40 patients with metastatic GIST who were being treated with tyrosine kinase inhibitors. The median age was 55 years and 45% were female (Table 1). The primary tumors originated mostly (85%) in the stomach or small intestine. At the initiation of tyrosine kinase inhibitor therapy, 8 patients had a primary GIST in addition to metastases, and the majority of patients had either extrahepatic intra-abdominal metastases alone (43%) or in combination with liver metastases (38%).
TABLE 1. Clinical Characteristics
The best response during preoperative tyrosine kinase inhibitor therapy was partial response or stable disease in all but 1 patient. At the time of surgery, all patients were being treated with imatinib mesylate (400–800 mg per day), except for 3 patients who had been switched to sunitinib. The median time of preoperative molecular therapy was shorter in patients with responsive disease (7 months) than in those with focal (21 months) or multifocal (26 months) resistance.
Surgical Results
Nearly all of the operations were performed on an elective basis. One patient had tumor debulking to control tumor hemorrhage induced by response to imatinib. A semi-urgent operation was performed for a patient with multifocal resistance in the liver who had gastrointestinal bleeding from the primary tumor. One operation was performed 3 weeks earlier than planned because of a contained bowel perforation. Two patients required 2 operations to remove all gross disease. Each had thefirst operation to clear all peritoneal disease followed by a hemi-hepatectomy to remove all liver disease.
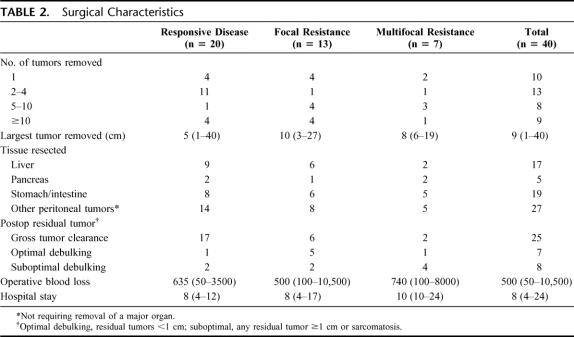
Only 25% of the patients had a solitary tumor and 43% had at least 5 tumors (Table 2). The median size of the largest tumor removed was 9 cm. Liver resections were performed in 43% of patients, pancreatic resections (including 2 Whipple procedures) in 13%, and stomach/intestine resections in 48%. Liver resections included 3 wedge resections, 7 segmental resections, 4 lobectomies, and 3 extended lobectomies. Peritoneal tumors not requiring resection of a major organ were removed in 68% of patients. Gross tumor clearance was achieved in 85% of patients with responsive disease, but only 46% of those with focal resistance and 29% of those with multifocal resistance.
TABLE 2. Surgical Characteristics
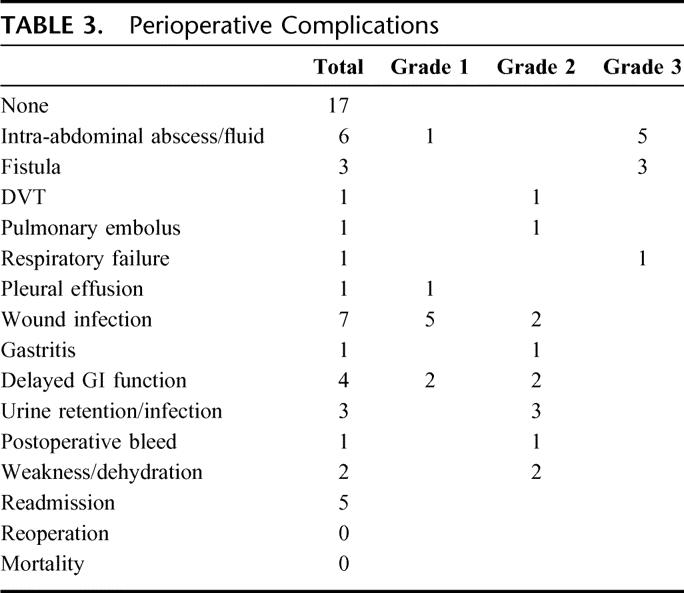
The median blood loss was 500 mL and exceeded 1 L in 8 patients. The median hospital stay was 8 days. There were no perioperative deaths. Complications occurred in 58% of patients but were mostly grade 1 or 2 (Table 3). There were 9 grade 3 complications, which occurred in 8 patients. Five patients required percutaneous drainage of a postoperative fluid collection or abscess. In addition, there were 2 pancreatic fistulas and 1 colonic fistula, all of which resolved with percutaneous drainage. One patient required intubation for transient respiratory failure. There were 5 hospital readmissions but no patient required reoperation for a complication.
TABLE 3. Perioperative Complications
Outcome
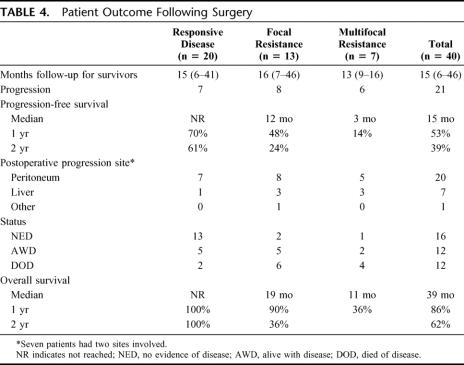
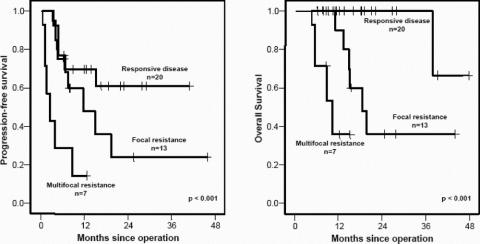
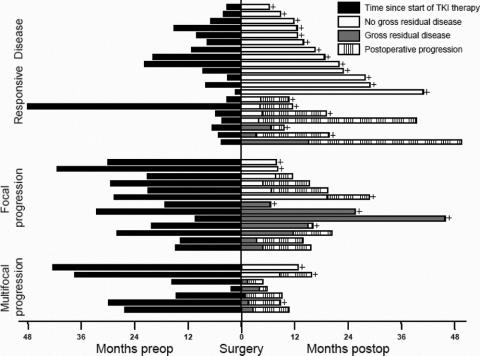
The median follow up time was 15 months (6–46 months) (Table 4). Progression-free survival was significantly different between the 3 patient groups (Fig. 1). The median time to progression was not reached in patients with responsive disease and the 1- and 2-year progression-free survival was 70% and 61%, respectively. Notably, 4 of the 7 patients in this group who had postoperative progression did not resume tyrosine kinase inhibitor therapy immediately after surgery because of patient refusal due to prior toxicity or delayed recovery from surgery. The other 3 patients in the responsive disease group who developed postoperative tumor progression had gross residual disease after surgery, 2 of whom had sarcomatosis. The 13 (65%) other patients with responsive disease underwent resection of all gross disease and have not developed progression at a median follow-up of 17 months (6–41 months). Patients with focal resistance had a median time to progression of 12 months, with a 1- and 2-year progression-free survival of 48% and 24%, respectively. Those with multifocal progression had a median time to postoperative progression of only 3 months. Overall, in the 21 patients with postoperative progression, the peritoneum was involved in 20, the liver in 7, and the lung in 1.
TABLE 4. Patient Outcome Following Surgery
FIGURE 1. Progression-free and overall survival after resection of metastatic GIST.
Twelve patients have died of disease, and no patient has died of another cause. Patients with responsive disease had a 2-year survival of 100%, which was significantly longer than that of the other 2 groups (Figs. 1, 2). Patients with focal resistance had a median survival of 19 months, and a 1- and 2-year survival of 90% and 36%, respectively. Those with multifocal resistance had a median survival of 11 months and a 1-year survival of 36%.
FIGURE 2. Timeline of treatment and outcome for 40 patients with metastatic GIST treated with molecular therapy and then surgery. Each patient is represented by a horizontal bar. The patients are grouped by their response at the time of surgery to tyrosine kinase inhibitor (TKI) therapy. The time period from the start of TKI therapy to surgery is shown by the black boxes. All patients were operated upon at time zero. After surgery, patients had either no gross residual disease (white boxes) or gross residual disease (gray boxes). Postoperative progression is shown by the striped boxes. A plus sign designates the patients who were alive at last follow-up; the other patients have died of disease.
DISCUSSION
Historically, the results of surgical resection alone in the treatment of metastatic GIST have been poor, with essentially all patients subsequently developing tumor recurrence.1,15 Therapy with tyrosine kinase inhibitors induce partial response or stable disease in over 80% of patients and has become the first-line treatment of metastatic GIST.7,8 However, there are several reasons to consider surgical resection in patients with metastatic GIST who are being treated with molecular therapy. While tyrosine kinase inhibitors control tumor growth in the majority of patients with metastatic GIST, complete responses are rarely achieved.7,8 Only 1 patient (who had a 3.1-cm peritoneal tumor) in the present study had no viable tumor upon microscopic examination of the resected specimen. Another reason to resect metastatic GIST is that tumors that appear nonviable by radiologic imaging usually still harbor alive cells, and these cells often have evidence of KIT protein activation.13 Furthermore, it is now clear that most patients who initially respond to tyrosine kinase inhibitors eventually acquire resistance. The median time to progression is approximately 2 years.8 The predominant mechanism of acquired resistance to imatinib is via additional mutations in KIT.16 Previously, we found that patients who developed a second KIT mutation had been treated for a median of 27 months of imatinib.13 Currently, once resistance to imatinib develops, there is only a small chance of rescuing the patient. Dose escalation of imatinib in 133 patients who progressed on 400 mg/day resulted in a median progression-free survival of only 81 days, with 18% progression-free at 1 year.17 Meanwhile, only about one fourth of patients who are switched to sunitinib will have responsive disease a year later.18
Based on the above rationale, we have used a combination of molecular therapy and surgery for the treatment of metastatic GIST in patients who have responsive disease. Our hypothesis is that the chance of resistance is proportional to the amount of residual GIST following therapy with tyrosine kinase inhibitors. Our general approach in responsive disease has been to treat patients for 3 to 6 months with tyrosine kinase inhibition and then consider them for surgery if their disease appears completely resectable. While it has been shown that tumor load can continue to decrease even after a year of imatinib therapy, the median time to best response is approximately 3.5 months, and there is little incremental tumor shrinkage after 9 months.8 It is critical to continue tyrosine kinase inhibition postoperatively to delay, or possibly even prevent, subsequent progression. Several of our patients with responsive disease who were rendered free of gross disease but progressed postoperatively had stopped their therapy because of prior toxicity or patient preference. These findings are in agreement with a French study, in which patients with metastatic GIST who were randomized to stop imatinib after 1 year of therapy subsequently developed disease progression.19 The optimal duration of tyrosine kinase inhibitor therapy after resection of metastatic GIST is unknown.
The patients with responsive disease who underwent surgery have fared well, with a 2-year actuarial survival of 100%. These data are consistent with a previous report of complete resection in 11 patients with metastatic GIST who were first treated with imatinib.20 The overall survival of patients with metastatic GIST treated with imatinib on a large randomized phase III clinical trial has been reported to be approximately 70% at 2 years.8 Nevertheless, the benefit of surgery in patients with metastatic GIST who have responsive disease remains unknown because our results are confounded by selection bias. We chose patients because of their limited disease, as demonstrated by our ability to remove all gross tumor in 85%. Furthermore, there is lead time bias in comparing our results in responsive disease to those in focal or multifocal resistance. The patients with responsive disease had a much shorter duration of preoperative therapy with tyrosine kinase inhibitors.
The patients with focal resistance (1 tumor increasing in size) had variable outcomes after surgery. Clearly, focal resistance portends subsequent progression at other sites, since the median time to progression after surgery was 12 months. It is likely that focal resistance signifies the presence of cumulative genetic damage in other seemingly quiescent tumors as a result of chronic (median, 21 months) tyrosine kinase inhibition. Nevertheless, the 1-year actuarial survival in this group was 90%. A subset of patients with focal resistance may have derived benefit from surgery, since in our series there have been 3 actual 2-year survivors following surgery, of whom 2 have not progressed.
We found that there was little benefit of surgical resection in patients with multifocal resistance (more than 1 tumor increasing in size). Their median time to progression was 3 months. While 2 patients have survived a year after surgery, nearly all patients with multifocal progression refractory to targeted therapy should be considered for clinical trials testing new agents or combinations of existing agents.
Many of the operations in this series were extensive as reflected by the number (17 patients had at least 5 tumors removed), size (median of 9 cm for largest tumor removed), and multifocality of tumors as well as the operative blood loss. In addition, there were 17 liver and 5 pancreatic resections. Overall, the rate of major complications was low and they consisted primarily of intra-abdominal fluid collections, pancreatic leaks, and 1 intestinal leak, all of which responded to nonoperative therapy. There were no perioperative deaths. It is particularly important to weigh the risks of operating electively on patients with responsive disease, since they are typically asymptomatic and can expect to live for at least several years on medical therapy alone.
Ideally, the question of whether surgery is beneficial for patients with metastatic GIST who have responsive disease to tyrosine kinase inhibition should be answered by a randomized clinical trial. After a defined period of targeted therapy (eg, 6 months), patients with a partial tumor response or stable disease who have resectable disease by cross-sectional imaging would be randomized to surgery or observation. All patients would continue tyrosine kinase inhibition. The endpoints would be time to tumor progression and overall survival. Given the accrual in the multicenter North American metastatic and adjuvant GIST trials, the trial should be feasible.
Footnotes
Supported in part by CA94503 and CA102613 (to R.P.D.), PO1 CA 47179 (to M.F.B.), and ACS MRSG CCE-106841 (to C.R.A.). Drs. DeMatteo and Maki have been consultants and received honoraria from Novartis Pharmaceuticals.
Reprints: Ronald P. DeMatteo, MD, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., New York, NY 10021. E-mail: dematter@mskcc.org.
REFERENCES
- 1.DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeMatteo RP, Heinrich MC, El Rifai WM, et al. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002;33:466–477. [DOI] [PubMed] [Google Scholar]
- 3.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. [DOI] [PubMed] [Google Scholar]
- 4.Rubin BP, Singer S, Tsao C, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001;61:8118–8121. [PubMed] [Google Scholar]
- 5.Antonescu CR, Sommer G, Sarran L, et al. Association of KIT exon 9 mutations with nongastric primary site and aggressive behavior: KIT mutation analysis and clinical correlates of 120 gastrointestinal stromal tumors. Clin Cancer Res. 2003;9:3329–3337. [PubMed] [Google Scholar]
- 6.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. [DOI] [PubMed] [Google Scholar]
- 7.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. [DOI] [PubMed] [Google Scholar]
- 8.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–1134. [DOI] [PubMed] [Google Scholar]
- 9.Demetri GD, Desai J, Fletcher JA, et al. SU11248, a multi-targeted tyrosine kinase inhibitor, can overcome imatinib (IM) resistance caused by diverse genomic mechanisms in patients with metastatic gastrointestinal stromal tumor (GIST). Proc Am Soc Clin Oncol. 2004;22:3001. [Google Scholar]
- 10.Fletcher JA, Corless CL, Dimitrijevic S, et al. Mechanisms of resistance to imatinib mesylate (IM) in advanced gastrointestinal stromal tumor (GIST). Proc Am Soc Clin Oncol. 2003;21:3275. [Google Scholar]
- 11.Chen LL, Trent JC, Wu EF, et al. A missense mutation in KIT kinase domain 1 correlates with imatinib resistance in gastrointestinal stromal tumors. Cancer Res. 2004;64:5913–5919. [DOI] [PubMed] [Google Scholar]
- 12.Debiec-Rychter M, Cools J, Dumez H, et al. Mechanisms of resistance to imatinib mesylate in gastrointestinal stromal tumors and activity of the PKC412 inhibitor against imatinib-resistant mutants. Gastroenterology. 2005;128:270–279. [DOI] [PubMed] [Google Scholar]
- 13.Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005;11:4182–4190. [DOI] [PubMed] [Google Scholar]
- 14.Martin RC, Brennan MF, Jaques DP. Quality of complication reporting in the surgical literature. Ann Surg. 2002;235:803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeMatteo RP, Shah A, Fong Y, et al. Results of hepatic resection for sarcoma metastatic to liver. Ann Surg. 2001;234:540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Zwan SM, DeMatteo RP. Gastrointestinal stromal tumor: 5 years later. Cancer. 2005;104:1781–1788. [DOI] [PubMed] [Google Scholar]
- 17.Zalcberg JR, Verweij J, Casali PG, et al. Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer. 2005;41:1751–1757. [DOI] [PubMed] [Google Scholar]
- 18.Maki RG, Fletcher JA, Heinrich MC, et al. SU11248, a multi-targeted tyrosine kinase inhibitor, can overcome imatinib (IM) resistance caused by diverse genomic mechanisms in patients (pts) with metastatic gastrointestinal stromal tumor (GIST). Proc Am Soc Clin Oncol. 2005;23:9011. [Google Scholar]
- 19.Blay JY, Berthaud P, Perol D, et al. Continuous vs intermittent imatinib treatment in advanced GIST after one year: a prospective randomized phase III trial of the French Sarcoma Group. Proc Am Soc Clin Oncol. 2004;22:9006. [Google Scholar]
- 20.Bauer S, Hartmann JT, de Wit M, et al. Resection of residual disease in patients with metastatic gastrointestinal stromal tumors responding to treatment with imatinib. Int J Cancer. 2005;117:316–325. [DOI] [PubMed] [Google Scholar]