Abstract
Objective:
To assess the morbidity after sentinel lymph node (SLN) biopsy compared with SLN and completion level I and II axillary lymph node dissection (ALND) in a prospective multicenter study.
Summary Background Data:
ALND after breast cancer surgery is associated with considerable morbidity. We hypothesized: 1) that the morbidity in patients undergoing SLN biopsy only is significantly lower compared with those after SLN and completion ALND level I and II; and 2) that SLN biopsy can be performed with similar intermediate term morbidity in academic and nonacademic centers.
Methods:
Patients with early stage breast cancer (pT1 and pT2 ≤ 3 cm, cN0) were included between January 2000 and December 2003 in this prospective Swiss multicenter study. All patients underwent SLN biopsy. In all patients with SLN macrometastases and most patients with SLN micrometastases (43 of 68) or isolated tumor cells (11 of 19), a completion ALND was performed. Postoperative morbidity was assessed based on a standardized protocol.
Results:
SLN biopsy alone was performed in 449 patients, whereas 210 patients underwent SLN and completion ALND. The median follow-ups were 31.0 and 29.5 months for the SLN and SLN and completion ALND groups, respectively. Intermediate-term follow-up information was available from 635 of 659 patients (96.4%) of enrolled patients. The following results were found in the SLN versus SLN and completion ALND group: presence of lymphedema (3.5% vs. 19.1%, P < 0.0001), impaired shoulder range of motion (3.5% vs. 11.3%, P < 0.0001), shoulder/arm pain (8.1% vs. 21.1%, P < 0.0001), and numbness (10.9% vs. 37.7%, P < 0.0001). No significant differences regarding postoperative morbidity after SLN biopsy were noticed between academic and nonacademic hospitals (P = 0.921).
Conclusions:
The morbidity after SLN biopsy alone is not negligible but significantly lower compared with level I and II ALND. SLN biopsy can be performed with similar short- and intermediate-term morbidity in academic and nonacademic centers.
This multicenter prospective study shows that the morbidity after sentinel lymph node (SLN) biopsy is significantly lower compared with axillary lymph node dissection. However, the morbidity after SLN biopsy is not negligible. Furthermore, this represents one of the first reports providing compelling evidence that SLN biopsy can be performed with similar morbidity in academic and nonacademic centers
The axillary lymph node status represents one of the most important prognostic factors in breast cancer patients and determines among others subsequent adjuvant treatment. The percentage of node positive patients who benefit from routine axillary lymph node dissection (ALND) is constantly decreasing as breast cancer is increasingly detected at an early stage. The sentinel lymph node (SLN) accurately reflects the status of the remaining axillary lymph nodes in patients with early-stage breast cancer with a very low false-negative rate. Therefore, most patients can be spared the considerable short- and long-term sequelae of ALND. First introduced in specialized, academic centers, the SLN biopsy has also become routine practice in the surgical therapy for breast cancer patients in nonacademic clinics and community hospitals.1–7
While the side effects and negative impact on quality of life of level I and II ALND have been extensively described in the literature,8–11 little is known about the intermediate-term morbidity after the SLN biopsy.7,12–14 Therefore, the objective of the present prospective multicenter study was to test 2 different hypotheses: first, that the morbidity in breast cancer patients undergoing SLN biopsy only is significantly lower compared with those after SLN and completion ALND level I and II; and second, that SLN biopsy can be performed with similar intermediate-term morbidity in academic and nonacademic centers.
PATIENTS AND METHODS
Between January 2000 and December 2003, a total of 698 patients with early-stage breast cancer were prospectively enrolled in the present multicenter investigation. Inclusion criteria for the present study were: 1) presence of palpable breast cancer, 2) tumor size histologically equal to or less than 3 cm in diameter, 3) absence of clinically palpable axillary lymph nodes, 4) no prior history of breast cancer or other malignancies, 5) no neoadjuvant therapy, and 6) no pregnancy. Thirty-nine patients did not meet the inclusion criteria and were therefore excluded. Written informed consent was obtained from all patients. The study was approved by all involved Local Ethic Committees. A total of 13 centers participated in this study, among them the Swiss University Hospitals in Basel, Berne, and Zurich, as well as different nonacademic institutions (community hospitals and private practices) in Switzerland.
Lymphatic Mapping and Operative Technique
SLN mapping was performed by using a combination of a radiolabeled colloid and a vital blue dye. 99mTc-labeled nanocolloid (Nanocoll, Nycomed AG, Wüädenswil, Switzerland) at a dose of 70 MBq was injected, peritumorally at 4 places, whereas at the injection site closest to the axilla half of the dose was injected peritumorally and subdermally. Lymphoscintigraphy was performed preoperatively to identify lymphatic flow to axillary and/or parasternal lymph nodes. Hot spots were marked on the skin. The SLN were intraoperatively identified first by the use of a handheld gamma probe (Navigator, USSC, RMD Waterton, MA; C-Trak, Care Wise Medical Products Corp., Morgan Hill, CA; or Neoprobe, Ethicon Endo-Surgery, Johnson & Johnson, Cincinnati, OH). Up to 5 mL of isosulfan blue (Lymphazurin, Ben Venue Labs Inc., Bedford, OH; and Hospital Pharmacy University Hospital Zurich, Zurich) or 2 to 4 mL of patent blue V (Guerbet Group, Roissy, France) were injected in the same fashion as the radioactive tracer 5 to 10 minutes prior to incision. Both isosulfan blue and patent blue V belong to the group of triarylmethan dyes and share the same formula. However, patent blue V has an additional hydroxyl group at position 5. Because of their nearly equal chemical structure patent blue V has very similar lymphatic penetration and flow properties compared with isosulfan blue. Patent blue V was commercially available in Switzerland and approved by Swissmedic, the Swiss Federal Agency for Therapeutic Products, whereas isosulfan blue was only temporarily admitted for specific use in this trial. The choice of the blue dye was left to the institutions’ preference. Hot and/or blue lymph nodes were excised and labeled separately as SLN. Dissection was continued until all hot and blue nodes had been removed and the background count of the axilla was less than 10% of the hottest lymph node ex vivo.
Prior to participation to this study, the SLN procedure had to be validated at all institutions based on at least 20 breast cancer patients in whom both SLN and completion level I and II ALND were performed. A SLN identification rate and a sensitivity of at least 95% were requirements for participating to this trial. All participating centers were supervised by the principal investigators (G.B., O.R.K., M.Z., all 3 being academic surgeons) for their first 20 cases. The results of the validation period (20 patients) were critically reviewed by one of the principal investigators. Only after adequate performance had been proven, the hospitals were allowed to enroll patients to the present study. The principal investigators were instructed at large medical centers abroad in institutions with vast experience for SLN technique.
Pathologic Examination of Lymph Nodes
Frozen sections were routinely performed intraoperatively. Lymph nodes larger than 5 mm in diameter were bisected, whereas lymph nodes less than or equal to 5 mm in diameter were not bisected but completely submitted for frozen section analysis. The SLN were intraoperatively examined at 3 levels with hematoxylin and eosin-stained sections at a cutting interval of 150 μm. The remaining tissue of the SLN was formalin-fixed and embedded in paraffin for histologic analysis. The residual tissue was then examined using step sectioning at a cutting interval of 250 μm. Step sections were stained with hematoxylin and eosin. If no carcinoma cells were detected, immunohistochemistry with cytokeratin antibody Lu-5 or CK 22 using a standard immunoperoxidase method (ABC Elite kit) was performed. Lu-5 (Bio Medicals, Augst, Switzerland) is a pan-cytokeratin monoclonal antibody that recognizes types I and II cytokeratin subfamilies of all epithelial and mesothelial cells.
Micrometastases are defined based on a size exceeding 0.2 mm and less than or equal to 2 mm in diameter according to the AJCC classification. Therefore, isolated tumor cells (ITC) or tumor cell clusters measuring less than or equal to 0.2 mm in diameter did not meet the definition of micrometastases. Patients with submicrometastases (≤0.2 mm) were considered node-negative in the present investigation. All patients with SLN macrometastases in frozen sections underwent immediate completion ALND. If no SLN macrometastases were found in frozen sections but in final histopathology, patients underwent delayed completion ALND. Because of the lack of clear data in the literature, the decision to perform completion ALND in patients with SLN micrometastases or isolated tumor cells was left to each hospital's directives. No completion ALND was performed in women with tumor-free SLN.
Adjuvant Therapy
After breast-conserving surgery patients received postoperative breast radiation therapy with 45 Gy over 5 weeks and a boost of 10 Gy to the tumor site, which had been clip-marked intraoperatively. Radiation to the axilla was only applied, if 4 or more lymph nodes had macrometastases. Adjuvant therapy consisted of hormonal treatment and/or chemotherapy. The indication for adjuvant therapy was based on the recommendations of the St. Gallen Consensus Conference.15
Postoperative Follow-up
The follow-up diagnostics included clinical examination of the breast and of the axilla every 3 months as well as annual mammography to detect local and axillary recurrences. Additional ultrasound of the breast was performed to clarify suspicious mammographic findings. A standardized study form was filled out: 1) before surgery, 2) on day 3 postoperatively or prior to discharge if patients were dismissed earlier, and 3) during every follow-up examination in the outpatient clinic. Subjective criteria such as pain (arm, shoulder, breast, and thorax), numbness, and restrictions in daily activities due to scar or arm problems were assessed as being present or not. Objective criteria included the preoperative and postoperative measurements of 1) range of motion in all directions of the shoulder according to the neutral-zero crossing method, and 2) the circumferences of both upper and lower extremities 15 cm above and 15 cm below the olecranon. The rationale for measuring 15 cm above and below the olecranon was to ensure measuring the circumferences in midupper- and midforearm. A deficit of range of motion over 20° to standard values and in comparison to the unaffected side was considered as abnormal. The diagnosis of lymphedema was based on either subjective symptoms or objective findings and measurements. Symptoms like swelling and heaviness of the affected arm were considered diagnostic for lymphedema as well as clinical findings such as indentation after skin impression or loss of skin folds. A 2-cm increase of the arm circumference compared with the ipsilateral baseline assessments as measured 15 cm above and 15 cm below the olecranon was considered abnormal.16 Differences in circumference of more than 2 cm to the measurements of the contralateral arm were also regarded as abnormal.
Statistical Analyses
Mann-Whitney U test was used for comparisons of continuous outcomes, while Fisher exact test was used for comparisons of dichotomous and categorical variables. The level of statistical significance was set at 0.05. All statistical tests were two-sided. For compilation of data Microsoft Access database Software (Microsoft Corporation, Redmond, WA) was used. Statistical analyses were performed with GraphPad InStat software version 3.05 (GraphPad Software, San Diego, CA).
RESULTS
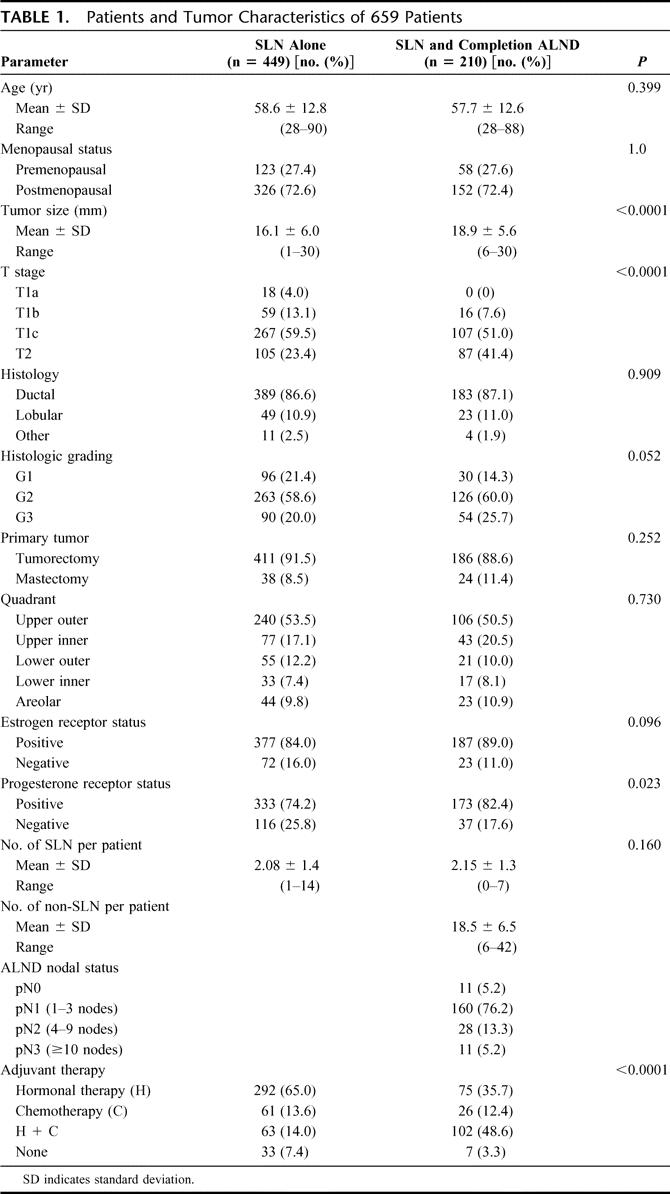
The patients’ characteristics of the SLN and SLN and completion ALND groups are listed in Table 1. According to the protocol most of the patients in the SLN and completion ALND group had SLN macrometastases or micrometastases. As increasing tumor size is associated with a higher probability of nodal metastases, there was a significant difference between both groups regarding primary tumor size.
TABLE 1. Patients and Tumor Characteristics of 659 Patients
Between January 2000 and December 2003, SLN biopsies were performed on 659 breast cancer patients meeting the inclusion criteria (Fig. 1). The overall SLN identification rate was 98.3% (648 of 659). A median number of 2 SLNs per patient were harvested in both groups. There was no difference in the number of harvested SLNs between the institutions using patent blue V or isosulfan blue (mean 2.11 ± 1.16 vs. 2.16 ± 1.44, P = 0.80) with a nearly equal SLN identification rate of 98.8% (338 of 342) and 97.7% (295 of 302), respectively (P = 0.36). The SLN were tumor-free in 416 patients (416 of 659, 63.1%). Macrometastases were detected in 145 patients (145 of 659, 22.0%), micrometastases in 68 patients (68 of 659, 10.3%), and isolated tumor cells in 19 patients (19 of 659, 2.9%, Fig. 1). All patients with SLN macrometastases and most with micrometastases or isolated tumor cells underwent completion level I and II ALND. SLN and completion ALND level I and II were performed in 210 patients (210 of 659, 31.9%). Of those, 145 patients had SLN macrometastases, 43 patients had SLN micrometastases, 11 patients had SLN isolated tumor cells, and in 11 patients, no SLN identification was possible (Fig. 1). In the remaining 449 women (449 of 659, 68.1%) no further axillary surgery was performed. Based on the frozen sections showing macrometastases (142 patients), micrometastases (7 patients), and in case of SLN identification failure (11 patients) an immediate completion ALND was performed in 160 patients (160 of 210, 76.2%). A delayed completion ALND was performed in 50 patients (50 of 210, 23.8%). Therefore, 7.6% (50 of 659) of the entire study population underwent delayed completion ALND.
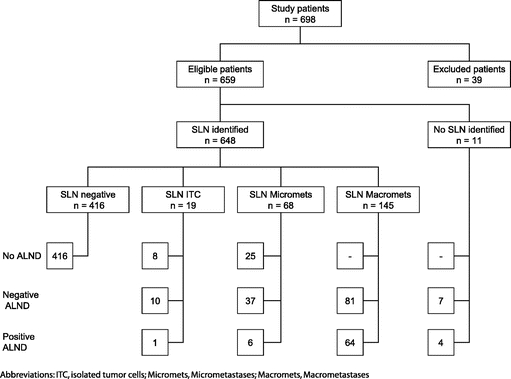
FIGURE 1. Study flow chart.
In-Hospital Morbidity and Mortality
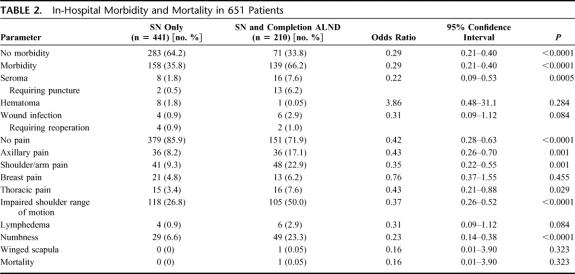
A total of 651 (651 of 659, 98.8%) patients were evaluated (Table 2). Eight patients (1.2%) were excluded because of incomplete data sheets. There was no reported morbidity in 35.8% and 66.2% in the SLN group and in the SLN and completion ALND group, respectively (P < 0.0001). The following postoperative sequelae were significantly less frequent in patients undergoing SLN procedure compared with those having SLN and completion ALND: shoulder range of motion (26.8% vs. 50.0%), shoulder/arm pain (9.3% vs. 22.9%), numbness (6.6% vs. 23.3%), axillary pain (8.2% vs. 17.1%), and seroma formation (all P values ≤ 0.001). Axillary seroma in the SLN and completion ALND group required more often a puncture (13 of 16 cases, 81%) than in the SLN group (2 of 8 cases, 25%). The wound infection rates were low in both groups (0.9% versus 2.9%, P = 0.08). One patient died on the second day after SLN and completion ALND of a myocardial infarction.
TABLE 2. In-Hospital Morbidity and Mortality in 651 Patients
Intermediate-term Morbidity and Mortality
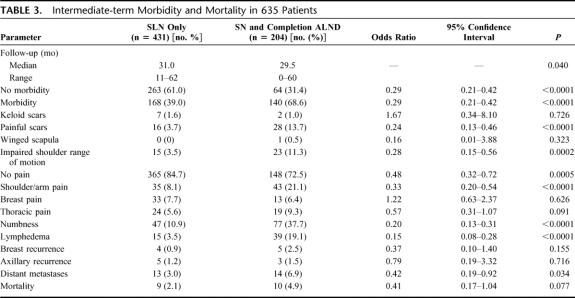
Intermediate-term follow-up information were collected for 635 of 659 patients (96.4%). The median (range) follow-up of patients undergoing SLN procedure and SLN and completion ALND were 31.0 (11–62) and 29.5 (0–60) months, respectively (Table 3). A total of 168 patients (168 of 431, 39.0%) and 140 patients (140 of 204, 68.6%) in the SLN and in the SLN and completion ALND group, respectively, suffered from at least one problem. Shoulder range of motion (3.5% vs. 11.3%), shoulder/arm pain (8.1% vs. 21.1%), painful scars (3.7% vs. 13.7%), numbness of the upper arm (10.9% vs. 37.7%), and lymphedema (3.5% vs. 19.1%) were significantly less frequent in the SLN group compared with the SLN and completion ALND group (all P values ≤0.0002). A total of 37 patients (37 of 204, 18.1%) in the SLN and completion ALND group received radiation therapy to the axilla. Of these, 18.9% (7 of 37) developed lymphedema during follow-up. However, even after excluding patients who received radiation therapy to the axilla, the difference in postoperative lymphedema formation between the SLN and SLN and completion ALND groups remained highly statistically significant (3.5% vs. 15.7%, P < 0.0001).
TABLE 3. Intermediate-term Morbidity and Mortality in 635 Patients
Tumor Recurrence
After median (range) follow-up of 31.0 (11–62) and 29.5 (0–60) months, there was no statistical difference regarding breast or axillary tumor recurrences between patients undergo- ing SLN biopsy and those having SLN and completion ALND (Table 3). Breast recurrence occurred in 0.9% and 2.5% in the SLN and SLN and completion ALND group, respectively (P = 0.155). Axillary recurrences were detected in 1.2% (5 of 431) in the SLN and 1.5% (3 of 204), in the SLN and completion ALND group (P = 0.716). All 8 patients with axillary recurrences were reoperated. Distant metastases occurred more frequently in the SLN and completion ALND group compared with patients having SLN biopsy only (P = 0.034). This can be explained by the fact that in the SLN and completion ALND group the patients were mostly node-positive.
Immediate Versus Delayed Completion ALND
Follow-up information for women undergoing completion ALND were gathered for 204 patients (204 of 210, 97.1%). Of those, 155 patients (155 of 204, 76.0%) received an immediate and 49 patients (49 of 204, 24.0%) a delayed completion ALND. There were no statistically significant differences between these 2 groups neither with respect to overall postoperative morbidity (68.4% vs. 69.4%, P = 1.0) nor regarding shoulder/arm pain (22.6% vs. 16.3%, P = 0.43), numbness (35.5% vs. 44.9%, P = 0.24), range of shoulder motion (11.6% vs. 10.2%, P = 1.0), and lymphedema (17.4% vs. 24.5%, P = 0.30).
Comparison of Outcomes Between Academic and Nonacademic Centers
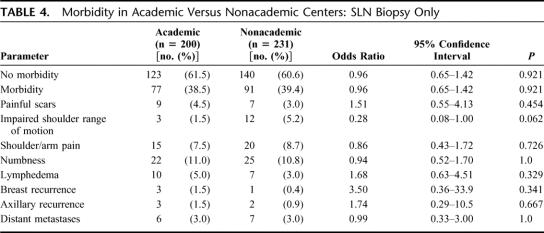
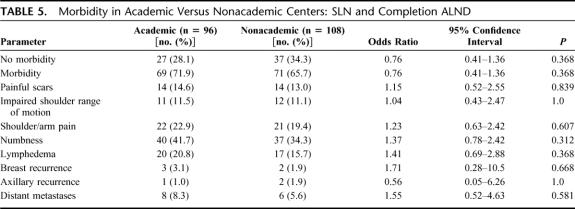
We compared the occurrence of postoperative complications between academic and nonacademic centers in both groups (SLN vs. SLN and completion ALND, Tables 4, 5). No difference was found with respect to postoperative morbidity, neither in patients having only SLN biopsy (P = 0.921) nor in those undergoing SLN and completion ALND (P = 0.368). No statistical difference regarding breast (P = 0.341) and axillary recurrences (P = 0.667) or distant metastases (P = 1.0) was found between academic and nonacademic centers (Tables 4, 5).
TABLE 4. Morbidity in Academic Versus Nonacademic Centers: SLN Biopsy Only
TABLE 5. Morbidity in Academic Versus Nonacademic Centers: SLN and Completion ALND
DISCUSSION
The present prospective multicenter investigation based on a large sample of breast cancer patients provides compelling evidence that postoperative morbidity after SLN procedure is significantly lower compared with SLN and completion ALND of level I and II. Therefore, patients with negative SLN clearly benefit from omitting formal ALND of level I and II. However, the morbidity after SLN biopsy is not negligible. Furthermore, this is one of the first reports in the literature showing that SLN biopsy can be performed with similar short- and intermediate-term morbidity in academic and nonacademic centers.
ALND is associated with significant morbidity that negatively impacts quality of life.17 In a retrospective study including 390 breast cancer patients undergoing ALND, lymphedema of the upper and lower arm were found in 13.2% and 8.4%, respectively, after a median follow-up of 62 months.11 In the aforementioned investigation, numbness occurred in 28%, hypertrophic scars in 17%, and shoulder pain in 15% of all patients. Maunsell et al assessed 223 patients 3 months after level I and II ALND; they reported that 82% of patients suffered from at least one arm problem, including swelling (24%), weakness (26%), limited arm movement (32%), stiffness (40%), pain (55%), and numbness (58%).18 Our results are in line with and confirm the prevalent occurrence of morbidity after ALND described in the literature.9
Lymphedema represents one of the major factors contributing to postoperative morbidity as it may result in decreased range of motion, pain, weakness, or stiffness of the affected extremity.17 The occurrence of lymphedema after level I and II ALND has been reported in the literature between 5% and 25%.19–23
Many factors have been cited to increase the risk of lymphedema following breast cancer surgery. The extent of axillary surgery and postoperative axillary irradiation are the 2 most common factors.22,24 No association between axillary metastases and the development of arm lymphedema was found in different studies.9,20,25,26 Similarly, chemotherapy was not an independent risk factor for arm lymphedema.7
Although there are generally accepted criteria to diagnose lymphedema, a precise definition is lacking and no consensus exists of what constitutes lymphedema.23 This renders the interpretation of the literature regarding the presence of lymphedema after breast cancer surgery difficult. Casley-Smith could show that circumferential measurements are highly correlated with the results of the more exact water displacement method.27 On the other hand, girth measurements do not always correlate with symptoms or quality of life.28 A combination of symptom assessment and limb measurement, as done in the present investigation, represents the most reliable clinical assessment to identify changes associated with postbreast cancer surgery lymphedema.29
Despite the fact that both academic and nonacademic institutions as well as high- and low-volume hospitals contributed to this study, the SLN identification rate in this trial remained over 98%. In another multicenter trial, Tafra et al examined the performance of 48 surgeons from academic and nonacademic centers after they underwent an SLN and lymphatic mapping course.30 Their SLN identification rate was 87%. In multivariable analysis, low surgeons’ experience and higher patient age were the only independent predictors of failed SLN identification. In the Z0010 American College of Surgeons Oncology Group (ACOSOG) study, in which both academic and teaching institutions as well as community practices participated, Posther et al showed that increased patients’ age, higher BMI, and fewer patients accrued to the study were independently associated with SLN identification failure.31 Conversely, specific skill qualification and the type of institution did not independently impact SLN identification failure.
In our patient sample, axillary recurrence occurred in 1.2% and 1.5% in the SLN and SLN and completion ALND groups, respectively. ALND provides excellent regional control with axillary recurrence rates ranging from 0% to 2%.32,33 However, the present prospective investigation provides compelling evidence that axillary recurrence rates in patients undergoing SLN biopsy only and in those having SLN and completion ALND did not significantly differ at a median follow-up of 30 months.
Although postoperative morbidity after SLN biopsy alone was significantly lower than after SLN and completion ALND in the present investigation, there were still 39% of patients in the former group who suffered from at least one problem. Because of the prospective design of our study and the use of a standardized protocol, we were able to assess the different morbidity parameters very accurately. Although one would think that the removal of a median number of 2 axillary lymph nodes through a small incision should not lead to problems or complications, the present investigations provides compelling evidence that morbidity is not negligible occur even after SLN biopsy alone. This finding is important in the assessment of a new surgical technique in comparison with a standard procedure, not only for quality assurance, but it enables also to provide accurate informed consent to patients undergoing breast cancer surgery.
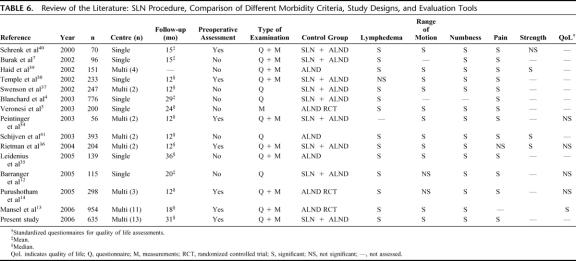
A variety of prospective studies have compared postoperative morbidity after SLN biopsy and ALND (Table 6), which show a clear advantage of the SLN technique for most of the variables and confirm the findings of our investigation.3,4,7,12–14,34–41
TABLE 6. Review of the Literature: SLN Procedure, Comparison of Different Morbidity Criteria, Study Designs, and Evaluation Tools
Methodologic limitations regarding the measurement of arm morbidity and quality of life may render the interpretation of study results difficult. The exclusive use of traditional objective measurements of arm morbidity while omitting patients self-reports may result in an underestimation of the extent of the problem.39,42 Other limitations include the assessment of a single (instead of multiple) postoperative assessments,36 the lack of preoperative baseline assessment potentially leading to an inadequate evaluation of change,2,3,7,38,43 a prohibitively short follow-up4,7,39,40 and the use of nonvalidated measurement instruments.3,4,7 In the present study, a preoperative baseline assessment was performed, the follow-up was carried out several times based on a standardized protocol, and reliable measurement instruments were used in the assessment of postoperative morbidity. Furthermore, both objective and subjective criteria were used to assess the postoperative morbidity as accurately as possible.
A critical review of the existing literature regarding the morbidity after SLN biopsy and ALND in breast cancer patients reveals many of the above-mentioned methodologic limitations (Table 6).
The present investigation was designed to include patients with early breast cancer who all underwent SLN biopsy. The control group consisted of patients with metastatic SLN or failure to identify a SLN who then underwent completion level I and II ALND. The rationale for this study design was as follows: First, most of breast cancer patients are well informed about the possibility of minimally invasive axillary surgery as represented by the SLN procedure. Second, based on the comparison of our own preliminary SLN results and historical ALND data, we were convinced that it would be unreasonable, even unethical, to perform a formal ALND on node-negative patients.1,11 Therefore, we refrained from performing a randomized controlled trial comparing SLN only with ALND. Third, in our study design patients undergoing level I and II completion ALND were mostly SLN positive and more likely to get adjuvant chemotherapy. As these factors are reportedly not associated with an increased morbidity after elective ALND, the differences in morbidity between the SLN and SLN and ALND group are mainly due to the type of surgical intervention.7
In the literature, there are only 3 randomized controlled clinical trials comparing SLN versus primary ALND.3,13,14 All these studies confirm the superiority of the SLN technique regarding physical and psychologic postoperative morbidity (Table 6).
Most recently, the results of the ACOSOG Z0010 trial regarding early morbidity of 5327 patients 30 days and 6 months after SLN biopsy were published.44 Both academic institutions as well as community practices contributed to this study. The rates for lymphedema, impaired shoulder range of motion, and paresthesia after SLN biopsy alone were similar to our results. There were no differences in early postoperative morbidity between academic and nonacademic centers.
In the present investigation the postoperative morbidity was compared between academic and nonacademic centers with a median follow-up over 30 months. No statistical difference was found between these 2 subgroups for overall morbidity, the different morbidity parameters as well as functional and oncologic outcome during the intermediate follow-up. This finding is among the most important of our analysis.
CONCLUSION
The present multicenter prospective study based on 659 breast cancer patients clearly shows that the morbidity after SLN biopsy alone is significantly lower compared with patients having SLN biopsy and completion level I and II ALND. However, the morbidity after SLN biopsy is not negligible. Furthermore, this represents one of the first reports in the literature that provides compelling evidence that SLN biopsy can be performed with similar short- and intermediate-term morbidity in academic and nonacademic centers.
ACKNOWLEDGMENTS
The authors thank Bernadette Von Felten, study nurse at the Department of Surgery, University Hospital Basel, for collecting patients’ data.
Footnotes
Reprints: Markus Zuber, MD, Department of Surgery, Kantonsspital Olten, Olten, Switzerland; e-mail: mzuber_ol@spital.ktso.ch.
REFERENCES
- 1.Langer I, Marti WR, Guller U, et al. Axillary recurrence rate in breast cancer patients with negative sentinel lymph node (SLN) or SLN micrometastases: prospective analysis of 150 patients after SLN biopsy. Ann Surg. 2005;241:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giuliano AE, Haigh PI, Brennan MB, et al. Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol. 2000;18:2553–2559. [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–553. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard DK, Donohue JH, Reynolds C, et al. Relapse and morbidity in patients undergoing sentinel lymph node biopsy alone or with axillary dissection for breast cancer. Arch Surg. 2003;138:482–487. [DOI] [PubMed] [Google Scholar]
- 5.Maggard MA, Lane KE, O'Connell JB, et al. Beyond the clinical trials: how often is sentinel lymph node dissection performed for breast cancer? Ann Surg Oncol. 2005;12:41–47. [DOI] [PubMed] [Google Scholar]
- 6.Hansen NM, Grube BJ, Giuliano AE. The time has come to change the algorithm for the surgical management of early breast cancer. Arch Surg. 2002;137:1131–1135. [DOI] [PubMed] [Google Scholar]
- 7.Burak WE, Hollenbeck ST, Zervos EE, et al. Sentinel lymph node biopsy results in less postoperative morbidity compared with axillary lymph node dissection for breast cancer. Am J Surg. 2002;183:23–27. [DOI] [PubMed] [Google Scholar]
- 8.Giuliano AE, Jones RC, Brennan M, et al. Sentinel lymphadenectomy in breast cancer. J Clin Oncol. 1997;15:2345–2350. [DOI] [PubMed] [Google Scholar]
- 9.Roses DF, Brooks AD, Harris MN, et al. Complications of level I and II axillary dissection in the treatment of carcinoma of the breast. Ann Surg. 1999;230:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borup CS, Lundgren E. Sequelae of axillary dissection vs. axillary sampling with or without irradiation for breast cancer: a randomized trial. Acta Chir Scand. 1989;155:515–519. [PubMed] [Google Scholar]
- 11.Harder L, Harder F. Outcome and complications after standard axillary lymph node dissection in breast cancer [Thesis]. Basel: University of Basel, 2002. [Google Scholar]
- 12.Barranger E, Dubernard G, Fleurence J, et al. Subjective morbidity and quality of life after sentinel node biopsy and axillary lymph node dissection for breast cancer. J Surg Oncol. 2005;92:17–22. [DOI] [PubMed] [Google Scholar]
- 13.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609. [DOI] [PubMed] [Google Scholar]
- 14.Purushotham AD, Upponi S, Klevesath MB, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol. 2005;23:4312–4321. [DOI] [PubMed] [Google Scholar]
- 15.Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. Seventh International Conference on Adjuvant Therapy of Primary Breast Cancer. J Clin Oncol. 2001;19:3817–3827. [DOI] [PubMed] [Google Scholar]
- 16.Bland KL, Perczyk R, Du W, et al. Can a practicing surgeon detect early lymphedema reliably? Am J Surg. 2003;186:509–513. [DOI] [PubMed] [Google Scholar]
- 17.Velanovich V, Szymanski W. Quality of life of breast cancer patients with lymphedema. Am J Surg. 1999;177:184–187. [DOI] [PubMed] [Google Scholar]
- 18.Maunsell E, Brisson J, Deschenes L. Arm problems and psychological distress after surgery for breast cancer. Can J Surg. 1993;36:315–320. [PubMed] [Google Scholar]
- 19.Ball AB, Waters R, Fish S, et al. Radical axillary dissection in the staging and treatment of breast cancer. Ann R Coll Surg Engl. 1992;74:126–129. [PMC free article] [PubMed] [Google Scholar]
- 20.Hoe AL, Ivens D, Royle GT, et al. Incidence of arm swelling following axillary clearance for breast cancer. Br J Surg. 1992;79:261–262. [DOI] [PubMed] [Google Scholar]
- 21.Lin PP, Allison DC, Wainstock J, et al. Impact of axillary lymph node dissection on the therapy of breast cancer patients. J Clin Oncol. 1993;11:1536–1544. [DOI] [PubMed] [Google Scholar]
- 22.Erickson VS, Pearson ML, Ganz PA, et al. Arm edema in breast cancer patients. J Natl Cancer Inst. 2001;93:96–111. [DOI] [PubMed] [Google Scholar]
- 23.Petrek JA, Pressman PI, Smith RA. Lymphedema: current issues in research and management. CA Cancer J Clin. 2000;50:292–307. [DOI] [PubMed] [Google Scholar]
- 24.Liljegren G, Holmberg L. Arm morbidity after sector resection and axillary dissection with or without postoperative radiotherapy in breast cancer stage I: results from a randomised trial. Uppsala-Orebro Breast Cancer Study Group. Eur J Cancer. 1997;33:193–199. [DOI] [PubMed] [Google Scholar]
- 25.Larson D, Weinstein M, Goldberg I, et al. Edema of the arm as a function of the extent of axillary surgery in patients with stage I–II carcinoma of the breast treated with primary radiotherapy. Int J Radiat Oncol Biol Phys. 1986;12:1575–1582. [DOI] [PubMed] [Google Scholar]
- 26.Ozaslan C, Kuru B. Lymphedema after treatment of breast cancer. Am J Surg. 2004;187:69–72. [DOI] [PubMed] [Google Scholar]
- 27.Casley-Smith JR. Measuring and representing peripheral oedema and its alterations. Lymphology. 1994;27:56–70. [PubMed] [Google Scholar]
- 28.Rampaul RS, Mullinger K, Macmillan RD, et al. Incidence of clinically significant lymphoedema as a complication following surgery for primary operable breast cancer. Eur J Cancer. 2003;39:2165–2167. [DOI] [PubMed] [Google Scholar]
- 29.Rockson SG, Miller LT, Senie R, et al. American Cancer Society Lymphedema Workshop. Workgroup III: diagnosis and management of lymphedema. Cancer. 1998;83:2882–2885. [DOI] [PubMed] [Google Scholar]
- 30.Tafra L, Lannin DR, Swanson MS, et al. Multicenter trial of sentinel node biopsy for breast cancer using both technetium sulfur colloid and isosulfan blue dye. Ann Surg. 2001;233:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Posther KE, McCall LM, Blumencranz PW, et al. Sentinel node skills verification and surgeon performance: data from a multicenter clinical trial for early-stage breast cancer. Ann Surg. 2005;242:593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fredriksson I, Liljegren G, Arnesson LG, et al. Consequences of axillary recurrence after conservative breast surgery. Br J Surg. 2002;89:902–908. [DOI] [PubMed] [Google Scholar]
- 33.Newman LA, Hunt KK, Buchholz T, et al. Presentation, management and outcome of axillary recurrence from breast cancer. Am J Surg. 2000;180:252–256. [DOI] [PubMed] [Google Scholar]
- 34.Peintinger F, Reitsamer R, Stranzl H, et al. Comparison of quality of life and arm complaints after axillary lymph node dissection vs sentinel lymph node biopsy in breast cancer patients. Br J Cancer. 2003;89:648–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leidenius M, Leivonen M, Vironen J, et al. The consequences of long-time arm morbidity in node-negative breast cancer patients with sentinel node biopsy or axillary clearance. J Surg Oncol. 2005;92:23–31. [DOI] [PubMed] [Google Scholar]
- 36.Rietman JS, Dijkstra PU, Geertzen JH, et al. Treatment-related upper limb morbidity 1 year after sentinel lymph node biopsy or axillary lymph node dissection for stage I or II breast cancer. Ann Surg Oncol. 2004;11:1018–1024. [DOI] [PubMed] [Google Scholar]
- 37.Swenson KK, Nissen MJ, Ceronsky C, et al. Comparison of side effects between sentinel lymph node and axillary lymph node dissection for breast cancer. Ann Surg Oncol. 2002;9:745–753. [DOI] [PubMed] [Google Scholar]
- 38.Temple LK, Baron R, Cody HS III, et al. Sensory morbidity after sentinel lymph node biopsy and axillary dissection: a prospective study of 233 women. Ann Surg Oncol. 2002;9:654–662. [DOI] [PubMed] [Google Scholar]
- 39.Haid A, Kuehn T, Konstantiniuk P, et al. Shoulder-arm morbidity following axillary dissection and sentinel node only biopsy for breast cancer. Eur J Surg Oncol. 2002;28:705–710. [DOI] [PubMed] [Google Scholar]
- 40.Schrenk P, Rieger R, Shamiyeh A, et al. Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer. 2000;88:608–614. [DOI] [PubMed] [Google Scholar]
- 41.Schijven MP, Vingerhoets AJ, Rutten HJ, et al. Comparison of morbidity between axillary lymph node dissection and sentinel node biopsy. Eur J Surg Oncol. 2003;29:341–350. [DOI] [PubMed] [Google Scholar]
- 42.Kuehn T, Klauss W, Darsow M, et al. Long-term morbidity following axillary dissection in breast cancer patients: clinical assessment, significance for life quality and the impact of demographic, oncologic and therapeutic factors. Breast Cancer Res Treat. 2000;64:275–286. [DOI] [PubMed] [Google Scholar]
- 43.Baron RH, Fey JV, Borgen PI, et al. Eighteen sensations after breast cancer surgery: a two-year comparison of sentinel lymph node biopsy and axillary lymph node dissection. Oncol Nurs Forum. 2004;31:691–698. [DOI] [PubMed] [Google Scholar]
- 44.Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13:491–500. [DOI] [PubMed] [Google Scholar]