Abstract
Background:
Some, but not all, studies using registry data have suggested a small but significant long-term survival advantage following a curative surgical resection of gastric cancer at hospitals where the volume of such surgeries is high. However, because such data may be significantly influenced by the impact of postoperative mortality, and may be imbalanced for factors important to survival, the true nature of this relationship remains uncertain.
Methods:
We conducted a nested volume-outcome study in a sample of 448 surgical survivors with stage IB through IV (M0) gastric and gastroesophageal junction adenocarcinoma, previously randomized to adjuvant chemoradiation after surgery or surgery alone, to measure the effect of hospital surgical volume, as assessed by Medicare claims data, on overall survival and gastric cancer recurrence.
Results:
In this selected sample of postoperative survivors, hospital surgical volume was not predictive of overall survival (P = 0.46) or disease-free survival (P = 0.43) at a median follow-up of 8.9 years. However, patients who underwent either a D1 or D2 dissection at a high- or moderate-volume center experienced an adjusted hazard ratio of 0.80 (95% CI, 0.53–1.20) for overall survival and 0.78 (95% CI, 0.53–1.14) for disease-free survival compared with those patients resected at a low-volume hospital; these results were not statistically significant. When a D0 resection was performed, hospital procedure volume showed no impact on survival.
Conclusions:
Excluding the impact of perioperative mortality by utilizing prospectively recorded data from a large postoperative adjuvant trial, hospital procedure volume had no overall effect on long-term gastric cancer survival. The potential benefit of moderate- to high-volume centers for patients who underwent a D1 or D2 dissection requires confirmation in larger studies.
This study evaluates the impact of hospital volume, as a proxy of surgical expertise, on recurrence and survival in patients who enrolled in U.S. Intergroup 0116, a randomized study that compared chemoradiotherapy after surgery with surgery alone in patients with adenocarcinoma of the stomach or gastroesophageal junction.
Surgery remains the primary curative treatment of gastric adenocarcinoma.1 There is a growing consensus in the surgical literature that enhanced surgical technique and expertise can improve outcomes for patients with curatively resected gastrointestinal cancer. Hospital procedure volume appears to be a reasonable proxy for the level of experience within a given medical center.2–16 Individual surgeon volume, of course, reflects a preferred measure, but such data are difficult to obtain. Hospital procedure volume has been shown to predict clinical outcome for both complex medical procedures and complex surgical procedures.13,16–20 Specifically, previous studies using registry databases have demonstrated that hospital procedure volume is predictive of clinical outcomes in patients undergoing complex operations such as pancreatectomy and esophagectomy, but also in more routine operations such as colectomy and lumpectomy.3,13,14,21–23
Previous volume-outcome studies of patients with resectable gastric cancer have relied primarily on cancer registry databases24–29 or on administrative claims-based sources.5,30,31 Higher hospital surgical volume for gastric cancer has been associated with decreased short-term27,28,31 and long-term mortality24,26,27,29 in some, but not in all5,25,28,30 publications. Many of these analyses are retrospective reviews,5,24,27,28,30,31 have limited patient information,5,24,27,28,30,31 lack details on the recurrence of gastric cancer,5,24,25,27,28,30,31 and none has uniform follow-up or detailed data on adjuvant treatment.
We therefore used data from a large randomized trial of patients with stage Ib through IV (M0) gastric cancer to examine the influence of hospital procedure volume on long-term outcome. Patients were identified for this trial 20 to 41 days after gastric cancer surgery, and surgery was performed prior to study enrollment at a variety of hospitals that had a wide range of surgical volume. Given the nature of enrollment, cases resulting in perioperative mortality were excluded. Since postoperative adjuvant therapy and follow-up were explicitly defined in this trial, and the impact of perioperative mortality was eliminated, we were able to assess the influence of hospital surgical volume on long-term survival and recurrence after accounting for potential confounding variables.
METHODS
Study Population
Patients for this analysis were drawn from the National Cancer Institute-sponsored Intergroup 0116 trial of adjuvant chemoradiotherapy for American Joint Committee on Cancer 1988 stage Ib through IV (M0) stomach and gastroesophageal junction cancer, conducted between August 1991 and July 1998 (Fig. 1). 32 The study, coordinated by the Southwest Oncology Group (SWOG), had a nationwide enrollment of 556 patients, with participation by institutions affiliated with one of the following cooperative groups: SWOG, Cancer and Leukemia Group B (CALGB), Radiation Therapy Oncology Group (RTOG), North Central Cancer Treatment Group (NCCTG), and Eastern Cooperative Oncology Group (ECOG). As previously described, 47 patients were deemed ineligible for this trial and were excluded from the analysis.32

FIGURE 1. Schema for INT-0116, a randomized phase III trial of observation or postoperative chemoradiotherapy for patients with resected gastric cancer.
Eligible patients had histologically confirmed adenocarcinoma of the stomach or gastroesophageal junction that had been resected en bloc with curative intent without evidence of residual gross or microscopic disease.32 Patients were eligible if there was evidence of extension of tumor beyond the muscularis propria or lymph node involvement (Ib-IV) and no evidence of metastatic disease.
All patients were required to give informed written consent and were registered between 20 and 41 days after surgery, with treatment beginning within 7 working days of registration.32 Patients must have had a SWOG performance status of 2 or less (ambulatory for at least 50% of the day or better). Patients were also required to have adequate bone marrow, renal, and hepatic function. In addition, patients had to have evidence of at least unilateral renal function and had to demonstrate a caloric intake of 1,500 kcal per day by oral or enterostomal alimentation. Patients with a prior malignancy within the past 5 years were excluded (except for adequately treated squamous or basal cell carcinoma of the skin or in situ carcinoma that had been fully resected). Patients who had received any previous chemotherapy, immunotherapy, or radiotherapy were also not eligible for entry into this trial.
For the present analysis, study staff, who were blinded to patient outcome, reviewed the operative reports of all eligible patients to identify the hospital where the prestudy primary stomach cancer surgery was performed. Since hospital procedure volume rankings were derived from U.S. Medicare claims, we excluded patients who underwent surgery at Veterans Administration, U.S. military, or Canadian hospitals (n = 110). Thus, after these exclusions, of 556 patients randomized in the original study, 448 patients were eligible for the current analysis.
Medicare Hospital Procedure Volume
Using the U.S. Medicare claims database, hospitals were ranked by volume according to the number of primary gastric cancer surgeries performed on all Medicare-enrolled patients between 1991 and 1998, the enrollment period inclusive of the clinical trial.14 Primary gastric cancer operations were defined using an International Classification of Disease (ICD) code of 150.x (esophageal cancer) or 151.x (gastric cancer) and either an ICD procedure code of 424.x, 425.x, 426.x (esophageal operations), 435, 436, 437, 438.x, or 439.x (gastric operations) for a particular hospitalization. Our results did not change when we used hospital volume rankings according to 1) patients with diagnosis of either esophageal or gastric cancer who underwent either an esophageal or gastric resection, 2) patients with diagnosis of esophageal or gastric cancer who underwent a gastric cancer resection, or 3) patients with a diagnosis of gastric cancer who underwent either an esophageal or gastric resection, as the 3 rankings were highly correlated (r > 0.90).
Validation of Medicare Procedure Volume
In previous studies, Medicare case volume was highly correlated with total hospital procedure volume.3 To validate our Medicare procedure volume rankings for gastric cancer surgery, we used the Nationwide Inpatient Sample (NIS) database from 1988 to 1992. The NIS database consists of a random sample of 750 to 900 hospitals per year from 11 states, approximating a 20% sample of U.S. community hospitals. Using the NIS database, we identified the total number of gastric cancer operations performed using the same ICD diagnostic and procedural codes, as described above, and created a parallel annual volume ranking for all hospitals that were represented in our cohort. Among hospitals that were jointly represented in our cohort study and the NIS database (667 hospitals), the Spearman rank correlation for annual gastric cancer surgery volume as measured by the Medicare claims database and the NIS database was 0.92 (P < 0.0001). This high correlation supports the use of procedure volume for gastric cancer surgery as calculated from the Medicare claims database as a valid measure of relative overall hospital procedure volume.
Follow-up
After completion of their therapy, patients were followed every 3 months from time of study entry for 2 years, then every 6 months for 3 years, and then annually thereafter. Data for treatment outcomes and therapeutic efficacy from INT-0116 were published in 2001.32 For the present analysis, patient data have been updated, with the median follow-up time for patients included in this report of 8.9 years.
Statistical Analysis
Surgical volume had a highly skewed distribution (Fig. 2), ranging from 0 to 205 gastrectomies per year. These were split into tertiles for analysis: low, (0–5), medium (6–13), and high (14+). Because of the large number of ties, the actual number of hospitals falling into each of the above categories is not equal (Table 1).

FIGURE 2. Distribution plot of hospitals by number of gastrectomies performed per year for the diagnosis of esophageal or gastric cancer (n = 304).
TABLE 1. Patient Characteristics for INT-0116 by Hospital Volume Tertile
The distribution of baseline characteristics across hospital procedure volume tertiles was evaluated using χ2 tests in the case of categorical variables and Wilcoxon rank sum tests in the case of continuous variables. Overall and disease-free survivals were plotted using the methods of Kaplan and Meier.33 These graphs are presented for descriptive purposes and do not include adjustments for treatment effects. Data were analyzed using Cox proportional hazards regression,34 with a priori inclusion in the model of treatment arm, and the stratification factors used in the original randomization: T-stage (T1 and T2 vs. T3 vs. T4), and involved lymph node status (none vs. 1–3 vs. 4+). This technique allows the assessment of the influence of hospital volume on outcome while adjusting for these factors, and also allows examination of the effects of potential confounders such as age, gender, race, location of primary, type of lymphadenectomy, and performance status. A robust sandwich estimator was used to adjust parameter standard errors for the clustering of cases within hospitals following the method of Lin and Wei.35 We used SAS 9.0 (SAS Institute, Cary, NC) for all statistical analyses. All P values are two-sided.
RESULTS
Baseline Characteristics by Hospital Tertiles
Among the 448 patients (age range, 25–87 years) included in our analysis, primary gastric cancer surgery was performed at 306 U.S. hospitals. Among these 306 hospitals, the mean and median number of annual gastric cancer surgeries were 14.0 and 9.0, respectively (range, 0–205; see Fig. 2 for distribution plot). The baseline characteristics of the cohort according to tertiles of hospital procedure volume are displayed in Table 1. Overall, the baseline characteristics did not differ substantially according to hospital procedure volume. There was a slight imbalance in race, location of primary tumor, and pattern of lymphadenectomy; patients who underwent surgical resection at high-volume hospitals were more likely to be white (P = 0.05), have their primary tumors in the corpus of the stomach (P = 0.03), and were less likely to have undergone a D0 lymph node dissection (P = 0.05).
Survival and Cancer Recurrence by Hospital Procedure Volume
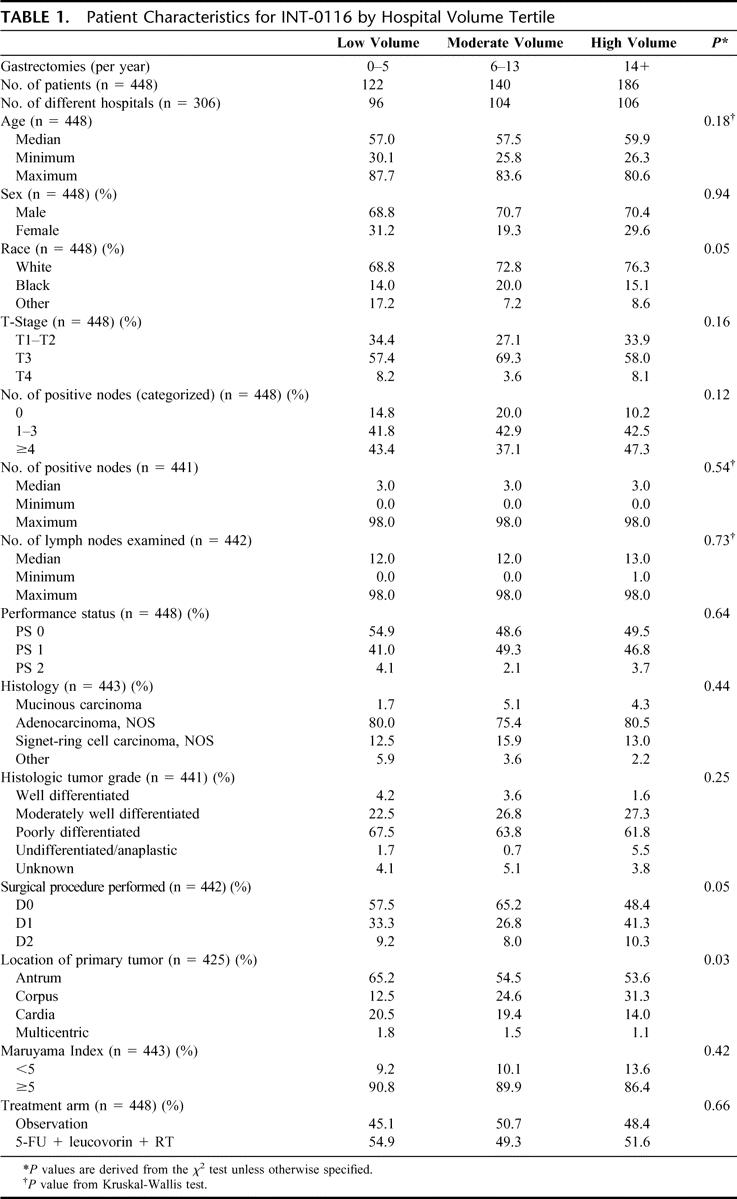
At the time of this analysis, median follow-up for the trial was 8.9 years, with a maximum of 13.2 years. For the entire cohort, overall survival and disease-free survival did not differ significantly by hospital procedure volume (Figs. 3, 4). Although 5-year survival was somewhat greater for patients resected at a high-volume center when compared with those resected at a low-volume center (36.7% vs. 30.8%, respectively), this difference was not statistically significant (Table 2). Using Cox proportional models, we further examined the influence of hospital procedure volume on the risk of overall mortality and cancer recurrence, after adjusting for treatment, T stage, and N stage (Table 3). Compared with patients treated at a low-volume center, those resected at a high-volume center had an adjusted hazard ratio for mortality of 0.88 (95% confidence interval [CI], 0.67–1.17), with similar values for the comparison of the middle-volume centers. When analyzed separately by treatment group, the corresponding hazard ratios between low- and high-volume centers were 1.03 (CI, 0.72–1.48) for the observation arm and 0.80 (CI, 0.48–1.32) for the combined modality arm. None of these differences was statistically significant. Similarly, we failed to observe a significant improvement in disease-free survival, after adjusting for these factors. Similar results were achieved if the models were expanded to include other covariates of interest, such as age, race, primary location, surgical procedure, histology, grade, and performance status. In particular, we investigated the effect of age at various cutpoints, to assess whether surgical volume influenced outcome in a very elderly subset (eg, >65, or >70, or >75). Similar results were obtained in this analysis as well.

FIGURE 3. Overall survival (OS) for all patients on INT-0116 divided by hospital procedure volume.
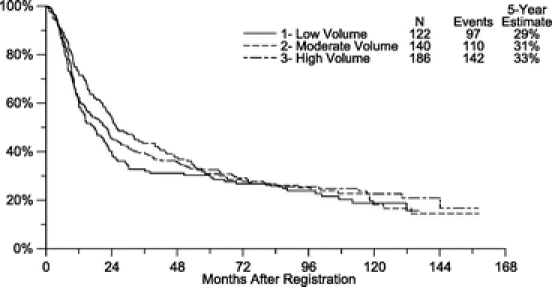
FIGURE 4. Disease-free survival (DFS) for all patients on INT-0116 divided by hospital procedure volume.
TABLE 2. Patient Cancer Recurrence and 5-Year Survival According to Hospital Surgical Volume (n = 448)

TABLE 3. Unadjusted and Adjusted Risk of Mortality and Recurrences According to Hospital Surgical Volume
We considered the possibility that differences in cancer recurrence may only be apparent at the extreme levels of hospital procedure volume. However, when we repeated our analysis after categorizing hospital volume according to quartiles or quintiles, the results were unchanged.
Influence of Lymphadenectomy
We explored the possibility that the influence of hospital volume on patient outcome may be modified by the extent of lymph node dissection at the time of cancer resection. Since few patients (10%) in the study underwent a D2 resection, we were unable to perform a meaningful volume-outcome analysis on only the D2 subset. We therefore stratified patients according to whether they underwent a D0 dissection (54% of patients) or a D1 or D2 dissection (46% of patients). Among patients who underwent a D0 dissection, resection at a high-volume center did not confer any significant improvement in overall or disease-free survival (Tables 4, 5; Fig. 5). However, among patients who underwent either a D1 or D2 dissection, patients treated at moderate or high-volume facilities experienced a trend toward improved overall survival (Tables 4, 5; Fig. 6). Compared with patients treated at a low-volume center, those resected at a moderate- or high-volume hospital experienced an adjusted hazard ratio of 0.80 (95% CI, 0.53–1.20) for overall survival and 0.78 (95% CI, 0.53–1.14) for disease-free survival. Nonetheless, despite this apparent trend, a test for statistical interaction between the extent of lymphadenectomy and hospital volume, at least within the limits of the aforementioned d-level groupings, did not reach statistical significance (P = 0.44 for overall survival).
TABLE 4. Risk of Death and Cancer Recurrence According to Hospital Surgical Volume and Type of Resection

TABLE 5. Unadjusted and Adjusted Risk of Mortality and Recurrences According to Hospital Surgical Volume and Type of Resection

FIGURE 5. Overall survival (OS) for all patients who underwent a D0 resection on INT-0116 divided by hospital procedure volume.
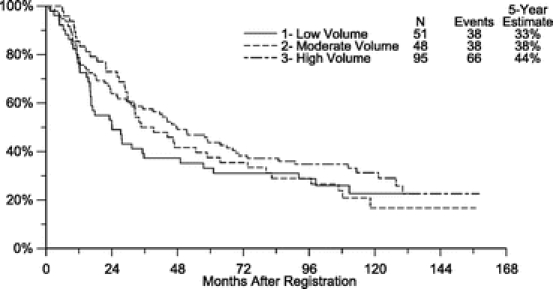
FIGURE 6. Overall survival (OS) for all patients who underwent a D1/2 resection on INT-0116 divided by hospital procedure volume.
Previous work indicated that the Maruyama Index of Unresected Disease (MI)36 was a significant predictor of patient outcome in the Intergroup 0116 trial.37 We therefore also assessed the relationship of this variable to surgical volume with respect to overall survival and disease-free survival. We were unable to detect any changes in our assessment of surgical volume and outcome with the inclusion of MI in the analysis.
DISCUSSION
In this large, postoperative adjuvant chemoradiation trial of operative survivors with stage Ib through IV (M0) stomach and gastroesophageal junction cancer, overall survival and disease-free survival following curative surgery did not differ significantly between low- and high-volume facilities. In exploratory analysis, for patients undergoing a D1 or D2 resection, we did observe a nonsignificant trend toward better outcome with higher volume. Hospital procedure volume had no influence on outcome among patients who underwent a D0 dissection.
Few studies have examined the relationship between hospital procedure volume and survival after a curative resection of gastric cancer. These reports are limited by the use of cancer registry databases24–29 or administrative claims-based sources,5,30,31 which often lack data on recurrences, adjuvant therapy, and important prognostic factors. Moreover, in most such studies, 30-day hospital postoperative mortality may obscure any differences in long-term survival associated with cancer recurrence.
The relationship between perioperative mortality and volume merits comment. Some,27,28,31 but not all,28,30 analyses have suggested an association between hospital volume and perioperative mortality after resection of gastric cancer. Among patients treated at (relatively uniform) Veterans Administration hospitals, in-hospital mortality was 6.9% at high-volume hospitals, 7.4% at medium-volume hospitals, and 8.7% at low-volume hospitals.30 However, these differences in short-term mortality failed to reach statistical significance when the authors adjusted for other patient and disease characteristics. Similarly, in a Swedish population-based study, Hansson et al found that 2-month postoperative mortality, adjusted for age, calendar time, and gender, was superior for patients with gastric cancer at “university” (high-volume) centers as compared with “local” (low-volume) hospitals.27 By contrast, in the Rotterdam Cancer Registry, perioperative mortality did not differ significantly according to hospital volume.28 The authors speculated that their results disagreed with the other reports because of the predominance of low-volume hospitals in their cohort.
Other studies have attempted to evaluate the association between hospital volume and long-term survival after gastrectomy. Most,24,26,27,29 but not all,25 of these analyses have suggested a relationship between hospital volume and long-term survival. A cancer registry study from Germany reported improved 5-year survival for patients undergoing radical gastrectomies at high-volume centers (>20 surgeries per year) compared with patients undergoing similar procedures at hospitals performing only 1 to 4 surgeries per year (10.6% vs. 3.4%).24 Similarly, Nomura et al examined the influence of hospital volume on long-term survival after curative resection of gastric cancer as reported to the Osaka Cancer Registry.27,29 They noted that during the period of 1975 to 1979, the hazard ratio for overall long-term survival was 1.0, 1.7, 1.8, and 2.5 for high-, medium-, low-, and very-low-volume hospitals, respectively. This advantage, however, decreased with time and disappeared completely during the period of 1990 to 1994 except for the very-low-volume hospitals, which still lagged behind with an adjusted hazard ratio of 1.7 (95% CI, 1.4–1.9). The authors suggested that this diminishing disparity between high- and low-volume hospitals over time might reflect surgical standardization and the gradual adoption of improved surgical techniques by the smaller institutions.
Although we failed to find an overall difference in survival between high- and low-volume hospitals in our analysis, we did observe a trend for improved outcome at moderate or high-volume centers when either a D1 or D2 dissection was performed. The test for statistical interaction between extent of lymphadenectomy and hospital volume did not reach statistical significance; however, it should be noted that we had a limited power to test for such an interaction in the relatively small patient population. Further, relatively few cases in our study had “low Maruyama Index surgery,”37 and our ability to study the relationship between this surgical variable and volume was limited. The extent of lymph node dissection for gastric cancer continues to be debated in the surgical literature. Although 4 randomized trials failed to demonstrate a survival benefit when Japanese-defined D2 or D2-plus lymphadenectomy was performed,38–41 other investigators suggest that the more extended resection does improve outcome when the procedure is conducted at experienced centers.36 Additionally, data from 2 separate trials have shown value for a “low Maruyama Index” lymphadenectomy.37,42 Conceivably, the benefits associated with a moderate- or high-volume surgical center may be most apparent when a more extended lymphadenectomy is attempted. In contrast, when a D0 procedure is chosen, the influence of hospital volume is minimized. Nonetheless, this subgroup analysis must be viewed cautiously.
Using clinical trial data to examine the influence of hospital procedure volume offers several advantages over the use of other data sources. First, the stage of disease is comparable within the constraints of the protocol entry criteria. Second, detailed information on adjuvant therapy and prognostic variables, such as location of primary tumor, the number of positive lymph nodes, and performance status, is routinely collected. Most importantly, the date and nature of cancer recurrence are prospectively recorded.
A limitation of our analysis is an inability to study the influence of individual surgeon volume on gastric cancer outcome. Intergroup Trial 0116 demonstrated that the use of postoperative fluorouracil-based chemotherapy and external beam radiotherapy significantly improved overall survival when compared with no postoperative therapy.32 As such, the administration of postoperative chemoradiotherapy to half of the patients in our study may have obscured the influence of hospital surgical volume on long-term patient outcome. However, we failed to find any differences between high- and low-volume hospitals among patients who were randomized to no postoperative therapy. Moreover, although our analysis offers several advantages compared with other data sources, our study was limited by the size of our patient sample. Thus, for instance, our analysis is not sufficiently powered to validate a potential difference in 5-year survival of 6% (Table 2; Fig. 3), which may (or may not) be present between patients treated at high- and low-volume centers. One may debate if such a potential difference in outcome would be substantial enough to channel all patients with localized gastric cancer to high-volume institutions.
Although there is no statistically significant difference in the Kaplan-Meier plots between the high- and low-volume surgery curves for overall survival (Fig. 3) or disease-free survival (Fig. 4) in our study, it is interesting to note that there appear to be larger differences in survival by volume during the first 3 years than during subsequent years of follow-up, in which the curves gradually converge. One possible explanation for this may be that volume-related differences in surgical quality may delay the time to recurrence but are ultimately trumped by the aggressive pathophysiology of gastric cancer. This potential phenomenon should be investigated further in future surgical studies of this disease.
As patients were identified for this clinical trial 20 to 41 days after primary surgical resection, perioperative (short-term) morbidity and mortality were not addressed in this study. However, we specifically sought to focus on the influence of hospital surgical volume on long-term gastric cancer outcomes. By utilizing data from a prospective clinical trial, our analysis may have eliminated variability in other potential explanatory variables, such as use of adjuvant therapy and other follow-up care. However, we were in the unique position of isolating the impact of hospital surgical volume from these other factors.
Since patients enrolled into a randomized clinical trial may not be representative of the broader population of gastric cancer patients nationwide, the generalizability of our findings could be questioned. However, gastric cancer surgery was performed prior to enrollment into this trial, and we did observe considerable variation in hospital procedure volume within our cohort. In addition, since the study population included patients in both community and academic medical centers, we think that the surgical treatment is reflective of the general U.S. population.
Our observation of a nonsignificant trend that hospital volume may influence patient survival only among patients who underwent D1 or D2 dissection merits further study. In an ongoing National Cancer Institute trial of adjuvant chemoradiation for gastric and gastroesophageal adenocarcinoma (CALGB 89803), the extent of surgical lymphadenectomy is prospectively recorded, and the effect of hospital procedure volume will be examined. As such, this additional trial should provide valuable insight into the interaction between hospital volume and the extent of gastric cancer surgery.
ACKNOWLEDGMENTS
The authors thank Michael Hadad (Beacon Healthcare Solutions, LLC) for his help in compiling the Medicare volume data.
Footnotes
Reprints: Peter Enzinger, MD, Dana-Farber Cancer Institute, 44 Binney Street, Boston, MA 02115. E-mail: peter_enzinger@dfci.harvard.edu.
REFERENCES
- 1.Fuchs C, Mayer R. Gastric carcinoma. N Engl J Med. 1995;333:32–39. [DOI] [PubMed] [Google Scholar]
- 2.Hodgson DC, Zhang W, Fuchs CS, et al. The effect of hospital volume and socioeconomic status on colostomy rates for rectal cancer. Proc Am Soc Clin Oncol, vol. 20. San Francisco, 2001:238a.
- 3.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–1751. [DOI] [PubMed] [Google Scholar]
- 4.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 5.Kelly JV, Hellinger FJ. Physician and hospital factors associated with mortality of surgical patients. Med Care. 1986;24:785–800. [DOI] [PubMed] [Google Scholar]
- 6.Khuri SF, Daley J, Henderson W, et al. Relation of surgical volume to outcome in eight common operations: results from the VA National Surgical Quality Improvement Program. Ann Surg. 1999;230:414–429; discussion 429–432. [DOI] [PMC free article] [PubMed]
- 7.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364–1369. [DOI] [PubMed] [Google Scholar]
- 8.Dardik A, Burleyson GP, Bowman H, et al. Surgical repair of ruptured abdominal aortic aneurysms in the state of Maryland: factors influencing outcome among 527 recent cases. J Vasc Surg. 1998;28:413–420; discussion 420–421. [DOI] [PubMed]
- 9.Hannan EL, O'Donnell JF, Kilburn H Jr, et al. Investigation of the relationship between volume and mortality for surgical procedures performed in New York State hospitals. JAMA. 1989;262:503–510. [PubMed] [Google Scholar]
- 10.Bach PB, Cramer LD, Schrag D, et al. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345:181–188. [DOI] [PubMed] [Google Scholar]
- 11.Birkmeyer JD, Warshaw AL, Finlayson SR, et al. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery. 1999;126:178–183. [PubMed] [Google Scholar]
- 12.Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care. J Clin Oncol. 2000;18:2327–2340. [DOI] [PubMed] [Google Scholar]
- 13.Roohan PJ, Bickell NA, Baptiste MS, et al. Hospital volume differences and five-year survival from breast cancer. Am J Public Health. 1998;88:454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrag D, Cramer LD, Bach PB, et al. Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA. 2000;284:3028–3035. [DOI] [PubMed] [Google Scholar]
- 15.Sosa JA, Bowman HM, Gordon TA, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg. 1998;228:429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao SL, Lu-Yao G. Population-based study of relationships between hospital volume of prostatectomies, patient outcomes, and length of hospital stay. J Natl Cancer Inst. 1999;91:1950–1956. [DOI] [PubMed] [Google Scholar]
- 17.Hannan EL, Racz M, Ryan TJ, et al. Coronary angioplasty volume-outcome relationships for hospitals and cardiologists. JAMA. 1997;277:892–898. [PubMed] [Google Scholar]
- 18.Edwards EB, Roberts JP, McBride MA, et al. The effect of the volume of procedures at transplantation centers on mortality after liver transplantation. N Engl J Med. 1999;341:2049–2053. [DOI] [PubMed] [Google Scholar]
- 19.McGrath PD, Wennberg DE, Dickens JD Jr, et al. Relation between operator and hospital volume and outcomes following percutaneous coronary interventions in the era of the coronary stent. JAMA. 2000;284:3139–3144. [DOI] [PubMed] [Google Scholar]
- 20.Canto JG, Every NR, Magid DJ, et al. The volume of primary angioplasty procedures and survival after acute myocardial infarction: National Registry of Myocardial Infarction 2 Investigators. N Engl J Med. 2000;342:1573–1580. [DOI] [PubMed] [Google Scholar]
- 21.Kee F, Wilson RH, Harper C, et al. Influence of hospital and clinician workload on survival from colorectal cancer: cohort study. BMJ. 1999;318:1381–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon TA, Burleyson GP, Tielsch JM, et al. The effects of regionalization on cost and outcome for one general high- risk surgical procedure. Ann Surg. 1995;221:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harmon JW, Tang DG, Gordon TA, et al. Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Ann Surg. 1999;230:404–411; discussion 411–413. [DOI] [PMC free article] [PubMed]
- 24.Slisow W, Marx G, Seifart W, et al. Arguments for regional centralized treatment of stomach cancer: a statistical study of national results. Zentralbl Chir. 1987;112:27–33. [PubMed] [Google Scholar]
- 25.Haugstvedt TK, Viste A, Eide GE, et al. Norwegian multicentre study of survival and prognostic factors in patients undergoing curative resection for gastric carcinoma: the Norwegian Stomach Cancer Trial. Br J Surg. 1993;80:475–478. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka H, Hiyama T, Hanai A, et al. Interhospital differences in cancer survival: magnitude and trend in 1975–1987 in Osaka, Japan. Jpn J Cancer Res. 1994;85:680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansson LE, Sparen P, Nyren O. Survival in stomach cancer is improving: results of a nationwide population-based Swedish study. Ann Surg. 1999;230:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damhuis RA, Meurs CJ, Dijkhuis CM, et al. Hospital volume and post-operative mortality after resection for gastric cancer. Eur J Surg Oncol. 2002;28:401–405. [DOI] [PubMed] [Google Scholar]
- 29.Nomura E, Tsukuma H, Ajiki W, et al. Population-based study of relationship between hospital surgical volume and 5-year survival of stomach cancer patients in Osaka, Japan. Cancer Sci. 2003;94:998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg. 2003;138:721–725; discussion 726. [DOI] [PubMed]
- 31.Wainess RM, Dimick JB, Upchurch GR Jr, et al. Epidemiology of surgically treated gastric cancer in the United States, 1988–2000. J Gastrointest Surg. 2003;7:879–883. [DOI] [PubMed] [Google Scholar]
- 32.Macdonald J, Smalley S, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 34.Cox D. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 35.Lin D, Wei L. The robust inference for the proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 36.Maruyama K, Sassako M, Kinoshita T, et al. Surgical treatment for gastric cancer: the Japanese approach. Sem Oncol. 1996;23:360–368. [PubMed] [Google Scholar]
- 37.Hundahl SA, Macdonald JS, Benedetti J, et al. Surgical treatment variation in a prospective, randomized trial of chemoradiotherapy in gastric cancer: the effect of undertreatment. Ann Surg Oncol. 2002;9:278–286. [DOI] [PubMed] [Google Scholar]
- 38.Dent D, Madden M, Price S. Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg. 1988;75:110–112. [DOI] [PubMed] [Google Scholar]
- 39.Robertson C, Chung S, Woods S, et al. A prospective randomized trial comparing R1 subtotal gastrectomy with R3 total gastrectomy for antral cancer. Ann Surg. 1994;220:176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069–2077. Epub 2004 Apr 13. [DOI] [PubMed]
- 42.Peeters KC, Hundahl SA, Kranenbarg EK, et al. Low Maruyama index surgery for gastric cancer: blinded reanalysis of the Dutch D1-D2 trial. World J Surg. 2005;29:1576–1584. [DOI] [PubMed] [Google Scholar]




