Abstract
Objective:
To develop a novel laparoscopic system of moveable instruments that are positioned intra-abdominally and “locked” into place by external permanent magnets placed on the abdomen.
Summary Background Data:
In conventional laparoscopy, multiple trocars are required because of the limited degrees of freedom of conventional instrumentation, and the limited working envelope (an inverted cone) created by the fulcrum motion around each port. While robotic systems can improve the number of degrees of freedom, they are restricted by even smaller working envelopes.
Methods:
A collaborative research group from the Department of Urology and the Automation & Robotics Research Institute of the University of Texas, Arlington built a prototype system of magnetically anchored instruments for trocar-less laparoscopy. The only design mandate was that the developed technology be able to pass into the abdomen through one existing 12-mm diameter trocar.
Results:
A transabdominal “magnetic anchoring and guidance system” (MAGS) platform was developed to incorporate instruments, retractors, and a controllable intra-abdominal camera. In vitro, the platform was able to anchor 375 and 147 g across porcine tissue 1.8 and 2.5 cm thick, respectively. The permanent magnet platforms were sufficiently strong to retract the porcine liver and securely anchor the camera. Its versatility was demonstrated by moving the camera to virtually any location in the peritoneum with no working envelope restrictions and the subsequent completion of porcine laparoscopic procedures with 2 trocars only.
Conclusions:
Trocar-less laparoscopy using magnetically anchored instruments is feasible and may expand intracorporeal instrument manipulation substantially beyond current-day capability. The ability to reduce the number of trocars necessary for laparoscopic surgery has the potential to revolutionize surgical practice.
A novel system of instruments and moveable camera was developed and introduced through a 12-mm trocar. These could be positioned virtually anywhere in the peritoneum without working envelope restrictions and locked into place by external permanent magnets. Laparoscopic surgery was successful in a porcine transperitoneal model with 2 trocars only.
Laparoscopic surgery is an increasingly common method to treat surgical disease. However, hand-eye dissociation, a two-dimensional field-of-view, and fixed instrumentation with limited degrees of freedom contribute to a steep learning curve.1–3 Another significant limitation of laparoscopy is the fixed working envelope surrounding each port that is created by the fulcrum motion around the insertion point of each trocar. This often necessitates placement of multiple ports to accommodate changes in instrument position for improved visibility and efficiency. Additional ports contribute to postoperative pain, diminish cosmesis, and carry a risk of bleeding, hernia formation, or organ damage.4,5
Current robotic systems can overcome some of these hurdles, but they still require multiple trocars and are restricted by even smaller working envelopes.6 To provide greater flexibility of endoscopic viewing, instrument usage and to further reduce morbidity, we have developed in prototype a novel adjunct laparoscopic system concept, a moveable, “lockable” platform that is positioned intra-abdominally and stabilized by an external permanent magnet placed on the abdominal skin. Our hypothesis is that a transabdominal “magnetic anchoring and guidance system” (MAGS) can be used to actively control an intra-abdominal endoscope and multiple working instruments introduced through a single trocar into the abdominal cavity (Fig. 1). This platform would allow unrestricted intra-abdominal movement of surgical instruments and has the potential to realize the benefits of single-keyhole surgery while meeting or exceeding the performance of current-day fixed-trocar laparoscopy. We present the initial development of a MAGS camera and tissue retractor for laparoscopic surgery.
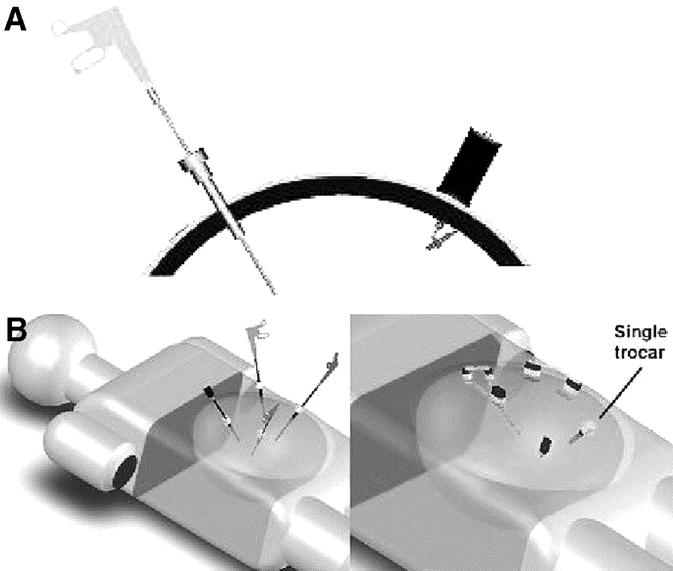
FIGURE 1. A, Schematic representation of conventional transabdominal trocar and instrument (left) and proposed magnetically anchored and guided instrument/camera (right). B, Schematic representation of typical multitrocar laparoscopic surgery (left) and proposed single trocar surgery through which multiple MAGS instruments are introduced and deployed.
MATERIALS AND METHODS
The Departments of Urology and Surgery at the University of Texas, Southwestern Medical Center, and the Automation & Robotics Research Institute of the University of Texas at Arlington formed a collaboration to build a prototype system of magnetically anchored instruments for trocar-less laparoscopy. The MAGS concept was advanced to a working prototype with a system of external magnetic anchors, 2 types of passive tissue retractors, and an internal camera system. The only design mandate was that the developed technology be able to deploy into the insufflated abdomen through one existing 12-mm-diameter trocar and then moved into position within the peritoneum by manipulating external magnets.
The MAGS platform (external magnet and internal magnet platform) coupling strength was determined across various distances of air gaps, across ex vivo porcine abdominal walls and commercially purchased bovine muscle of various thicknesses. The coupling strength was tested in air by attaching the external magnetic platform to a load cell and increasing the distance from the internal platform, which was locked to the base. The coupling strength across bovine muscle was accomplished with the sample frozen and with both the external and internal magnetic platforms in direct contact with the tissue. The load was increased on the internal platform until decoupling occurred. The prototype camera enclosure and passive tissue retractors were then designed, manufactured, and evaluated in vivo, in a porcine laparoscopic nephrectomy model. Unlike conventional laparoscopes, which provide their own illumination, the prototype camera was not designed with its own light source. Therefore, a 12-mm trocar was modified by wrapping it in fiberoptic cables (resulting in an outer diameter of 15 mm) and coupled to a conventional light source (Fig. 2). Using 100-lb domestic pigs, the MAGS camera and tissue retractors were inserted through a 12-mm trocar port that was removed once the tools were securely held and located off site. The illuminating trocar was then inserted into the same incision to introduce the tools. The tools were evaluated by attempting laparoscopic nephrectomies using conventional laparoscopic scissors and graspers.
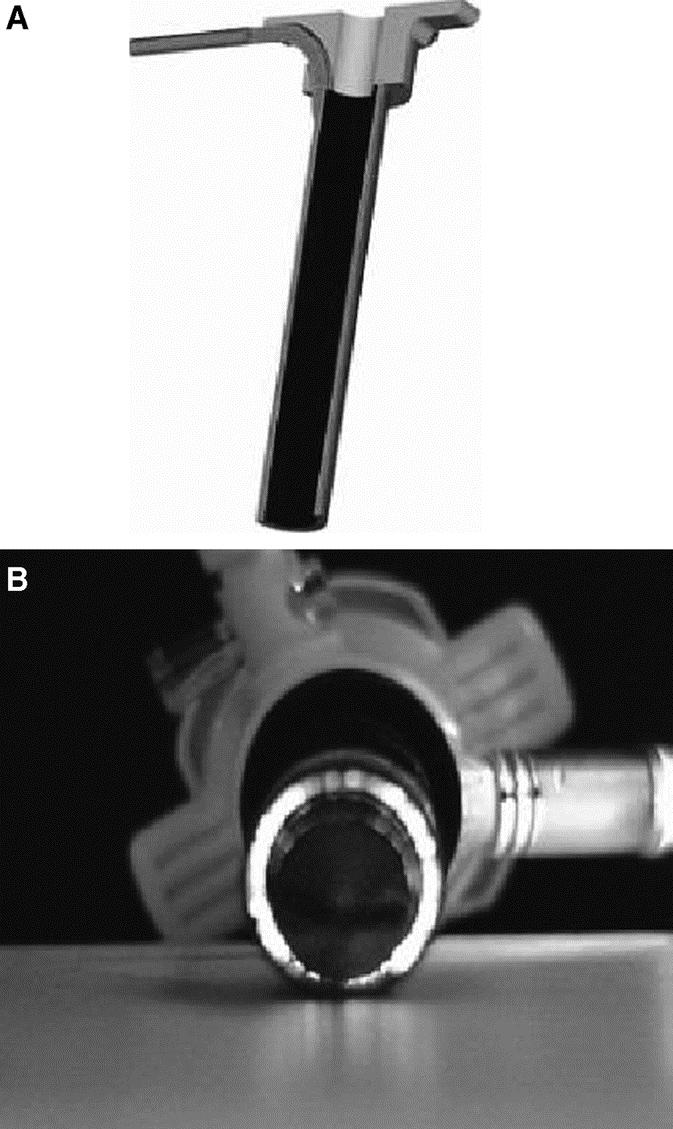
FIGURE 2. A, Schematic representation of “light” trocar. Inner diameter 12-mm conventional trocar modified by wrapping with fiberoptic cords attached to conventional light source. B, Prototype “light” trocar demonstrating illumination capability (intentionally dimmed).
RESULTS
The MAGS working prototype consists of a stack of neodymium-iron-boron (NdFeB) permanent magnets in an external enclosure approximately 3.2 cm in diameter by 5.7 cm tall placed on the outer abdominal wall (Fig. 3). Intra-abdominal instruments have corresponding NdFeB permanent magnets 0.95 cm in diameter attached to the base, with force-distance properties depicted in Figure 4. Two types of passive tissue retractors were designed for successful introduction through the 12-mm inner diameter of a standard trocar. To elevate and move organs such as the liver or spleen, a sling retractor consisting of latex tubing of various lengths was attached to 2 internal MAGS platforms. By manipulating the pair of external anchors, the surgeon was capable of positioning the sling under an organ to elevate it (Fig. 5). To either elevate or retract laterally, a prototype paddle-type retractor attached to a pair of internal MAGS platforms that contain 2 coupling magnets was designed. By manipulating the pair of external magnets, the surgeon was able to position the retractor and then by either pushing them apart or closer was able to actively depress and/or elevate the internal retractor. The three-fingered “paddle” was spring-loaded to deploy upon entry (Fig. 6). The sling retractor weighed 25 g and the paddle-type retractor weighed 45 g.

FIGURE 3. Porcine surgery with 2 conventional laparoscopic trocars and 2 MAGS external components coupling to retractor.
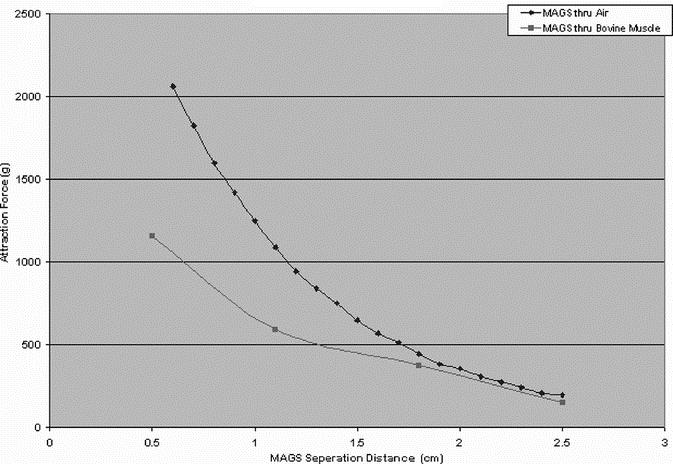
FIGURE 4. Attractive force of MAGS across varying distances between internal and external components.
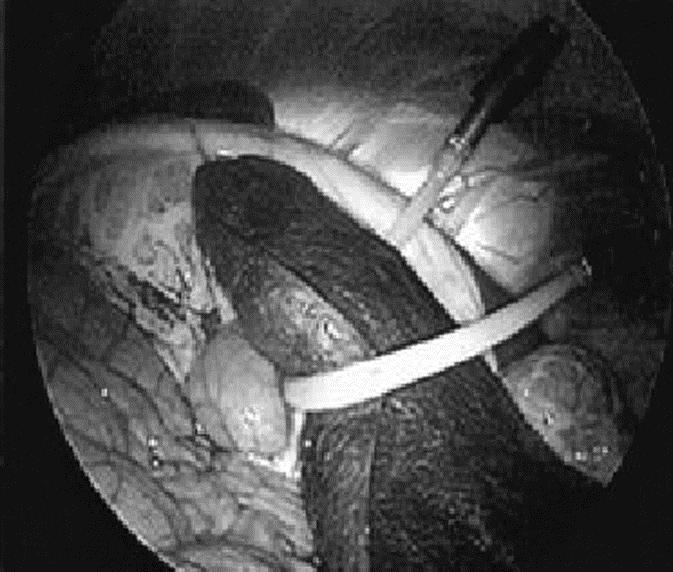
FIGURE 5. Internal view of prototype sling-type retractor elevating porcine spleen.
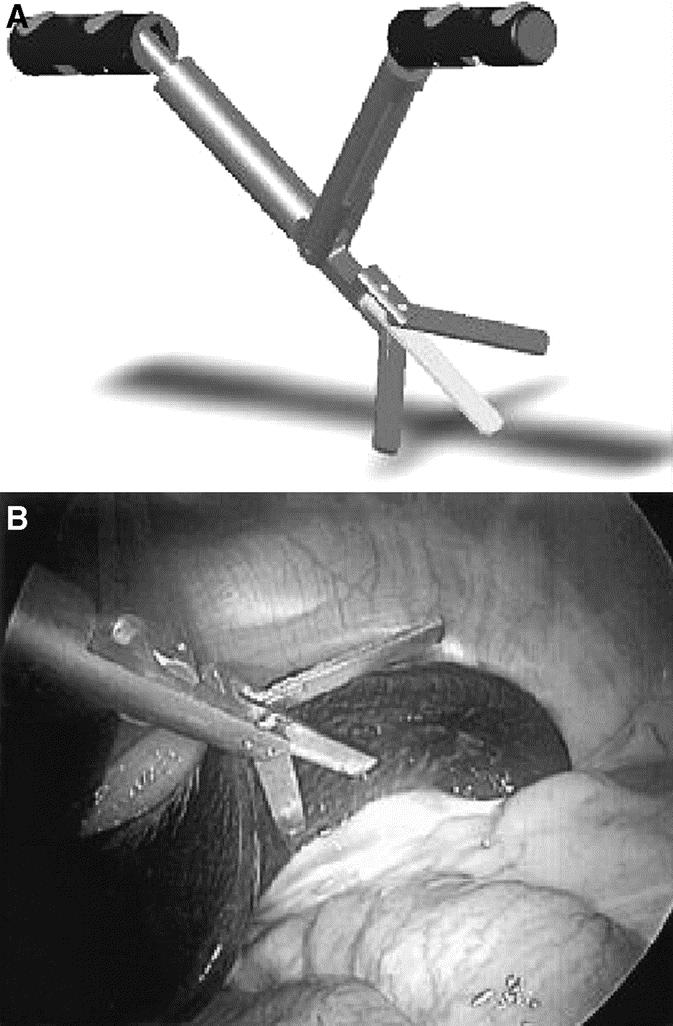
FIGURE 6. A, Schematic representation of prototype paddle-type retractor fully deployed. B, Internal view of prototype elevating porcine spleen.
A commercially available miniature camera (QN42H, Elmo, Plainview, NY) was packaged within a customized aluminum enclosure attached to a pair of internal MAGS platforms. The camera was inserted through a conventional 12-mm trocar and coupled to the external magnets. The introductory trocar was then removed and replaced with the customized “light” trocar allowing the camera cable (3 mm in diameter) to exit the abdomen alongside it. Similar to the paddle-type retractor, the assistant surgeon manipulated the pair of external magnets to orient the camera horizontally (yaw) and then by either pushing the external magnets apart or together was able to actively point the camera lens upward or downward (pitch) (Fig. 7). The 2 anchoring magnets along the longitudinal axis essentially fixed the 30° camera to the anterior peritoneum preventing spontaneous yaw or roll (horizontal or rotational movement). A digital editing system (Prizm Video Workstation 386, Pinnacle, Mountain View, CA) was used to perform real-time digital zoom and further adjust rotation (horizon/roll correction). The camera, its enclosure, and internal MAGS weighed 35 g. Because of its intended nonmedical design, the camera resolution was expectedly inferior to conventional laparoscopes (Fig. 8).
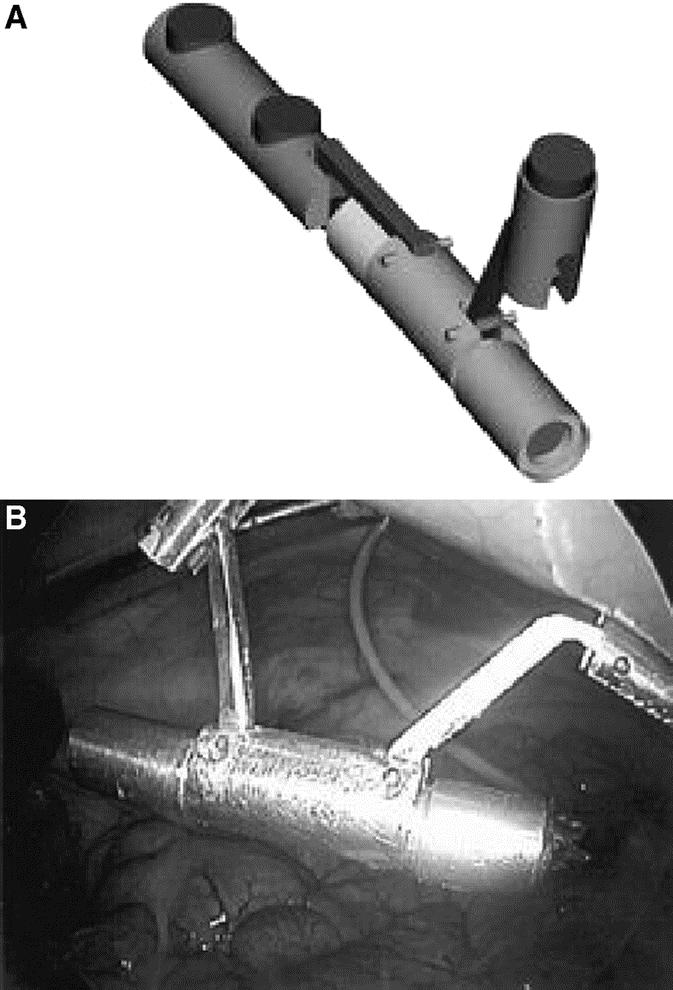
FIGURE 7. A, Schematic representation of prototype internal camera fully deployed. B, Internal view of camera fully deployed.

FIGURE 8. Image captured from video footage taken with prototype internal camera. Image is of left kidney within retroperitoneum. Glare from “light” trocar.
After development in the laboratory, these instruments were tested in vivo, in a porcine laparoscopic surgery model (Fig. 3). The average porcine abdominal wall thickness was 1.5 cm, and the MAGS platform was sufficient to securely anchor the tissue retractors and camera. These instruments could also be moved within the abdomen without loss of coupling. In particular, the camera could be manipulated to any desired location inside the peritoneum, although it did require a few minutes for the assistant surgeon to get familiarized with the MAGS. In laboratory tests and in the porcine model, removal of the prototype camera and retractors through conventional 12-mm ports was not problematic. Newcomers to the MAGS platform did require some practice in un-deploying and alignment with the trocar at removal, but otherwise no special maneuvers were required.
Two nonsurvival porcine laparoscopic nephrectomies were successfully completed without complications using the prototype MAGS camera, “light” trocar, MAGS paddle-type tissue retractor, and a 5-mm conventional trocar. As such, only 2 trocars were necessary, with the 15-mm “light” trocar site used for specimen extraction. The renal artery and vein were transected with a conventional laparoscopic stapler introduced through the “light” trocar. Procedure time was not recorded and blood loss was minimal.
DISCUSSION
The introduction of laparoscopic procedures in the mid 1980s ushered in an era that has arguably had the greatest impact on the surgical treatment of human disease in the past 30 years. Conversion from an open to a closed operation has substantially decreased pain, morbidity, hospitalization, and cost for many of the most commonly performed procedures. While there have been major strides in developing minimally invasive versions of traditionally open operations (eg, fundoplication, prostatectomy, etc.), conventional laparoscopy has required a 1:1 ratio between instruments and trocars. As such, this research aimed to further decrease morbidity by introducing multiple functional tools through a single port, and anchoring them magnetically.
Our objective has been to further minimize the invasiveness of laparoscopic surgery by utilizing controlled magnetic coupling to manipulate intracorporeal surgical instruments, which are delivered via a single keyhole incision in the patient. In conventional pure laparoscopy, the surgeon places multiple ports to retract tissue or to accommodate the required changes in position and approach angles of the instruments and laparoscope to maximize visibility and efficiency. However, each working port contributes to postoperative pain, carries morbidity risks (bleeding, hernia and/or internal organ damage), and incrementally diminishes cosmesis. Cosmesis is particularly important in procedures on pediatric patients and is also demanded by sophisticated adult patients.7,8
The introduction of laparoscopic surgical robots for computer-assisted surgery over the past 5 years has been a tremendous advance, facilitating complex laparoscopic procedures.9,10 In particular, they have enabled surgeons to overcome the challenges of eye-hand dissociation, have replaced two-dimensional with three-dimensional fields-of-view, and have improved dexterity by increasing the degrees of freedom of working instruments. Despite these advances, each computer-assisted laparoscopic instrument still requires a conventional transabdominal port and, as such, is still limited by the trocar-working envelope. In addition to their often prohibitively high expense, the issues of trocar site-associated pain, morbidity, and cosmesis are not addressed by these computer-assisted surgery systems, leading some investigators to question its overall value.11,12
We think that the development of magnetically controlled and anchored, intracorporeal surgical instruments and camera introduced through a single trocar can further advance surgical practice and patient care by realizing the benefits of single-keyhole surgery while meeting or exceeding the performance of current-day fixed-trocar laparoscopy. We have successfully designed, manufactured, and implemented this technology to complete 2 porcine laparoscopic nephrectomies utilizing only 2 trocars (for left and right hand instruments). The prototypes developed and 2 cases completed serve as “proof of concept” for this technology.
This technology will be a significant advance only if the following objectives/advantages are demonstrated. First, instruments must retain the advantages of conventional instrumentation and robotic surgery systems while being easily repositioned as necessary whenever the working envelope is disadvantageous by moving the external anchoring devices. Second, surgical pain should be reduced by the elimination of possibly all but one trocar. Positioning a single trocar at the umbilicus where it does not penetrate the abdominal wall muscles could result in potentially more outpatient-based operations and lower hospitalization costs. Lastly, visibly unappealing surgical scars should be eliminated or minimized further by utilizing a single umbilical trocar.13 Clearly, our experience to date cannot address the postoperative pain and perceived cosmetic advantages as we only performed pilot porcine experiments. However, the ability to position and use the prototype camera and retractor during surgery demonstrates the potential for this technology to use the entire insufflated abdomen as the working envelope similar to open surgery performed through a large incision. Further work to engineer a miniaturized robotic arm introduced through a 12-mm trocar, coupled to an MAGS platform, and manipulated with computer-assistance would allow replacement of a third trocar realizing the potential for true single trocar/keyhole surgery.
Before widespread adoption of this novel platform, clinical and engineering limitations must be addressed. Surgeons must become familiar with MAGS components in the dry laboratory and in animal models. As for all new technologies, there will be an associated learning curve, and MAGS surgeons will also have to develop new “MAGS techniques” by modifying techniques originally developed for traditional laparoscopic operations (eg, laparoscopic cholecystectomy, laparoscopic nephrectomy). The coupling strength of magnets, whether electromagnetic or permanent magnets, decreases as a decaying exponential with respect to the distance between the source magnet and its target. Because of this decrease, tissue thicknesses in excess of 1.5 cm limits the effectiveness of the paddle retractor, while the camera due to no-load conditions can be supported up to tissue thicknesses of 2.5 cm. As a result, potential clinical deployment of the prototypes developed thus far would be limited to thin or pediatric patients. Future directions include development of electromagnets to generate significantly stronger magnetic fields. Theoretically, unlimited magnetic attractive force could be generated; eg, electromagnets found in modern MRI machines generate fields 30,000 times that of earth, and in principle, their electromagnets could be miniaturized to a size that could be used for MAGS. Electromagnets may avoid some procedural difficulties encountered with permanent magnets.
The maximum number of external magnets in the operating area is another unknown. Theoretically, it is limited only by the working area (abdominal wall) minus the area used by the external magnet, and its minimum distance to the next magnet. The minimum separation distance of our current external platforms is 3 cm, before between-magnet attraction becomes problematic. This 3-cm distance was not problematic because, as in traditional laparoscopic surgery, optimal instrument positioning requires triangulation, with the camera centered on the operative site. In both human and porcine laparoscopic nephrectomy, intertrocar distance in the insufflated anterior abdominal wall is at least 5 cm. Any shorter distances not only will have magnet-magnet interference but also operator hand-magnet collisions; clearly, these issues will have to be elucidated during the “learning curve” phase of MAGS development before widespread utilization.
Furthermore, we must extend and broaden the capabilities of existing prototypes to define long-term directions of development. If articulating computer-assisted instruments are to be developed, the MAGS must provide local or wireless sources of power and control to said instruments, as well as a stable platform and fulcrum for each instrument across greater tissue thicknesses. Increased coupling strength may require exploration of alternative technologies and techniques such as electromagnets and magnetic focusing to optimize magnetic field shape and penetration. Finally, other important hurdles include engineering the MAGS instruments for waterproofing during sterilization.
CONCLUSION
To further minimize the invasiveness of surgery, we have developed a novel magnetic anchoring and guidance system for prototype intra-abdominal instruments introduced into the abdomen through a single trocar. Pilot studies in a porcine model have demonstrated that laparoscopic surgery using an MAGS-linked camera and retractor is feasible. Magnetic anchoring-based surgery expands intracorporeal instrument manipulation substantially beyond current-day capability and may facilitate new surgical approaches such as natural orifice transgastric endoscopic surgery or permit the development of new minimally invasive procedures performed in a manner never accomplished before.
Footnotes
Reprints: Jeffrey A. Cadeddu, MD, Department of Urology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., MC 9110, Dallas, TX 75390-9110. E-mail: Jeffrey.cadeddu@utsouthwestern.edu.
REFERENCES
- 1.Shapiro K, Patel S, Abdo Z, et al. Laparoscopic adjustable gastric banding: is there a learning curve? Surg Endosc. 2004;18:48–50. [DOI] [PubMed] [Google Scholar]
- 2.Hwang H, Turner LJ, Blair NP. Examining the learning curve of laparoscopic fundoplications at an urban community hospital. Am J Surg. 2005;189:522–526; discussion 526. [DOI] [PubMed]
- 3.Frede T, Erdogru T, Zukosky D, et al. Comparison of training modalities for performing laparoscopic radical prostatectomy: experience with 1,000 patients. J Urol. 2005;174:673–678; discussion 678. [DOI] [PubMed]
- 4.Lowry PS, Moon TD, D'Alessandro A, et al. Symptomatic port-site hernia associated with a non-bladed trocar after laparoscopic live-donor nephrectomy. J Endourol. 2003;17:493–494. [DOI] [PubMed] [Google Scholar]
- 5.Marcovici I. Significant abdominal wall hematoma from an umbilical port insertion. Jsls. 2001;5:293–295. [PMC free article] [PubMed] [Google Scholar]
- 6.Gutt CN, Oniu T, Mehrabi A, et al. Robot-assisted abdominal surgery. Br J Surg. 2004;91:1390–1397. [DOI] [PubMed] [Google Scholar]
- 7.Dunker MS, Stiggelbout AM, van Hogezand RA, et al. Cosmesis and body image after laparoscopic-assisted and open ileocolic resection for Crohn's disease. Surg Endosc. 1998;12:1334–1340. [DOI] [PubMed] [Google Scholar]
- 8.Teoh TA, Reissman P, Weiss EG, et al. Enhancing cosmesis in laparoscopic colon and rectal surgery. Dis Colon Rectum. 1995;38:213–214. [DOI] [PubMed] [Google Scholar]
- 9.Mohr CJ, Nadzam GS, Curet MJ. Totally robotic Roux-en-Y gastric bypass. Arch Surg. 2005;140:779–786. [DOI] [PubMed] [Google Scholar]
- 10.Killewich LA, Cindrick-Pounds LL, Gomez G. Robot-assisted laparoscopic aortic reconstruction for occlusive disease: a case report. Vasc Endovascular Surg. 2004;38:83–87. [DOI] [PubMed] [Google Scholar]
- 11.Braumann C, Jacobi CA, Menenakos C, et al. Computer-assisted laparoscopic colon resection with the Da Vinci system: our first experiences. Dis Colon Rectum. 2005;48:1820–1827. [DOI] [PubMed] [Google Scholar]
- 12.Bodner J, Lucciarini P, Fish J, et al. Laparoscopic splenectomy with the da Vinci robot. J Laparoendosc Adv Surg Tech A. 2005;15:1–5. [DOI] [PubMed] [Google Scholar]
- 13.Toth A, Graf M. The center of the umbilicus as the Veress needle's entry site for laparoscopy. J Reprod Med. 1984;29:126–128. [PubMed] [Google Scholar]


