Abstract
Objective:
To compare sentinel lymph node (SLN)-positive breast cancer patients who had completion axillary dissection (ALND) with those who did not, with particular attention to clinicopathologic features, nomogram scores, rates of axillary local recurrence (LR), and changes in treatment pattern over time.
Background:
While conventional treatment of SLN-positive patients is to perform ALND, there may be a low-risk subgroup of SLN-positive patients in whom ALND is not required. A multivariate nomogram that predicts the likelihood of residual axillary disease may assist in identifying this group.
Methods:
Among 1960 consecutive SLN-positive patients (1997–2004), 1673 (85%) had ALND (“SLN+/ALND”) and 287 (15%) did not (“SLN+/no ALND”). We compare in detail the clinicopathologic features, nomogram scores, and rates of axillary LR between groups.
Results:
Compared with the SLN+/ALND group, patients with SLN+/no ALND were older, had more favorable tumors, were more likely to have breast conservation, had a lower median predicted risk of residual axillary node metastases (9% vs. 37%, P < 0.001), and had a marginally higher rate of axillary LR (2% vs. 0.4%, P = 0.004) at 23 to 30 months’ follow-up; half of all axillary LR in SLN+/no ALND patients were coincident with other local or distant sites. For patients in whom intraoperative frozen section was either negative or not done, the rate of completion ALND declined from 79% in 1997 to 62% in 2003 to 2004 but varied widely by surgeon, ranging from 37% to 100%. For 10 of 10 evaluable surgeons, the median nomogram scores in the SLN+/no ALND group were ≤10.5.
Conclusions:
SLN+/no ALND breast cancer patients, a selected group with relatively favorable disease characteristics, had a 9% predicted likelihood of residual axillary disease by nomogram but an observed axillary LR of 2%. A gradual and significant decline over time in the rate of completion ALND is associated with, but not entirely explained by, the institution of a predictive nomogram. It is reasonable to omit ALND for a low-risk subset of SLN-positive patients.
While standard care for sentinel lymph node positive breast cancer patients is to perform axillary dissection, for a low-risk subgroup this may not be required. Among 287 selected SLN-positive patients who did not have axillary dissection, we predicted a 9% rate of residual axillary metastases but observed axillary local recurrence in 2% at 23 months follow-up.
At many centers in the United States and worldwide, sentinel lymph node (SLN) biopsy has largely replaced axillary lymph node dissection (ALND) for breast cancer staging. Compared with ALND, the staging accuracy of SLN biopsy is greater,1 and the morbidity of SLN biopsy (while not zero) is less.2–4 The SLN is falsely negative in about 5% of node-positive patients,5 but the observed rate of axillary local recurrence (LR) after a negative SLN biopsy is vanishingly small (0.3% in 10 clinical series and 0.12% in our own experience6), and SLN-negative patients do not require ALND. In contrast, among SLN-positive patients about half have metastases in the remaining non-SLN,5 and completion ALND is still considered to be standard care in this setting.7,8
A growing literature has asked whether completion ALND can be avoided in a low-risk subset of SLN-positive patients,9 and in general demonstrates that those factors which predict metastases to the non-SLN are the same as those that predict metastases to the SLN: tumor size, tumor grade, and lymphovascular invasion (LVI). Additional predictors include volume of SLN metastasis, number of positive SLN, and number of negative SLN. While most such studies use relatively few predictive factors, typically two or three, the risk of non-SLN involvement is best predicted on the basis of multiple variables and a carefully validated multivariate nomogram developed at Memorial Sloan-Kettering Cancer Center (MSKCC) by Van Zee et al9 (“MSKCC nomogram”) has proven particularly useful for this purpose. Here we examine in detail 1673 SLN-positive patients who had ALND (“SLN+/ALND”) and 287 who did not (“SLN+/no ALND”), comparing clinicopathologic features, nomogram scores, and rates of axillary LR. Over time a growing proportion of SLN-positive patients in our practice have chosen not to have ALND, and we hypothesize that this trend may in part reflect increasing clinical application of the MSKCC nomogram. Accordingly, we also compare, across our entire service and by individual surgeon, the rates of completion ALND for the “prenomogram” and “postnomogram” time periods.
METHODS
At MSKCC, we performed 7692 consecutive SLN biopsies for breast cancer between September 1996 and December 2004, and all were entered prospectively in the Breast Service SLN database; this study is a retrospective analysis conducted under a Waiver of Authorization from our Institutional Review Board.
We excluded 1876 patients, including those with a planned “backup” ALND, failed mapping, nonmalignant lesions, nonmammary cancers, pure ductal carcinoma in situ, inflammatory cancer, bilateral cancers, prophylactic mastectomy, nonaxillary SLN, and male breast cancer, leaving 5816 patients for analysis. Of these, 1960 were SLN-positive; 1673 (85%) had ALND (“SLN+/ALND”), and 287 (15%) did not (“SLN+/no ALND”).
SLN biopsy was performed as described in detail previously,10,11 using a combination of isotope and blue dye. In general, an immediate ALND was performed for all patients whose SLN were positive on intraoperative frozen section (FS). Standard pathologic examination for SLN proved negative on FS and included an FS control (recorded as “routine hematoxylin and eosin”), and 2 adjacent sections: one stained with hematoxylin and eosin and the other with anticytokeratin (AE1:AE3) immunohistochemistry (IHC), taken from the paraffin block at each of 2 levels 50 μm apart (recorded as “serial sections/IHC”). A median of 5 slides were examined per SLN. Non-SLNs were examined with routine single-section hematoxylin and eosin. ALND was defined on the basis of surgeon intent and not the number of nodes removed.
Clinicopathologic features were compared between groups, and nomogram scores (ie, the predicted percent likelihood of non-SLN involvement in SLN-positive patients) were calculated for all SLN-positive patients whose pertinent data were complete (1627 of 1960, 83%), using the MSKCC nomogram (www.mskcc.org/nomograms).9 Rate of ALND was calculated based on patients in whom intraoperative FS was either negative or not done. SLN-positive patients were categorized as refusing ALND when this information was clearly documented in their records, and otherwise categorized as being in agreement with their surgeon.
Median follow-up, based on patients seen ≥6 months postoperatively, was 23 months (range, 6–87) in the SLN+/no ALND group and 30 months (range, 6–93) in the SLN+/ALND group. Statistical comparisons included, as appropriate, the χ2, Fisher exact test, and Wilcoxon rank-sum tests, using SPSS 12.0 for Windows and StatXact 5. The significance of time trends in rate of ALND and in nomogram scores was evaluated with the Cochran-Armitage trend and the Jonckheere-Terpstra tests, respectively.
RESULTS
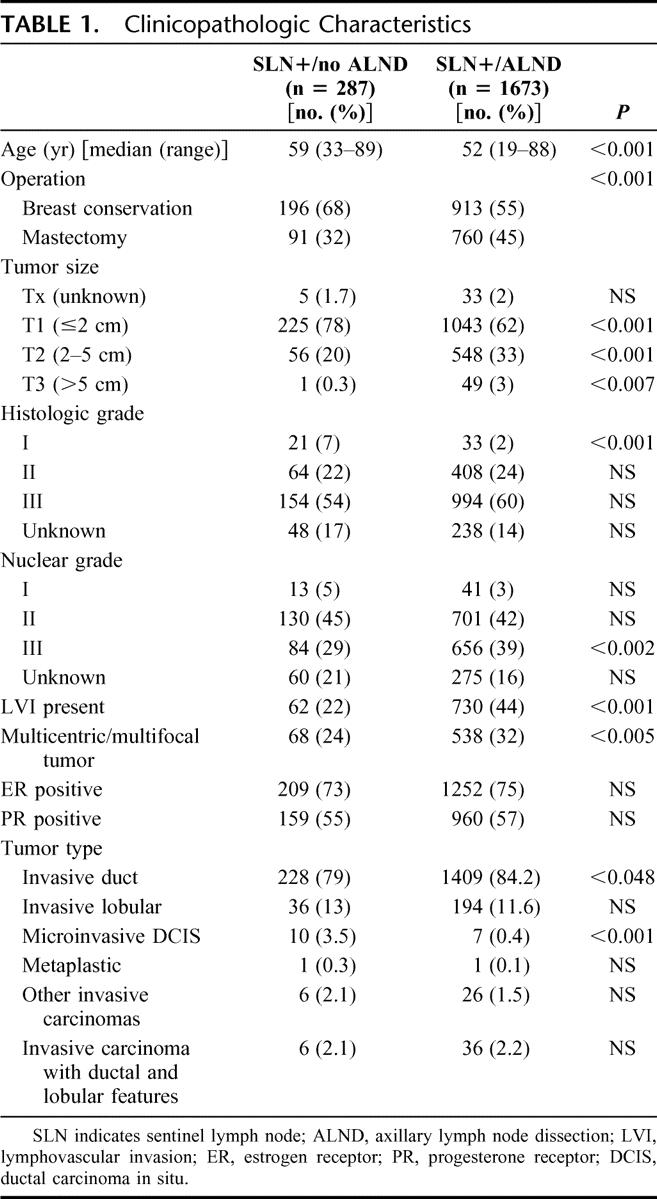
Patients in the SLN+/no ALND group were significantly older, were more likely to have breast conservation, and had more favorable tumors. Tumor size was smaller, low grade lesions were more frequent, and both LVI and multicentricity were less frequent (Table 1). The proportion of invasive lobular cancers was similar in each group.
TABLE 1. Clinicopathologic Characteristics
The number of SLN removed was similar between groups (Table 2) and the extent of nodal involvement was greater in the SLN+/ALND group (Table 2). Although 97% of the SLN+/ALND patients’ positive SLN were identified within the first 3 SLN sampled, 15% had involvement of 4 to 9 nodes, and 7% had involvement of ≥10 nodes overall.
TABLE 2. Number of Nodes Excised and Number of Positive Nodes
Median nomogram scores were significantly different between groups (9% vs. 37%, P < 0.001), indicating a high degree of selectivity in the use of ALND for SLN-positive patients. This selectivity is also reflected in the distribution of nomogram scores (Table 3); 86% of SLN+/no ALND patients had nomogram scores of ≤20%, while 74% of SLN+/ALND patients had nomogram scores of >20% (Fig. 1, P < 0.001).
TABLE 3. Distribution of Median Nomogram Scores
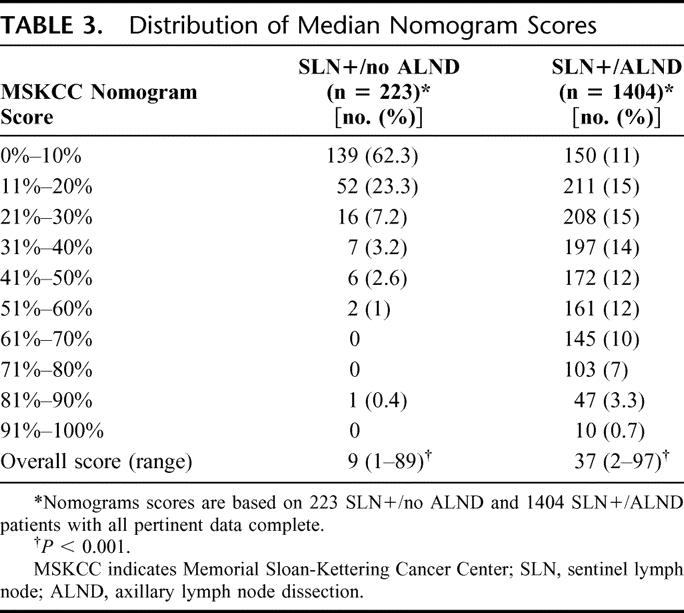
FIGURE 1. Comparison of nomogram score distributions for the SLN+/no ALND and SLN+/ALND groups. *P < 0.001.
A substantial majority of SLN+ patients had a completion ALND (1673 of 1960, 85%), but for patients in whom intraoperative FS was either negative or not done, the rate of completion ALND decreased significantly over time (Fig. 2), and there was no significant trend in median nomogram scores for the same patients (Fig. 3).
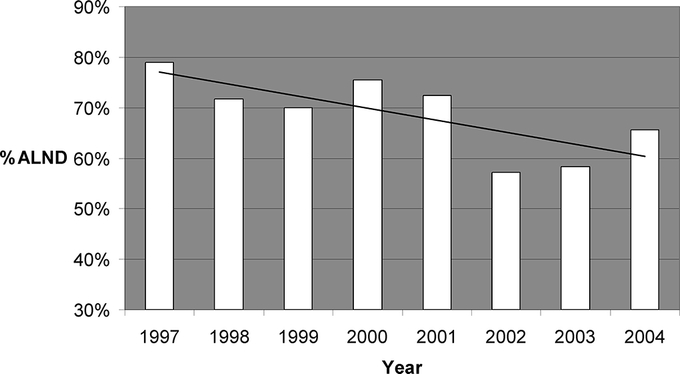
FIGURE 2. Rate of completion ALND for SLN+ patients in whom intraoperative FS was either negative or not done, showing a significant trend over time toward fewer ALND. *P < 0.001.
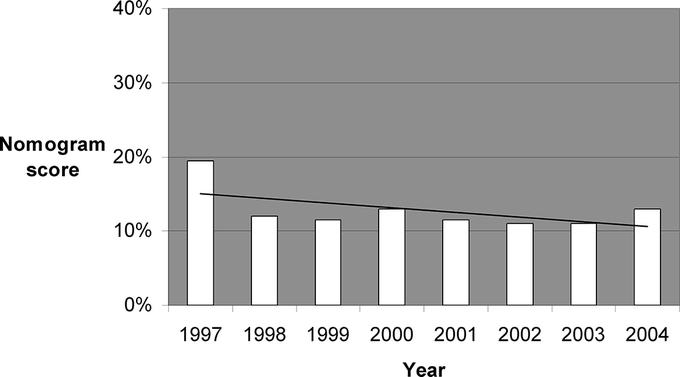
FIGURE 3. Median nomogram scores over time for SLN+ patients in whom intraoperative FS was either negative or not done, showing no significant trend in nomogram scores over time. *P = not significant.
The MSKCC nomogram was fully incorporated into our clinical practice in 2003, and comparing the “prenomogram” (1997–2002) and “postnomogram” (2003–2004) time periods, the proportion of ALND declined from 69% to 62% (Table 4, P < 0.05). For the 7 surgeons (“A–G”) with a volume of more than 50 procedures, the rate of ALND by surgeon varied widely, both prenomogram (55%–86%) and postnomogram (51%–77%), but was lower for 5 of 7 surgeons in the postnomogram period, with 1% to 16% fewer ALND performed (Table 4).
TABLE 4. Rate of ALND Prenomogram and Postnomogram
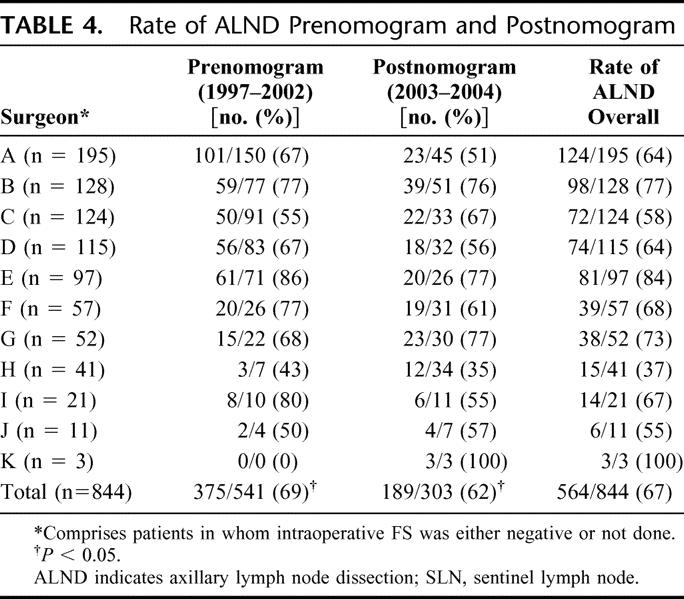
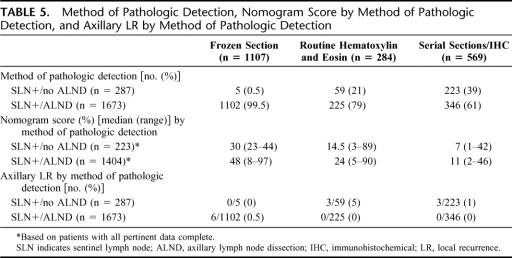
Table 5 categorizes the 2 cohorts by method of pathologic detection. For patients whose SLN were positive by FS, by routine hematoxylin and eosin, or by serial sections/IHC, ALND was performed in 99.5%, 79%, and 61% of cases, respectively (Table 5).
TABLE 5. Method of Pathologic Detection, Nomogram Score by Method of Pathologic Detection, and Axillary LR by Method of Pathologic Detection
Nomogram scores were overall higher when SLN metastases were detected by FS than by routine hematoxylin and eosin, than by serial sections/IHC (Table 5). Regardless of detection method, nomogram scores were consistently higher in the ALND than in the no ALND cohort. Axillary LR was infrequent overall, but occurred in 5% of SLN+/no ALND patients (3 of 59) who were positive on routine hematoxylin and eosin (Table 5).
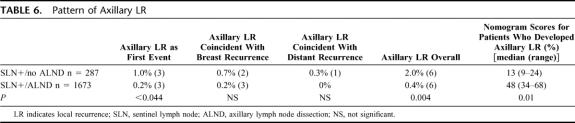
Twelve patients developed axillary LR at a median follow-up of 23 to 30 months: 6 in the SLN+/ALND group and 6 in the SLN+/no ALND group (0.4% vs. 2%, respectively, P = 0.004). Half of all axillary LR were the initial site of treatment failure, and the remainder was coincident with ipsilateral breast LR or with distant relapse (Table 6). Median nomogram scores were marginally higher among the 6 patients in each cohort who developed LR than for that cohort as a whole (48% vs. 37% in the ALND and 13% vs. 9% in the no ALND groups).
TABLE 6. Pattern of Axillary LR
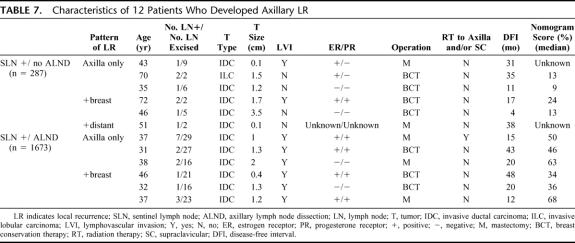
Among the 12 patients who developed axillary LR, there was no consistent pattern of clinicopathologic features, except perhaps for LVI, which was present in all of the SLN+/ALND patients who developed axillary LR (Table 7). Among the SLN+/no ALND group, 15% of patients with complete follow-up (41 of 269) received additional radiotherapy to the axilla and/or supraclavicular nodes.
TABLE 7. Characteristics of 12 Patients Who Developed Axillary LR
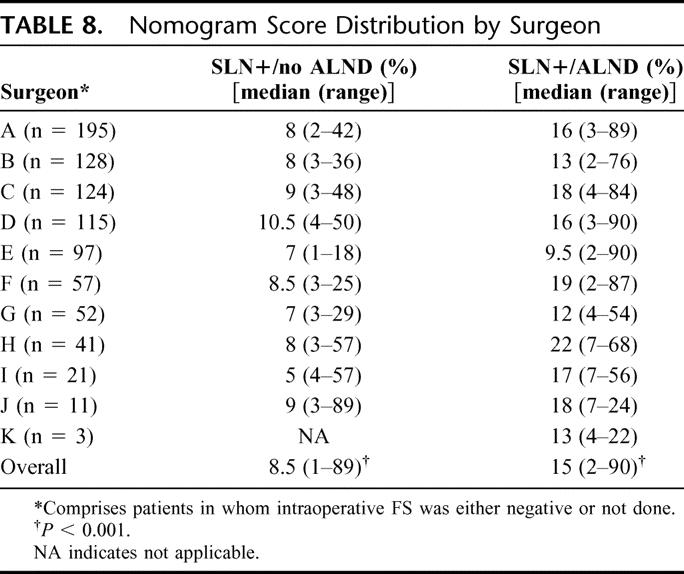
Table 8 compares, by surgeon, the median nomogram scores for SLN+/no ALND and SLN+/ALND patients. Nomogram scores were significantly lower in the SLN+/no ALND group (8.5% vs. 15%, P < 0.001) Among the SLN+/no ALND group, the predicted rate of non-SLN involvement was ≤10.5 for all 10 evaluable surgeons.
TABLE 8. Nomogram Score Distribution by Surgeon
Among the 223 SLN+/no ALND patients with complete nomogram data, we compared 33 (15%) who chose no ALND against their surgeon's recommendation with 190 (85%) who made the decision in agreement with their surgeon. The distribution of nomogram scores was different between groups (Fig. 4). For patients who disagreed with their surgeons, compared with those who agreed, median nomogram scores were higher (11% vs. 8%, P < 0.001) and the range of scores was greater (3–89 vs. 1–50).
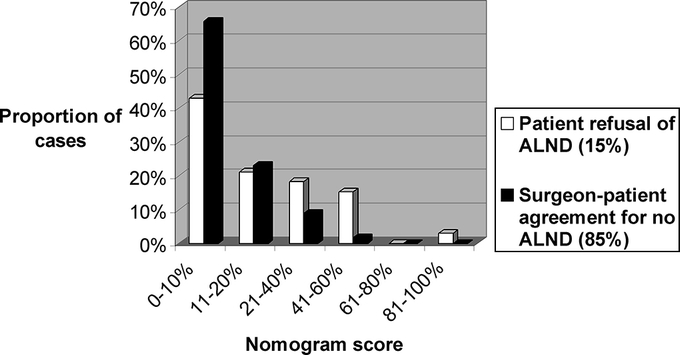
FIGURE 4. Distribution of nomogram scores for SLN+/no ALND patients who either refused ALND (15%) or made the decision in agreement with their surgeon (85%).
DISCUSSION
SLN biopsy, if negative, is adequate axillary staging for virtually all patients with noninflammatory invasive breast cancer.12 While the SLN will be falsely negative in about 5% of node-positive patients,5 these false negatives do not appear to result in axillary LR. In our own experience,6 axillary LR occurred in 0.04% of SLN-negative patients (1 of 2340) as a first event, 0.04% (1 of 2340) coincident with other sites of LR, and 0.04% (1 of 2340) coincident with distant relapse. It remains uncertain whether false-negative SLN procedures will adversely affect breast cancer survival. Three randomized trials2,13,14 aim to answer this question, but based on such low rates of observed axillary LR, it seems almost certain that they will not. A recent meta-analysis15 by the Early Breast Cancer Trialists Collaborative Group (EBCTCG) of 78 randomized trials from the pre-SLN era (comprising >42,000 patients) clearly demonstrates that local control and survival are related, but found no effect on 15-year survival for treatment comparisons in which LR was reduced by less than 10%.
Among SLN-positive patients, ALND remains standard care.7,8 In an overview of 69 reports of SLN biopsy validated by a backup ALND,5 residual axillary metastases were found in 53% of SLN-positive cases. Such metastases, if left behind, would seem to constitute a higher risk for axillary LR than that of SLN-negative patients, but this may not in fact be true. An important prospective trial, the American College of Surgeons Oncology Group Z0011,16 aimed to answer this question by randomizing SLN-positive patients between ALND and observation, but closed early because of slow accrual (886 of a projected 1800 patients) and a lower-than-expected rate of events. Observational studies of SLN+/no ALND patients have reported very low rates of axillary LR, with 3 reports17–19 comprising 150 patients having no axillary LR at 27 to 32 months’ follow-up. While 2 of the 3 studies do not provide a comparison group, Jeruss et al19 show that the SLN+/no ALND patients had smaller tumor and SLN metastasis size than patients who had ALND. We have previously reported axillary LR in 1.4% (3 of 210) of SLN+/no ALND patients at 25 months’ follow-up,6 and here we attempt to further characterize this group.
First, we demonstrate selectivity in the decision to omit ALND in SLN-positive patients. Compared with the SLN+/ALND group, the SLN+/no ALND patients were older, more likely to have breast conservation, and had smaller tumor size, fewer high grade lesions, and less LVI (Table 1). This selectivity is evident in a striking difference in nomogram scores overall (9% vs. 37%, P < 0.001, Table 3), and in a nearly inverse nomogram score distribution (Fig. 1). The nomogram prediction of non-SLN metastases in 37% of SLN+/ALND patients closely matches an observed rate of 38% (Table 2).
Second, we demonstrate a statistically significant decline in the rate over time of ALND in SLN-positive patients (Fig. 2) for whom intraoperative FS was either negative or not done. This decline is not due to a progressively more favorable patient population, as the nomogram scores of all SLN-positive patients do not change significantly over the same time period (Fig. 3).
Third, we demonstrate a significantly lower rate of ALND in the postnomogram compared with the prenomogram years (69% vs. 62%, P < 0.05). We also show wide variation between surgeons in the rates of ALND, both prenomogram and postnomogram, and no clear correlation between rate of ALND and surgeon volume (Table 4). For 7 of 10 evaluable surgeons, the rate of ALND was lower in the postnomogram years, by 1–25%.
Fourth, we show a relationship between method of pathologic detection and the performance of ALND. Our practice is to perform ALND on all patients whose SLN are positive on FS; ALND was done in 99.5% of FS-positive patients (Table 5). By contrast, ALND was performed in 79% of those whose SLN were positive on routine hematoxylin and eosin, and 61% of those whose SLN were positive on serial sections/IHC.
Fifth, we show a relation between method of pathologic detection and nomogram scores (Table 5). Nomogram scores were highest among FS-positive patients, lower for those detected by routine hematoxylin and eosin, and lowest for those detected by serial sections/IHC. For the latter group, the difference in nomogram scores between SLN+/ALND and SLN+/no ALND patients was small (11% vs. 7%).
Sixth, we show very low rates of axillary LR, both by method of detection (Table 5) and overall (Table 6). The highest rate of axillary LR was in the subgroup of SLN+/no ALND patients whose SLN were positive on routine hematoxylin and eosin (5%, 3 of 59). Overall, axillary LR was more frequent in the SLN+/no ALND patients (2% vs. 0.4%, P = 0.004). While statistically significant, the clinical significance of this difference is arguable, especially as half of the LR in both groups were coincident with other sites of local or distant relapse.
Seventh, we do not identify any clinicopathologic features which reliably predict axillary LR in SLN+/no ALND patients (Table 7). LVI was present in all 6 patients who developed axillary LR after ALND, but these 6 comprise only 0.4% of the entire SLN+/ALND group. Of all patients with axillary LR, only 1 of 12 received axillary or supraclavicular RT (and this patient had an ALND).
Eighth, a comparison of median nomogram scores in the SLN+/no ALND and SLN+/ALND groups by surgeon demonstrates median nomogram scores in the SLN+/no ALND patients of ≤10.5 for all 10 evaluable surgeons. We wish to emphasize that this observation does not support the concept of a nomogram “cutoff” for performing ALND, as the range of nomogram scores in both groups, and for all surgeons, was wide (Table 8). In our practice, the decision to perform ALND is individualized and not based on nomogram score alone.
Finally, we show that 15% of SLN+/no ALND patients chose this approach against their surgeon's recommendation. Their distribution of nomogram scores is different (Fig. 4) and their predicted likelihood of residual axillary disease is higher than that of the remaining 85% who chose in agreement with their surgeons, 11% vs. 8% (P < 0.001).
Some caveats apply to our findings. This is an observational, nonrandomized study. We compare 2 groups of patients who (despite standardization of their SLN biopsy procedures) are otherwise quite different. Follow-up is longer for the SLN+/ALND than for the SLN+/no ALND group (30 vs. 23 months), reflecting the trend toward fewer ALND in more recent years, and is relatively short. While we expect that the number of axillary LR will increase over time, the NSABP B-04 trial20 found that 75% of axillary LR (among patients treated by mastectomy without ALND) appeared within the first 2 years, and the EBCTCG meta-analysis15 found that about 75% of all LR events occurred within the first 5 years of follow-up. This study spans a time period during which our criteria for performing ALND were changing, and varied widely by surgeon. In recent years, the incidence of axillary LR after a negative (or a positive) SLN biopsy has proved in our experience and in that of others to be surprisingly low, and this finding may itself have influenced the declining rate of ALND. We are unable to determine the effect of radiotherapy and/or systemic adjuvant therapy, independently of surgery, on the rate of axillary LR. Finally, we have not examined the extent to which ALND was recommended by a medical oncologist, to identify a subset of SLN-positive patients for whom systemic therapy might be changed based on the finding of additional positive nodes.
CONCLUSION
While ALND is considered standard care for SLN-positive patients, the risk of residual axillary disease in this group varies widely and is accurately predicted by a multivariate nomogram. Our SLN+/no ALND patients (compared with those who had ALND) were characterized by more favorable tumor characteristics, lower nomogram scores, and a slightly higher rate of axillary LR (2% vs. 0.4%). Axillary LR was most frequent (5%) among SLN+/no ALND patients whose SLNs were positive by routine hematoxylin and eosin. A trend toward fewer ALND in SLN-positive patients is associated with, but not entirely explained by, the institution of a multivariate nomogram. It is reasonable to omit ALND for a low-risk subset of SLN-positive patients.
Footnotes
Reprints: Hiram S. Cody, III, MD, Breast Service, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10021. E-mail: codyh@mskcc.org.
REFERENCES
- 1.Giuliano AE, Dale PS, Turner RR, et al. Improved staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995;3:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–553. [DOI] [PubMed] [Google Scholar]
- 3.Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13:491–500. [DOI] [PubMed] [Google Scholar]
- 4.Temple LK, Baron R, Cody HS III, et al. Sensory morbidity after sentinel lymph node biopsy and axillary dissection: a prospective study of 233 women. Ann Surg Oncol. 2002;9:654–662. [DOI] [PubMed] [Google Scholar]
- 5.Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma. Cancer. 2006;106:4–16. [DOI] [PubMed] [Google Scholar]
- 6.Naik AM, Fey J, Gemignani M, et al. The risk of axillary relapse after sentinel lymph node biopsy for breast cancer is comparable with that of axillary lymph node dissection: a follow-up study of 4008 procedures. Ann Surg. 2004;240:462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–7720. [DOI] [PubMed] [Google Scholar]
- 8.American Society of Breast Surgeons. Consensus statement on guidelines for performing sentinel lymph node dissection in breast cancer. http://breastsurgeons.org/slnd.shtml 2004. Accessed on November 3, 2006.
- 9.Van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10:1140–1151. [DOI] [PubMed] [Google Scholar]
- 10.Cody HS, Borgen PI. State-of-the-art approaches to sentinel node biopsy for breast cancer: study design, patient selection, technique, and quality control at Memorial Sloan-Kettering Cancer Center. Surg Oncol. 1999;8:85–91. [DOI] [PubMed] [Google Scholar]
- 11.Brogi E, Torres-Matundan E, Tan LK, et al. The results of frozen section, touch preparation, and cytological smear are comparable for intraoperative examination of sentinel lymph nodes: a study in 133 breast cancer patients. Ann Surg Oncol. 2005;12:173–180. [DOI] [PubMed] [Google Scholar]
- 12.Cody HS III. Sentinel lymph node biopsy for breast cancer: does anybody not need one? Ann Surg Oncol. 2003;10:1131–1132. [DOI] [PubMed] [Google Scholar]
- 13.Krag DN. Protocol B-32: a randomized phase III clinical trial to compare sentinel node resection to conventional axillary dissection in clinically node-negative breast cancer patients. http:www.nsabp.pitt.edu/B-32.htm 2003; Available from: http://www.nsabp.pitt.edu/B-32.htm.
- 14.Clarke D, Khonji NI, Mansel RE. Sentinel node biopsy in breast cancer: ALMANAC trial. World J Surg. 2001;25:819–822. [DOI] [PubMed] [Google Scholar]
- 15.Clarke M, Collins R, Darby S, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. [DOI] [PubMed] [Google Scholar]
- 16.Giuliano AE. Z0011: a randomized trial of axillary node dissection in women with clinical T1 or T2 N0 M0 breast cancer who have a positive sentinel node. http://www.acosog.org/studies/synopses/Z0011_Synopsis.pdf 2003.
- 17.Fant JS, Grant MD, Knox SM, et al. Preliminary outcome analysis in patients with breast cancer and a positive sentinel lymph node who declined axillary dissection. Ann Surg Oncol. 2003;10:126–130. [DOI] [PubMed] [Google Scholar]
- 18.Guenther JM, Hansen NM, DiFronzo LA, et al. Axillary dissection is not required for all patients with breast cancer and positive sentinel nodes. Arch Surg. 2003;138:52–56. [DOI] [PubMed] [Google Scholar]
- 19.Jeruss JS, Winchester DJ, Sener SF, et al. Axillary recurrence after sentinel node biopsy. Ann Surg Oncol. 2005;12:34–40. [DOI] [PubMed] [Google Scholar]
- 20.Fisher B, Redmond C, Fisher E. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. N Engl J Surg. 1985;312:674–681. [DOI] [PubMed] [Google Scholar]