Abstract
Objective:
To examine the diagnostic agreement of preoperative needle core biopsy (NCB) grading of hepatocellular carcinoma (HCC) compared with the final surgical pathologic tumor grade.
Summary Background Data:
Some centers have adopted protocols for selecting patients with HCC for transplantation based on tumor grade as determined by preoperative NCB. The validity of NCB to predict final tumor grade has not been previously assessed.
Methods:
A total of 211 patients who underwent hepatic resection, open radiofrequency, or transplantation for HCC between 1998 and 2004 were identified. Clinicopathologic, NCB, and surgical data were collected and analyzed using χ2 and κ statistics.
Results:
A total of 120 (67.4%) of the 178 who underwent resection or transplantation had an NCB. On preoperative NCB, the majority of HCC cases were classified as well-differentiated (n = 35; 37.6%) or moderately differentiated (n = 44; 47.3%), while 14 (15.1%) cases were categorized as poorly differentiated. In contrast, when tumor grading was based on the final surgical specimen, there was a significantly higher proportion of HCC cases graded as poorly differentiated (well-differentiated, n = 34; 36.6%; moderately differentiated, n = 33; 35.5%; poorly differentiated, n = 26; 27.9%) (P < 0.05). The overall percent agreement of NCB and surgical pathology to determine tumor grade was poor (κ = 0.18, P < 0.0001). Whereas final pathologic tumor grade predicted the presence of microscopic vascular invasion (well, 15.7%; moderate; 31.9%, poor; 58.4%; P = 0.001), NCB grade did not (well, 23.7%; moderate, 28.0%; poor, 25.4%; P = 0.65).
Conclusions:
Selection of candidates for transplantation based on NCB tumor grade may be misleading, as NCB tumor grade often did not correlate with grade or presence of microscopic vascular invasion on final pathology. Clinicomorphologic criteria (tumor size, number) should remain the major determinants of eligibility for transplantation.
The diagnostic agreement of preoperative needle core biopsy (NCB) grading of hepatocellular carcinoma (HCC) versus the final surgical pathologic tumor grade was analyzed. We report that the overall percent agreement and sensitivity of NCB to determine tumor grade was poor (κ = 0.18, sensitivity = 34.6%; both P < 0.0001). NCB tumor grade may be misleading, as NCB tumor grade often does not correlate with grade or presence of microscopic vascular invasion on final pathology.
Hepatic transplantation is the therapeutic modality of choice for many patients with hepatocellular carcinoma (HCC). However, due to poor outcomes early in the transplantation era, the eligibility criteria for transplantation have become more stringent over the last decade. Specifically, early results of transplantation for HCC were associated with recurrence rates that ranged from 60% to 70%,1,2 and the 5-year survival rate was less than 30%.3,4 More recently, survival data published from Milan and Barcelona revealed 4-year survival rates of 75% after liver transplantation.5,6 These improved survival rates were due to careful selection of patients with specific morphologic criteria: only patients with HCC and cirrhosis who had ≤3 tumor nodules that were ≤3 cm in maximum diameter or a single tumor ≤5 cm and no clinically apparent signs of vascular invasion were considered acceptable for transplantation.7 With the adoption of these criteria, survival rates after liver transplantation have been similar to those after resection for noncirrhotic patients with lesions of similar stage.8,9
There is concern, however, that allocation of organs to patients based on such strict morphologic criteria may potentially exclude some patients who, despite not meeting the Milan criteria, may still benefit from transplantation.10–13 Several groups14–16 have suggested that preoperative tumor grade be incorporated into the transplantation selection criteria for those patients who do not meet the Milan criteria. In support of this argument, these investigators point to the fact that tumor grade has previously been shown to influence survival after resection for HCC11,17–19; patients with poorly differentiated HCC do worse than patients with moderately or well-differentiated tumors.16,17 As such, Cillo et al14 have adopted a protocol for selecting HCC patients for transplantation based on moderately or well-differentiated tumor grade as determined by preoperative needle biopsy, even in those patients who do not meet the Milan criteria.
The 2 most widely used and accepted methods of needle biopsy include fine needle aspiration (FNA) cytology and needle core biopsy (NCB).20 FNA specimens are usually acquired using 20 to 25 gauge needles, whereas NCB specimens are obtained using larger 14 to 18 gauge needles. While FNA generally provides a sample adequate for cytologic examination, NCB specimens provide a core of tissue that can undergo histologic assessment. The overall diagnostic sensitivity and specificity of FNA have been reported to range from 67% to 100% and 80% to 100%, respectively.21–24 The accuracy of cytologic grading using FNA has been questioned,25 but the validity of NCB to predict the final nuclear grade of the tumor in the pathologic specimen has not been previously assessed. Before preoperative tumor grade can even be considered as a transplant criterion, the overall accuracy of assessing nuclear grade by NCB needs to be determined. Therefore, the objective of the current study was to examine the validity of preoperative NCB to assess tumor grade. Specifically, we analyzed the diagnostic agreement of preoperative NCB grading of HCC compared with the final surgical pathologic tumor grade as the reference or “gold standard.”
PATIENTS AND METHODS
A total of 211 patients who underwent hepatic resection, open radiofrequency ablation, or transplantation for HCC at Johns Hopkins Hospital between January 1998 and December 2004 were identified from our institutional database. Standard demographic and clinicopathologic data were collected on all patients, including age, sex, laboratory data (α-fetoprotein [AFP] level and hepatitis serology), tumor size and number, presence of vascular invasion (major and microscopic), parenchymal cirrhosis, and histologic grade. The institutional review board at Johns Hopkins Hospital approved this study.
A blinded review of both the preoperative needle biopsy and the final surgical specimen was performed by a single pathologist with hepatobiliary expertise (R.A.A.). Evaluation for vascular invasion was performed only on the final surgical pathology specimen, as vascular invasion cannot be accurately assessed on needle biopsy.24 Microscopic vascular invasion was defined as the presence of tumor emboli within the central vein, the portal vein, or large capsular vessels or involvement of the lobar or segmental branches of the portal vein or the hepatic veins.26,27
Tumor grade was assessed on both the preoperative NCB and the final surgical pathology specimen. Tumor grade was scored using the modified nuclear grading scheme outlined by the Edmondson and Steiner, with tumor grade categorized as low, intermediate, or high.28,29 Specifically, modified Edmondson-Steiner grades 1 and 2 were defined as well-differentiated, grade 3 as moderately differentiated, and grade 4 as poorly differentiated (Fig. 1). In all cases, tumor grade was defined by the poorest degree of differentiation identified within the tumor upon pathologic analysis of the entire specimen.

FIGURE 1. Tumor grade was scored using the modified Edmonson and Steiner nuclear grading scheme in which grades 1 and 2 were defined as well-differentiated, grade 3 as moderately differentiated, and grade 4 as poorly differentiated.
Summary statistics were obtained using established methods. χ2 and Fisher exact test tests were used for comparing categorical variables, and the Kruskal-Wallis test was used to compare continuous variables among the groups. Multivariate analyses to assess long-term prognosis were performed using the Cox proportional hazards model and reported as the hazard ratio (HR) with 95% confidence intervals (CI). Validity was evaluated by comparing preoperative NCB tumor grade using surgical pathology tumor grade as the “gold standard.” Agreement was reported using the κ statistics to account for agreement that may have occurred by chance alone. κ values were calculated using the formula:
 |
where PO was the proportion of observed agreement and PE was the chance agreement, that is, the proportion of agreement expected to occur by chance alone.30 To further evaluate the ability of preoperative NCB to predict the final surgical pathologic tumor grade, a receiver operating characteristic (ROC) curve analysis was used and the area under the curve (AUC) was calculated to assess the performance of preoperative NCB.
RESULTS
Clinicopathologic Characteristics and NCB Diagnosis
Table 1 shows the clinicopathologic features of the 211 patients in the study. There were 128 men and 83 women, for a male-to-female ratio of 1.54:1. The median patient age was 62 years (range, 23–80 years). The median number of treated lesions was 1 (range, 1–10), with most patients having a solitary HCC lesion (n = 178; 84.4%). The median size of the largest lesion was 7.0 cm (range, 2–23 cm). A total of 108 (51.2%) patients had cirrhosis. Roughly one half of patients had viral hepatitis (n = 105; 49.8%) (hepatitis B, n = 35; hepatitis C, n = 63; hepatitis B and C, n = 7). Thirty-three patients underwent open radiofrequency ablation alone, while 144 underwent hepatic resection. The extent of the hepatic resection included an extended hepatectomy (n = 23; 15.9%), hemihepatectomy (n = 53; 36.8%), less than a hemihepatectomy (n = 51; 35.4%), and wedge (n = 17; 11.8%). Thirty-four patients underwent liver transplantation.
TABLE 1. Clinical and Pathologic Features of Patients (n = 211)

Patients were clinically staged according to the system of the Cancer of the Liver Italian Program Investigators (CLIP),31 which incorporates Child-Pugh stage, tumor morphology, AFP level, and presence of portal vein thrombosis. Of the 211 patients included in the study, most (47.9%) had an aggregate CLIP score of 0 (Table 2). Of those patients who underwent either resection or transplantation (n = 178), the majority of patients had node negative (98.9%), T1 disease (71.9%) on final American Joint Committee on Cancer32 pathologic staging (Table 2).
TABLE 2. Clinical and Pathologic Staging of Patients

Of the 211 patients, 33 patients were treated with open radiofrequency ablation alone and therefore were excluded from further analyses because no ex vivo tumor was available to allow comparison with the preoperative NCB. Of the remaining 178 patients who underwent either resection or transplantation, 120 (67.4%) had a preoperative NCB. Of the 120 cases in which a preoperative NCB was performed, 97 (80.8%) had both the preoperative NCB and surgical pathology specimen available for review, allowing for a direct comparison of the 2 specimens. Of the 97 cases in which both the NCB and the final pathologic specimen were available, the NCB was positive in 93 patients, yielding a diagnostic sensitivity for preoperative NCB of 95.9%.
Overall, no clinical factor predicted which patients were more likely to have undergone a preoperative NCB. However, among the subset of patients with cirrhosis, patients with a tumor ≤2 cm tended to undergo preoperative NCB more often than those patients with cirrhosis and a tumor >2 cm (tumor size ≤2 cm, 100% vs. tumor size >2 cm, 70.1%; P = 0.09). Similarly, patients with cirrhosis and a normal AFP (<20 ng/mL) were more likely to undergo a preoperative NCB than patients with cirrhosis and an elevated AFP (normal AFP, 95.4% vs. elevated AFP, 64.3%; P = 0.01).
Concordance of Tumor Grade Assessment
Table 3 shows the degree of concordance between preoperative NCB and final pathologic tumor grade. On preoperative NCB, the majority of HCC cases were classified as either well-differentiated (n = 35; 37.6%) or moderately differentiated (n = 44; 47.3%), while only 14 (15.1%) cases were categorized as poorly differentiated. In contrast, when tumor grading was based on the final surgical specimen, there was a significantly higher proportion of HCC cases graded as poorly differentiated (well-differentiated, n = 34; 36.6%; moderately differentiated, n = 33; 35.5%; poorly differentiated, n = 26; 27.9%) (P < 0.05). Of the 93 cases, there was complete concordance between the preoperative NCB grade and the final surgical specimen grade in only 42 (45.2%) cases (well-differentiated, n = 17; moderately differentiated, n = 16; poorly differentiated, n = 9) (Table 3). Therefore, using a 3-tier grading system (well-differentiated, moderate differentiated, poorly differentiated), preoperative NCB misclassified tumor grade in 54.8% of cases. The corresponding κ statistic for concordance was 0.18 (P < 0.0001).
TABLE 3. Concordance of Tumor Grade on Preoperative Needle Core Biopsy Versus Final Surgical Pathology Using 3-Tier Grading System (κ statistic = 0.18)

Although well- and moderately differentiated tumors have similar long-term survival outcomes following transplantation for HCC, patients with poorly differentiated tumors have a significantly worse outcome following transplantation.17 As such, distinguishing well-/moderately differentiated tumors from poorly differentiated tumors has more important clinical implications. A second analysis was therefore performed that compared tumor grade concordance between preoperative NCB and the final pathologic specimen using a 2-tier grading scheme: well-/moderately differentiated vs. poorly differentiated (Table 4). Using this grouping, preoperative NCB still misclassified 23.7% of the cases and the κ statistic for concordance was only 0.38 (P < 0.001). Overall, the sensitivity and specificity of preoperative NCB to identify poor histologic grade were 34.6% and 92.5%, respectively.
TABLE 4. Concordance of Tumor Grade on Preoperative Needle Core Biopsy Versus Final Surgical Pathology Using 2-Tier Grading System (κ statistic = 0.37)

To better assess whether preoperative NCB could predict poor tumor grade on the final surgical specimen, a sensitivity and specificity analysis was performed using an ROC curve. The AUC for preoperative NCB was 0.74 (Fig. 2).
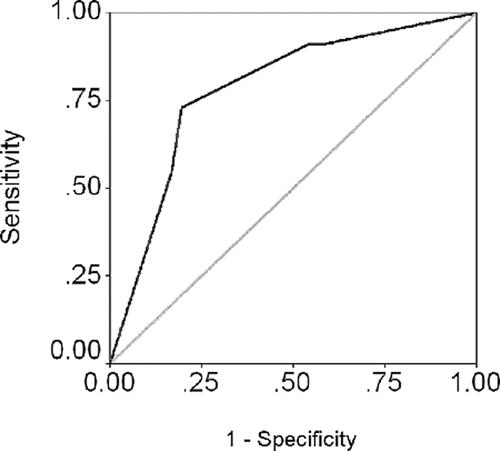
FIGURE 2. ROC curve analysis displaying the limited ability of NCB to discriminate poor tumor grade on the final surgical specimen (AUC = 0.74).
Preoperative NCB Versus Final Surgical Specimen as Predictor of Microscopic Vascular Invasion
Tumor grade is important because multiple studies33–35 have shown that tumor grade is the main predictor of microscopic vascular invasion, one of the most powerful independent predictors of prognosis following transplantation for HCC.26,36,37 We therefore examined the association of tumor grade as determined on preoperative NCB versus final surgical pathology with the presence of microscopic vascular invasion in the final tumor specimen.
Determination of tumor grade on preoperative NCB was not associated with the presence of microscopic vascular invasion in the final tumor specimen (Fig. 3). Approximately the same number of patients were found to have microscopic vascular invasion regardless of the degree of histologic differentiation as determined by preoperative NCB (well-differentiated, 23.7%; moderately differentiated, 28.0%; poorly differentiated, 25.4%) (P = 0.65). In contrast, tumor grade on the final surgical specimen was significantly associated with the presence of microscopic vascular invasion. Specifically, HCC tumors classified as poorly differentiated based on the final surgical specimen were more than 3 times (risk ratio = 3.7) more likely to have microscopic vascular invasion compared with well-differentiated tumors (well-differentiated, 15.7%; moderately differentiated, 31.9%; poorly differentiated, 58.4%) (P = 0.001) (Fig. 3).

FIGURE 3. Although preoperative NCB tumor grade was not associated with microscopic vascular invasion, tumor grade on the final surgical specimen was significantly associated with the presence of microscopic vascular. *P = 0.001.
Preoperative NCB Versus Final Surgical Specimen as Predictor of Prognosis
To evaluate the relative prognostic importance of tumor grade compared with standard clinicopathologic prognostic variables, multivariate analyses were performed. Specifically, the relative prognostic importance of the preoperative clinical stage (eg, CLIP score31) vs. tumor grade was assessed. In multivariate analyses, tumor grade on final surgical assessment was a significantly more powerful predictor of prognosis than the preoperative CLIP score (Table 5). While patients with poorly differentiated tumors had a 4-fold increased risk of disease-specific death (HR = 4.07; 95% CI, 1.63–10.21; P = 0.003), the CLIP score failed to be prognostically significant (HR = 1.12; 95% CI, 0.80–1.56; P = 0.50). However, when tumor grade on preoperative NCB was analyzed in a multivariate model that included CLIP score, tumor grade lost its prognostic power (poorly differentiated on NCB: HR = 1.25; 95% CI, 0.62–2.51; P = 0.53) and the CLIP score became significant (HR = 1.60; 95% CI, 1.16–2.22; P = 0.004). An analysis of tumor grade versus established pathologic prognostic variables (eg, tumor number, tumor size, presence of vascular invasion) was also performed. A similar pattern of the relative prognostic impact of tumor grade was noted. Tumor grade on preoperative NCB was not significant compared with traditional pathologic factors; however, in contrast, tumor grade on final pathologic examination was a powerful predictor of disease-specific death (poorly differentiated on final pathology, HR = 2.66; 95% CI, 1.16–6.12; P = 0.02) (Table 5).
TABLE 5. Relative Prognostic Importance of Tumor Grade Versus Established Clinical and Pathologic Variables
DISCUSSION
The establishment of strict morphologic criteria has significantly improved survival following liver transplantation for HCC.5,6 This careful allocation of organs has been a guiding principle in transplantation to optimize benefits to patients who undergo transplantation and to avoid transplanting patients unlikely to derive a benefit from the treatment. Notwithstanding the dramatic improvements in outcome, some investigators have suggested, however, that such strict criteria also carry the risk of refusing a potentially curative option to some patients may who actually benefit from transplantation.10–15 These investigators caution that exclusive reliance on a tumor's macromorphologic characteristics may be inaccurate and limited by imaging techniques.12,14,38 For example, in the seminal report by Mazzaferro et al,6 27% of patients exceeded the original study entry criteria at histologic examination of the explanted liver. In a more recent study by Cillo et al,14 the pretransplant stage was nonconcordant with the posttransplant stage in 48% of patients according to TNM stage and in 14% of patients according to the Milan criteria. As such, the macromorphologic characteristics of HCC may give an imprecise estimate of the tumor's aggressiveness. Indeed, Kirimlioglu et al39 and Jonas et al17 noted that 17% to 40% of patients had aggressive histologic features on the explant following transplantation, despite being chosen for transplantation using the Milan criteria. In light of these data, there has been an interest in identifying a more direct indicator of biologic progression, and subsequent risk of recurrence, in patients with HCC who are being considered for transplantation.
Tumor grade has been shown to influence survival after transplantation for HCC.11,17 Patients with poorly differentiated HCC do worse than patients with moderately or well-differentiated tumors following transplantation.16,17 Tamura et al16 reported that histologic differentiation was an independent predictor of survival following transplantation. In that study, patients with small (≤5 cm) tumors that were well- to moderately differentiated had a 3-year survival of 82% compared with only 67% for patients with poorly differentiated tumors. In addition, patients with large (>5 cm) well- or moderately differentiated tumors had a 3-year survival of 62.5% compared with no survivors (0%) for those patients with large poorly differentiated tumors. Because needle biopsy can routinely provide tumor grade preoperatively, several investigators14–16 have suggested that preoperative histologic grade may be a worthwhile criterion for selecting candidates for transplantation.
The results of the current study are important because they demonstrate that preoperative NCB of HCC to determine histologic grade is inaccurate. Using either a 3-tier (well-, moderately, or poorly differentiated) or 2-tier (well-/moderately or poorly differentiated) grading scheme, the concordance of HCC histologic grade on preoperative NCB versus the final surgical specimen was poor (κ = 0.18 and 0.38, respectively). Indeed, using the 3-tier grading scheme, over one half of the cases were misclassified with regard to histologic grade. Even when using a 2-tier grading system (well-/moderate vs. poor), the overall sensitivity of preoperative NCB was only 34.6%. The AUC for the preoperative NCB ROC curve was 0.74, which generally is considered to be in the fair range of diagnostic accuracy. However, given the proposed clinical implications of the preoperative NCB to help determine transplant eligibility, such a low diagnostic accuracy is clearly problematic.
One of the reasons for the poor diagnostic accuracy of preoperative NCB to predict tumor grade is that HCCs are commonly very heterogeneous with regard to tumor differentiation.40 For example, a portion of the tumor may be well-differentiated while another area may be moderately differentiated (Fig. 4A) or poorly differentiated (Fig. 4B). These types of HCC lesions can be associated with sampling errors on preoperative NCB. Of note, several investigators41–43 have reported that the risk of tumor dedifferentiation, and histologic heterogeneity, increases as the HCC gets larger. In the current study, the fact that the median tumor size was 7.0 cm may have contributed to the poor concordance of tumor grade on preoperative NCB versus final surgical pathology. The association of tumor size with histologic heterogeneity is important because preoperative NCB is being proposed in this population of patients with large tumors (ie, those who do not meet the Milan criteria), the very subset of patients most likely to have histologic heterogeneity.

FIGURE 4. Tumor grade in HCC can be heterogeneous. A, A portion of tumor is well-differentiated while an adjacent area, which contains markedly enlarged oncocytic hepatocytes with some nuclear pleomorphism and angulation, is moderately differentiated. B, In this example, an area of moderate differentiation is adjacent to an area of poor differentiation.
Multiple studies26,36,37 have shown that vascular invasion, macroscopic or microscopic, is one of the strongest predictors of tumor recurrence after liver transplantation. Llovet et al36 reported that microscopic vascular invasion detected at pathologic examination in the explant specimen was associated with no disease-free survivors at 3 years, whereas 94% of patients without vascular invasion were disease-free after 3 years. Iwatsuki et al44 similarly reported that microscopic vascular invasion was associated with a 4.4-fold increased risk of recurrence following transplantation for HCC. Microvascular invasion, however, is a histopathologic diagnosis and cannot be made prior to removal of the liver specimen. Because microvascular invasion has a significant impact on recurrence and survival after transplantation, tumor grade has been investigated as a possible surrogate marker of microscopic invasion. In an analysis of tumors less than 5 cm, Esnaola et al35 reported that poor histologic differentiation predicted microscopic vascular invasion in patients with HCC who were candidates for liver transplantation. Similarly, Pawlik et al33 reported that high histologic tumor grade predicted occult vascular invasion in tumors larger than 5 cm. In the current study, however, tumor grade on preoperative NCB was not able to predict the presence of microscopic vascular invasion in the final tumor specimen. Indeed, the same number of patients (roughly 25%–30%) was found to have microscopic vascular invasion regardless of tumor grade on preoperative biopsy. In contrast, as expected, tumor grade on the final surgical specimen was associated with the presence of microscopic vascular invasion, with poorly differentiated HCC being significantly more likely to have evidence of vascular invasion. These data have important implications as they suggest that the established association between final HCC tumor grade and risk of vascular invasion33,35 cannot be extrapolated to tumor grade as determined by preoperative NCB.
To place tumor grade on preoperative NCB versus full pathologic examination in some perspective, we assessed the relative impact of tumor grade compared with standard clinical and pathologic prognostic variables. In multivariate analyses, the prognostic power of tumor grade was only significant when the model included tumor grade based on final pathologic examination. However, while tumor grade on final surgical pathology was an important prognostic factor compared with either the CLIP score or traditional pathologic characteristics, tumor grade on preoperative NCB was not (Table 5). These data suggest that the relative prognostic importance of tumor grade versus clinical/pathologic staging depends on how the information on tumor grade was ascertained. Specifically, our data emphasize that tumor grade on preoperative NCB cannot be used as a prognostic factor, unlike tumor grade on final pathologic examination, which has established prognostic importance.11,17
In the current study, the analyses and findings were based on a single NCB of the HCC mass. As such, we could not address whether increasing the number of preoperative NCBs would have improved the overall accuracy and concordance of NCB versus final histologic grade. Multiple NCBs may be inadvisable, however, as Saborido et al45 recently reported that preoperative aspiration-biopsy may be associated with a larger incidence of tumor recurrence in patients following liver transplantation for HCC. In the current study, the overwhelming majority (84.4%) of patients had a solitary lesion, and we were therefore not able to investigate the accuracy of preoperative NCB in the setting of multiple HCCs. It is most plausible, however, that the overall accuracy and concordance of preoperative NCB with final surgical pathology would most likely suffer as tumor number increased. The current study also did not examine the issue of interobserver discordance in grading the preoperative NCB, as all the biopsies were reviewed by a single pathologist. Therefore, the concordance values presented in the current study reflect only intraobserver variability and therefore probably represent a “best case scenario” for grade concordance between preoperative NCB and final pathology (ie, no interobserver variability).
CONCLUSION
Our data suggest that selection of candidates for transplantation based on preoperative NCB tumor grade may be misleading. As such, the findings of the current study call into question any proposed use of preoperative NCB in an eligibility algorithm to decide which patients outside the Milan criteria should be transplanted. Rather clinicomorphologic criteria should currently remain the major determinants for liver transplantation. In the future, identification of novel biomarkers,46–48 not simply tumor grade, on preoperative biopsy may better help to identify patients for hepatic transplantation.
Footnotes
Presented at the American Hepato-Pancreato-Biliary Association 2006 Annual Meeting, March 10, 2006, Miami, FL.
Reprints: Timothy M. Pawlik, MD, MPH, Department of Surgery, Johns Hopkins Hospital, 600 North Wolfe Street, Halsted 614, Baltimore, MD 22187-6681. E-mail: tpawlik1@jhmi.edu.
REFERENCES
- 1.Iwatsuki S, Gordon RD, Shaw BW Jr, et al. Role of liver transplantation in cancer therapy. Ann Surg. 1985;202:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Grady JG, Polson RJ, Rolles K, et al. Liver transplantation for malignant disease: results in 93 consecutive patients. Ann Surg. 1988;207:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ismail T, Angrisani L, Gunson BK, et al. Primary hepatic malignancy: the role of liver transplantation. Br J Surg. 1990;77:983–987. [DOI] [PubMed] [Google Scholar]
- 4.Olthoff KM, Millis JM, Rosove MH, et al. Is liver transplantation justified for the treatment of hepatic malignancies? Arch Surg. 1990;125:1261–1266; discussion 1266–1268. [DOI] [PubMed]
- 5.Llovet JM, Bruix J, Fuster J, et al. Liver transplantation for small hepatocellular carcinoma: the tumor-node-metastasis classification does not have prognostic power. Hepatology. 1998;27:1572–1577. [DOI] [PubMed] [Google Scholar]
- 6.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 7.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002;8:765–774. [DOI] [PubMed] [Google Scholar]
- 8.Khakhar A, Solano E, Stell D, et al. Survival after liver transplantation for hepatocellular carcinoma. Transplant Proc. 2003;35:2438–2441. [DOI] [PubMed] [Google Scholar]
- 9.Cha CH, Ruo L, Fong Y, et al. Resection of hepatocellular carcinoma in patients otherwise eligible for transplantation. Ann Surg. 2003;238:315–321; discussion 321–323. [DOI] [PMC free article] [PubMed]
- 10.Roayaie S, Frischer JS, Emre SH, et al. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235:533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klintmalm GB. Liver transplantation for hepatocellular carcinoma: a registry report of the impact of tumor characteristics on outcome. Ann Surg. 1998;228:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. [DOI] [PubMed] [Google Scholar]
- 13.Salizzoni M, Zamboni F, Lupo F, et al. Liver transplantation for early-detected, multifocal hepatocellular carcinoma. Br J Surg. 2001;88:1194–1195. [DOI] [PubMed] [Google Scholar]
- 14.Cillo U, Vitale A, Bassanello M, et al. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg. 2004;239:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Carlis L, Giacomoni A, Lauterio A, et al. Liver transplantation for hepatocellular cancer: should the current indication criteria be changed? Transpl Int. 2003;16:115–122. [DOI] [PubMed] [Google Scholar]
- 16.Tamura S, Kato T, Berho M, et al. Impact of histological grade of hepatocellular carcinoma on the outcome of liver transplantation. Arch Surg. 2001;136:25–30. [PubMed] [Google Scholar]
- 17.Jonas S, Bechstein WO, Steinmuller T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–1086. [DOI] [PubMed] [Google Scholar]
- 18.Wayne JD, Lauwers GY, Ikai I, et al. Preoperative predictors of survival after resection of small hepatocellular carcinomas. Ann Surg. 2002;235:722–730; discussion 730–731. [DOI] [PMC free article] [PubMed]
- 19.Regimbeau JM, Abdalla EK, Vauthey JN, et al. Risk factors for early death due to recurrence after liver resection for hepatocellular carcinoma: results of a multicenter study. J Surg Oncol. 2004;85:36–41. [DOI] [PubMed] [Google Scholar]
- 20.Stewart CJ, Coldewey J, Stewart IS. Comparison of fine needle aspiration cytology and needle core biopsy in the diagnosis of radiologically detected abdominal lesions. J Clin Pathol. 2002;55:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samartunga H, Wright G. Value of FNA biopsy cytology in the diagnosis of discrete hepatic lesions suspicious for malignancy. Aust NZ J Surg. 1992;62:540–544. [DOI] [PubMed] [Google Scholar]
- 22.Schwerk WB, Schmitz-Moormann P. Ultrasonically guided fine-needle biopsies in neoplastic liver disease: cytohistologic diagnoses and echo pattern of lesions. Cancer. 1981;48:1469–1477. [DOI] [PubMed] [Google Scholar]
- 23.Walker AN, Feldman PS, Covell JL, et al. Fine needle aspiration under percutaneous transhepatic cholangiographic guidance. Acta Cytol. 1982;26:767–771. [PubMed] [Google Scholar]
- 24.Hertz G, Reddy VB, Green L, et al. Fine-needle aspiration biopsy of the liver: a multicenter study of 602 radiologically guided FNA. Diagn Cytopathol. 2000;23:326–328. [DOI] [PubMed] [Google Scholar]
- 25.Kulesza P, Torbenson M, Sheth S, et al. Cytopathologic grading of hepatocellular carcinoma on fine-needle aspiration. Cancer. 2004;102:247–258. [DOI] [PubMed] [Google Scholar]
- 26.Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–1536. [DOI] [PubMed] [Google Scholar]
- 27.Ikai I, Yamamoto Y, Yamamoto N, et al. Results of hepatic resection for hepatocellular carcinoma invading major portal and/or hepatic veins. Surg Oncol Clin N Am. 2003;12:65–75. [DOI] [PubMed] [Google Scholar]
- 28.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. [DOI] [PubMed] [Google Scholar]
- 29.Nzeako UC, Goodman ZD, Ishak KG. Comparison of tumor pathology with duration of survival of North American patients with hepatocellular carcinoma. Cancer. 1995;76:579–588. [DOI] [PubMed] [Google Scholar]
- 30.Szklo M, Nieto FJ. Quality assurance and control. In: Szklo M, Nieto FJ, eds. Epidemiology Beyond the Basics. Sudbury, MA: Jones and Bartlett, 2004:375–380. [Google Scholar]
- 31.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. [DOI] [PubMed] [Google Scholar]
- 32.Liver (including intrahepatic bile ducts). In: Greene FL, Page DL, Fleming ID, et al, eds. American Joint Committee on Cancer Staging Manual. Philadelphia: Lippincott Raven, 2002:131–138. [Google Scholar]
- 33.Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. [DOI] [PubMed] [Google Scholar]
- 34.Adachi E, Maeda T, Kajiyama K, et al. Factors correlated with portal venous invasion by hepatocellular carcinoma: univariate and multivariate analyses of 232 resected cases without preoperative treatments. Cancer. 1996;77:2022–2031. [DOI] [PubMed] [Google Scholar]
- 35.Esnaola NF, Lauwers GY, Mirza NQ, et al. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg. 2002;6:224–232; discussion 232. [DOI] [PubMed]
- 36.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. [DOI] [PubMed] [Google Scholar]
- 37.Tsai TJ, Chau GY, Lui WY, et al. Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery. 2000;127:603–608. [DOI] [PubMed] [Google Scholar]
- 38.Kaihara S, Kiuchi T, Ueda M, et al. Living-donor liver transplantation for hepatocellular carcinoma. Transplantation. 2003;75(suppl 3):37–40. [DOI] [PubMed] [Google Scholar]
- 39.Kirimlioglu H, Dvorchick I, Ruppert K, et al. Hepatocellular carcinomas in native livers from patients treated with orthotopic liver transplantation: biologic and therapeutic implications. Hepatology. 2001;34:502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.An FQ, Matsuda M, Fujii H, et al. Tumor heterogeneity in small hepatocellular carcinoma: analysis of tumor cell proliferation, expression and mutation of p53 AND beta-catenin. Int J Cancer. 2001;93:468–474. [DOI] [PubMed] [Google Scholar]
- 41.Kenmochi K, Sugihara S, Kojiro M. Relationship of histologic grade of hepatocellular carcinoma (HCC) to tumor size, and demonstration of tumor cells of multiple different grades in single small HCC. Liver. 1987;7:18–26. [DOI] [PubMed] [Google Scholar]
- 42.Sugihara S, Nakashima O, Kojiro M, et al. The morphologic transition in hepatocellular carcinoma: a comparison of the individual histologic features disclosed by ultrasound-guided fine-needle biopsy with those of autopsy. Cancer. 1992;70:1488–1492. [DOI] [PubMed] [Google Scholar]
- 43.Kojiro M, Yano H, Nakashima O. Pathology of early hepatocellular carcinoma: progression from early to advanced. Semin Surg Oncol. 1996;12:197–203. [DOI] [PubMed] [Google Scholar]
- 44.Iwatsuki S, Dvorchik I, Marsh JW, et al. Liver transplantation for hepatocellular carcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 2000;191:389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saborido BP, Diaz JC, de Los Galanes SJ, et al. Does preoperative fine needle aspiration-biopsy produce tumor recurrence in patients following liver transplantation for hepatocellular carcinoma? Transplant Proc. 2005;37:3874–3877. [DOI] [PubMed] [Google Scholar]
- 46.Telomerase mRNA: a novel marker for hepatocellular carcinoma. Nat Clin Pract Gastroenterol Hepatol. 2005;2:343. [Google Scholar]
- 47.Shih WL, Yu MW, Chen PJ, et al. Localization of a susceptibility locus for hepatocellular carcinoma to chromosome 4q in a hepatitis B hyperendemic area. Oncogene. 2006;25:3219–3224. [DOI] [PubMed] [Google Scholar]
- 48.Chen CF, Yeh SH, Chen DS, et al. Molecular genetic evidence supporting a novel human hepatocellular carcinoma tumor suppressor locus at 13q12.11. Genes Chromosomes Cancer. 2005;44:320–328. [DOI] [PubMed] [Google Scholar]



