Abstract
Objective:
To assess the effectiveness of antibiotic prophylaxis in mesh hernioplasty.
Background:
Antibiotic prophylaxis use in mesh inguinal hernioplasty is controversial. Available evidence is nonconclusive because of the low number of clinical trials assessing its effectiveness. Some trials have a small sample size that could overestimate or underestimate the real effectiveness of this intervention. Meta-analysis is a good method to improve these methodological flaws.
Methods:
Meta-analysis intended to measure the benefits of antibiotic prophylaxis on surgical site infection rate in adult patients scheduled for mesh inguinal hernioplasty. Six randomized clinical trials were found. Quality was assessed using Cochrane Collaboration criteria.
Results:
A total of 2507 patients were analyzed. Surgical site infection frequency was 1.38% in the antibiotic group versus 2.89% in the control group (odds ratio = 0.48; 95% confidence interval, 0.27–0.85). There was no statistical heterogeneity. Sensitivity analysis by quality did not show differences in overall results.
Conclusion:
Antibiotic prophylaxis use in patients submitted to mesh inguinal hernioplasty decreased the rate of surgical site infection by almost 50%.
A meta-analysis on prophylactic antibiotics in mesh hernia repair that included 6 randomized controlled trials showed that antibiotics decrease the frequency of surgical site infection by approximately 50%.
Surgical site infection (SSI) is the most frequent complication in inguinal herniorrhaphy.1 Some studies have identified risk factors for SSI such as sex (greater in women), age (older than 70 years), comorbidity, operative time, and routine use of drainage and prostheses.2–5 SSI is related with an increase in length of stay and costs and a decrease in quality of life.6,7 In the 1970s, it was demonstrated that antibiotic prophylaxis for clean-contaminated surgery was the most cost-effective intervention to prevent SSI. But some authors have recommended its use in clean procedures as inguinal herniorrhaphy. However, the recognition of the free tension herniorrhaphy concept and the current introduction of mesh hernioplasty made the use of antibiotic prophylaxis more critical because of the infection risk when prosthetic materials are used. To prove the effectiveness of antibiotic prophylaxis in these procedures, it is necessary to conduct randomized clinical trials (RCTs) with large numbers of patients, which are difficult and sometimes unfeasible.
Available evidence related to the effectiveness of antibiotic prophylaxis for mesh inguinal hernia repair is found in a meta-analyses, including few RCTs. SSI rate was 1.2% and 3.3%, in the prophylaxis and placebo group, respectively (odds ratio = 0.28; 95% confidence interval [CI], 0.02–3.14). These results concluded there were no statistical differences between groups, so antibiotic prophylaxis was not recommended. However, new RCTs including patients with mesh hernioplasty have been published in the last years, increasing the number of patients evaluated. We decided to conduct a new meta-analysis to assess the effectiveness of antibiotic prophylaxis in mesh hernioplasty.
MATERIALS AND METHODS
All RCTs, which compared antibiotic prophylaxis versus no antibiotics or placebo in adults scheduled for mesh inguinal hernioplasty, including those without contraindications for antibiotic use and without immunosuppression caused by diseases or medicaments, were included. Clinical trials published since 1985 up to date, without restrictions by language or number of patients were included. Quasi-randomized trials were excluded.
Search was made using the MESH terms and free text terms (-#1: antibiotic*, antimicrobial*, anti-infective*, -#2: prophylactic*, prophylaxis*, prevention*-, #3: #1 and #2-, -#4: clean and (surgery or tech* or proced*)-, -#5: herni*-, -#6: (wound infection) and #4-, -#7: #3 and (#4 or #5 or #6) as suggested by Sanchez-Manuel and Seco-Gil8, in databases of Cochrane Hernia Trialists Collaboration, Cochrane Collaboration, MEDLINE, EMBASE and LILACS. Outcome selected was SSI as defined by Center for Disease Control [CDC], recorded as present or not.9 Outcomes follow-up should be until 1 year.
Articles search and data extraction was performed independently by 2 researchers, and conflicts were solved by consensus. Variables selected for extraction were descriptive (population, intervention, control use, intervention, design, and outcomes) and methodologic quality related as recommended by Cochrane Collaboration.10–13 Statistical analysis was made with Stata 8.0 software and OR (95% CI) was calculated for each outcome, using Mantel-Haenszel fixed-effects model. A sensitivity analysis adjusting by quality was also made. Number needed to treat was calculated using Cates method.14 Heterogeneity was assessed with the Q test (significant if P < 0.1), and the influence of heterogeneity on OR value was made with I2 test.15 Results for intervention effect were presented with a forest plot graphic and funnel plot graphic16 and Begg test was used to assess publication bias.17
RESULTS
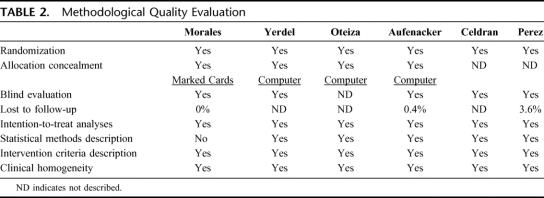
Literature search found in 90 potential studies, and only 6 RCTs that exclusively evaluated mesh inguinal hernia repair that accomplished inclusion and exclusion criteria were selected.18–23 Study characteristics and quality evaluation of each selected study are shown in Tables 1 and 2. They were homogeneous in clinical and methodologic criteria.
TABLE 1. Included Studies Description
TABLE 2. Methodological Quality Evaluation
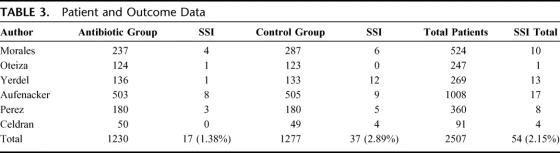
The RCTs selected included 2507 patients: 1230 in the antibiotic group and 1277 in the control group (Table 3). SSI rate was 17 in 1230 (1.38%) patients in the antibiotic group and 37 in 1277 (2.89%) in the control group. OR value was 0.48 (95% CI, 0.27–0.85). Statistical heterogeneity was not identified (Q test for heterogeneity, P = 0.17) (Fig. 1). Number needed to treat was 66 (95% CI, 38–258). Funnel plot (Fig. 2) showed asymmetry in favor of studies with protective effect. So, we decided to expand the search, but could not identify new trials. Begg test, a more specific test for publication bias showed a P value of 0.348, which discarded this bias (Fig. 3).
TABLE 3. Patient and Outcome Data
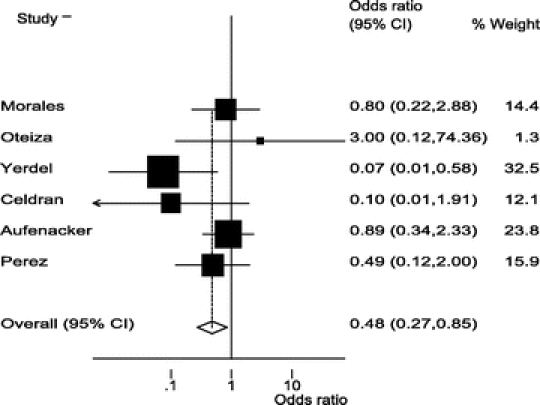
FIGURE 1. Forest plot for effectiveness of prophylactic antibiotics in mesh hernioplasty.
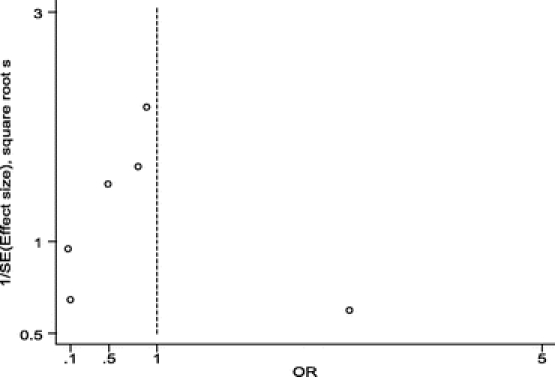
FIGURE 2. Funnel plot for studies assessing effectiveness of prophylactic antibiotics in mesh hernioplasty.
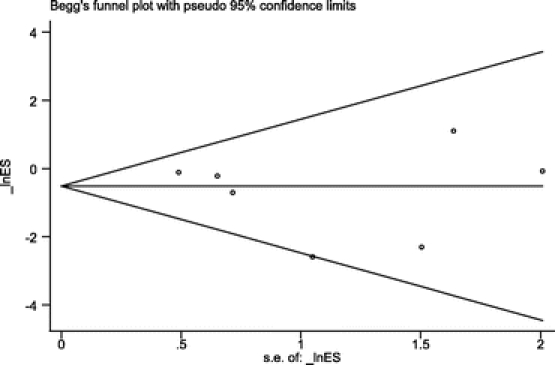
FIGURE 3. Begg method plot for publication bias evaluation.
A sensitivity analysis by quality, excluding the Celdran et al study23 because of its early finishing and low number of patients, did not show a difference with overall OR (0.54; 95% CI, 0.30–0.96) (Fig. 4).
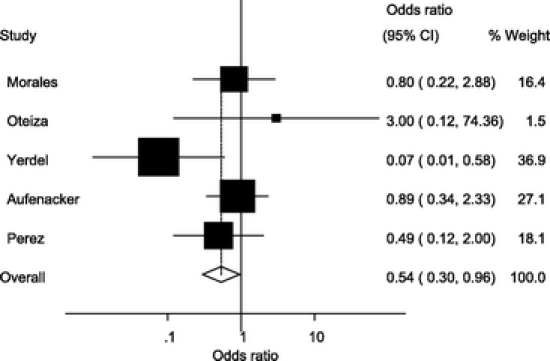
FIGURE 4. Forest plot in sensitivity analysis by quality, excluding the Celdran et al study.23
DISCUSSION
Inguinal hernia is the most common surgical abdominal entity in adults. Although many patients are asymptomatic, most of them have local symptoms and the hernia itself has potential complications (such as incarceration or strangulation). All this forces surgical repair. Of the available treatments, herniorrhaphy is the gold standard because of its low morbidity and high rate of success.
Several surgical techniques have been described, with access to anterior or posterior spermatic cord, by the open or laparoscopic approaches, and with or without prosthetic materials. Since 1975, mesh has been often used for hernia repair, first for recurrent hernias and second for all kind of hernias, because of the principle of free tension repair.24
Surgical site infection is the most common complication secondary to mesh hernia repair, and antibiotic prophylaxis has been suggested as the most effective way to prevent it. Main objectives of antibiotic prophylaxis are to reach high serum levels of antibiotic at the surgical site to avoid colonization by skin microorganisms and secondary infection. It is broadly accepted that antibiotic prophylaxis must be administered in surgical procedures classified as clean-contaminated. However, some authors recommend its use in clean procedures, as inguinal hernioplasty.
Nonetheless, antibiotic prophylaxis in inguinal hernia surgery is controversial, especially after the increasing use of mesh. For some authors, hernia and breast surgery are clear examples of the benefits of antibiotic prophylaxis in clean surgery.25–27 Others consider that low frequency of SSI in hernia surgery does not justify prophylaxis.28–30 A previous meta-analysis by Sanchez-Manuel and Seco-Gil8 for the Cochrane Collaboration, including 8-high quality RCTs, reported no statistical difference in SSI rates between antibiotic and no antibiotic groups. However, a subgroup analysis suggested that, in mesh hernia repair, a protective effect could exist, undetectable because of the small sample size.
SSI rates in inguinal hernia repair vary from 0.06% to 5.3%, with a mean value around 3%.31 So, to perform a RCT with enough power to detect a 50% decrease in SSI rates, it will be necessary to include 1600 to 3000 patients, depending on the sample size formula used and the base rate of SSI. Larger sample sizes will be needed if this percentage decreases even more. Obviously, to perform this ideal study is difficult. However, a meta-analysis is a design that allows merging results of small RCTs, increasing the possibility of detecting an intervention effect. Antibiotic prophylaxis in mesh inguinal hernioplasty is an example of an intervention where meta-analysis could help to detect a beneficial effect.
Results from this meta-analysis clearly show a 50% protective effect of prophylactic antibiotics on decreasing the SSI rate in patients submitted to mesh inguinal hernioplasty and adds evidence favoring the routine use of prophylactic antibiotics in these patients. Besides, neither clinical nor statistical differences were found between included studies, which makes the results stronger and generalizable.
On the other hand, the discussion about the clinical use of antibiotic prophylaxis is still open. It must be specifically determined if prophylactic antibiotics must be administered to all patients, or if there are some risk factors that could help to select the best candidates. Studies included only assessed low-risk patients, so conclusions could be applied solely to this kind of patients. However, as can be seen at Figure 1, studies with higher rates of SSI showed a stronger positive response with prophylactic antibiotic use, and these finding suggest that improvement is higher in these clinical settings. Therefore, surgeons and hospitals must assess their own SSI rates to define if prophylactic antibiotics must be widely used in all patients. For clinical settings with low rates of SSI, selective use of antibiotics based on patients’ basal risk factors could be a better therapeutic strategy. Nonetheless, this strategy can only be probed with a specific designed study.
Another subject that must be assessed in antibiotic prophylaxis is cost-effectiveness. As can be seen, SSI rate could be as low as 1% in some centers. In these cases, the costs of antibiotic administration must be carefully evaluated against the potentials benefits. Only studies particularly designed to answer this question could solve it.
ACKNOWLEDGMENTS
The authors thank Professor Jose Felix Patiño, MD, FACS, for style and grammar review.
Footnotes
Reprints: Alvaro Sanabria, MD, MSc, Hospital Universitario San Ignacio, Cra 7, No. 40-62 of 718, Bogotá, Colombia. E-mail: alvarosanabria@gmail.com.
REFERENCES
- 1.Bendavid R. Complications of groin hernia surgery. Surg Clin North Am. 1998;78:1089–1103. [DOI] [PubMed] [Google Scholar]
- 2.Abo RE. Perioperative antibiotic prophylaxis in abdominal surgery for hernia repair: retrospective study of 1,524 consecutive patients. J Chemother. 1998;10:248–253. [DOI] [PubMed] [Google Scholar]
- 3.Taylor EW, Duffy K, Lee K, et al. Surgical site infection after groin hernia repair. Br J Surg. 2004;91:105–111. [DOI] [PubMed] [Google Scholar]
- 4.Deysine M. Pathophysiology, prevention, and management of prosthetic infections in hernia surgery. Surg Clin North Am 1998;78:1105–1115. [DOI] [PubMed] [Google Scholar]
- 5.Amid PK. Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia. 1997;1:15–21. [Google Scholar]
- 6.Barie PS. Modern surgical antibiotic prophylaxis and therapy–less is more. Surg Infect (Larchmt). 2000;1:23–29. [DOI] [PubMed] [Google Scholar]
- 7.Weed HG. Antimicrobial prophylaxis in the surgical patient. Med Clin North Am. 2003;87:59–75. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Manuel FJ, Seco-Gil JL. Antibiotic prophylaxis for hernia repair. Cochrane Database Syst Rev. 2004;CD003769. [DOI] [PubMed] [Google Scholar]
- 9.Garner JS, Jarvis WR, Emori TG, et al. APIC Infection Control and Applied Epidemiology: Principles and Practice. St. Louis: Mosby, 1996. [Google Scholar]
- 10.Cochrane Reviewers’ Handbook, vol. 44. 2.2 [updated December 2003]. Chichester, UK: John Wiley & Sons, 2004. [Google Scholar]
- 11.Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609–613. [DOI] [PubMed] [Google Scholar]
- 12.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med. 2001;135:982–989. [DOI] [PubMed] [Google Scholar]
- 13.Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. [DOI] [PubMed] [Google Scholar]
- 14.Cates CJ. Simpson's paradox and calculation of number needed to treat from meta-analysis. BMC Med Res Methodol. 2002;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 16.Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 18.Morales R, Carmona A, Pagán A, et al. Utility of antibiotic prophylaxis in reducing wound infection in inguinal or femoral hernia repair using polypropylene mesh. Cir Esp. 2000;67:51–59. [Google Scholar]
- 19.Oteiza F, Ciga MA, Ortiz H. Antibiotic prophylaxis in inguinal hernioplasty. Cir Esp. 2004;75:69–71. [Google Scholar]
- 20.Yerdel MA, Akin EB, Dolalan S, et al. Effect of single-dose prophylactic ampicillin and sulbactam on wound infection after tension-free inguinal hernia repair with polypropylene mesh: the randomized, double-blind, prospective trial. Ann Surg. 2001;233:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aufenacker TJ, van GD, van MT, et al. The role of antibiotic prophylaxis in prevention of wound infection after Lichtenstein open mesh repair of primary inguinal hernia: a multicenter double-blind randomized controlled trial. Ann Surg. 2004;240:955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez AR, Roxas MF, Hilvano SS. A randomized, double-blind, placebo-controlled trial to determine effectiveness of antibiotic prophylaxis for tension-free mesh herniorrhaphy. J Am Coll Surg. 2005;200:393–397. [DOI] [PubMed] [Google Scholar]
- 23.Celdran A, Frieyro O, de la Pinta JC, et al. The role of antibiotic prophylaxis on wound infection after mesh hernia repair under local anesthesia on an ambulatory basis. Hernia. 2004;8:20–22. [DOI] [PubMed] [Google Scholar]
- 24.Patino JF, Garcia-Herreros LG, Zundel N. Inguinal hernia repair: the Nyhus posterior preperitoneal operation. Surg Clin North Am. 1998;78:1063–1074. [DOI] [PubMed] [Google Scholar]
- 25.Lewis RT, Weigand FM, Mamazza J, et al. Should antibiotic prophylaxis be used routinely in clean surgical procedures: a tentative yes. Surgery. 1995;118:742–746. [DOI] [PubMed] [Google Scholar]
- 26.Platt R. Antibiotic prophylaxis in clean surgery: does it work? Should it be used if it does? New Horiz. 1998;6(suppl):53–57. [PubMed] [Google Scholar]
- 27.D'Amico DF, Parimbelli P, Ruffolo C. Antibiotic prophylaxis in clean surgery: breast surgery and hernia repair. J Chemother. 2001;13:108–111. [DOI] [PubMed] [Google Scholar]
- 28.Gupta R, Sinnett D, Carpenter R, et al. Antibiotic prophylaxis for post-operative wound infection in clean elective breast surgery. Eur J Surg Oncol. 2000;26:363–366. [DOI] [PubMed] [Google Scholar]
- 29.Knight R, Charbonneau P, Ratzer E, et al. Prophylactic antibiotics are not indicated in clean general surgery cases. Am J Surg. 2001;182:682–686. [DOI] [PubMed] [Google Scholar]
- 30.Leaper DJ, Melling AG. Antibiotic prophylaxis in clean surgery: clean non-implant wounds. J Chemother. 2001;13:96–101. [DOI] [PubMed] [Google Scholar]
- 31.Cheek CM, Williams MH, Farndon JR. Trusses in the management of hernia today. Br J Surg. 1995;82:1611–1613. [DOI] [PubMed] [Google Scholar]