Abstract
Objective:
The aim of this study was to assess the efficacy and safety of enteral vancomycin in controlling MRSA endemicity in an intensive care burn unit.
Summary Background Data:
MRSA is a serious clinical and epidemiologic problem. It is not uncommon that the traditional maneuvers, detection and isolation of carriers, fail to control endemicity due to MRSA.
Methods:
All patients admitted to an Intensive Care Burn unit from January 1995 to February 2004 have been included in this prospective cohort study comprised 2 different periods. During period 1 (January 1995 to January 2000), barrier and isolation measures were enforced. During period 2 (February 2000 to February 2004), patients received enteral vancomycin 4 times daily in addition to selective digestive decontamination.
Results:
A total of 777 patients were enrolled into the study: 402 in period 1, and 375 in period 2. There were no significant differences in the characteristics of patients between the 2 periods, except for the total body surface burned area, 30.3% in period 1 and 25.61% in period 2 (P = 0.009). There was a significant reduction in the incidence of patients who acquired MRSA from 115 in period 1 to 25 in period 2 (RR, 0.22; 95% confidence interval [CI], 0.15–0.34). Similar reductions were observed in the number of patients with wound (RR, 0.20; 95% CI, 0.12–0.32), blood (RR, 0.13; 95% CI, 0.04–0.35), and tracheal aspirate (RR, 0.07; 95% CI, 0.03–0.19), samples positive for MRSA. There was no emergence of either vancomycin-resistant enterococci or Staphylococcus aureus with intermediate sensitivity to glycopeptides in period 2.
Conclusions:
Enteral vancomycin is an effective and safe method to control MRSA in intensive care burn units without VRE.
The aim of this prospective before-after study is to assess the efficacy and long-term safety of enteral vancomycin in controlling methicillin-resistant Staphylococcus aureus endemicity in an intensive care burn unit. The study comprises a 9 year-period and 777 patients. Vancomycin was associated with a reduction of methicillin-resistant Staphylococcus aureus acquisition (RR, 0.22; 95% CI, 0.15–0.34). There were no cases of either vancomycin-resistant enterococci or Staphylococcus aureus with intermediate sensitivity to glycopeptides.
Thermal injury is a serious trauma requiring intensive care in a specialized unit. Figures from the United States show that, of the 100,000 burn patients who require intensive care, approximately 12,000 die per year due to thermal injury.1 Infection is the cause of death in over half the severely burned patients.2 Thermal injury gives rise to immunosuppression following massive release of inflammatory mediators, including cytokines, prostaglandins, and leukotrienes.3 Inhalation injury, mechanical ventilation, and the lack of immediate adequate parenteral antimicrobials are independent risk factors for pneumonia in burn patients.4 Recently, we observed a 48% incidence of pneumonia and 25% mortality rate in a prospective study of 56 severely burned patients.5
The loss of the natural cutaneous barrier to infection, and the presence of coagulated protein and other microbial nutrients in the burn wound, combined with avascularity of the wound tissue, lead to microbial colonization.6 Colonization is invariably followed by invasion of microorganisms, giving rise to burn wound infection. The burn wound and bloodstream infection rates were 14 and 54 infections per 100 patients, respectively, in the previously mentioned study.7
Lower airway, wound, and bloodstream infections can be caused by any of the potentially pathogenic microorganisms (PPM), both normal “community” bacteria, including Streptococcus pneumoniae, methicillin-sensitive Staphylococcus aureus, and Haemophilus influenzae, and abnormal “hospital” bacteria, such as aerobic Gram-negative bacilli (AGNB) and methicillin-resistant Staphylococcus aureus (MRSA).8
MRSA is a serious diagnostic and epidemiologic problem.9 Patients with burns acquire MRSA more frequently than other acutely ill surgical patients.10 S. aureus, both sensitive and resistant to methicillin, has been recognized as the predominant PPM in burn patients.11,12 Burn patients can import or acquire MRSA that contaminates the environment and the hands of health care workers. Patient to patient transmission via hands is the most common mode for spread and subsequent outbreaks leading to endemicity.
In our recent study, MRSA was the cause of 30% (11 of 37) of pneumonias, 25% (7 of 28) of bloodstream infections, and 75% (6 of 8) of burn wound infections.7 The mortality rate due to MRSA infections in burn patients was 30%. Whether the mortality associated with MRSA is higher than that of methicillin-sensitive S. aureus is unclear.13
Control of MRSA transmission has usually relied on 5 maneuvers: 1) hand disinfection; 2) isolation; 3) personal protective equipment (use of gloves, gowns, and aprons); 4) care of patient equipment; and 5) care of the environment.14 It is not uncommon that these 5 traditional maneuvers fail to prevent and control outbreaks and subsequent endemicity due to MRSA.15–19 There is a low level of evidence for the efficacy of the traditional approach in the control of MRSA outbreaks.19–21 Additionally, “early” debridement and wound grafting have been shown to be more effective in the prevention of MRSA and in improving patients outcome compared with the 5 traditional infection control interventions. Systemic antibiotics do not control MRSA outbreaks in severe burn patients.22,23
Another approach to control MRSA colonization and infection is the administration of enteral vancomycin. Recent studies in ICU patients demonstrate the efficacy and safety of enteral vancomycin in controlling MRSA overgrowth, transmission, and subsequent outbreak.24,25 The impact of this intervention has never been assessed in patients with severe burns, who are at high risk of MRSA acquisition.
In a previous randomized clinical trial, we demonstrated that SDD (enteral polymixin, tobramycin, and amphotericin plus parenteral cefotaxime) 1) reduced mortality in severe burn patients and 2) was associated with a trend toward an increase in MRSA infections.28 Just after finishing this clinical trial, we considered that all the patients fulfilling the inclusion criteria must be treated with SDD. Based on the results of the previous studies,25,28 we hypothesized that enteral vancomycin was efficacious and safe to control MRSA acquisition in severe burn patients treated with SDD in an intensive care burn unit (ICBU) with a high incidence of MRSA.
This before/after study was undertaken to contrast the hypothesis that enteral vancomycin: 1) reduces acquisition of MRSA and 2) does not increase the incidence of vancomycin-resistant enterococci (VRE) and of S. aureus with intermediate sensitivity to glycopeptides (GISA).
MATERIALS AND METHODS
Patients
All consecutive patients admitted to the ICBU at the University Hospital of Getafe over a period of 110 months (from January 1995 to February 2004) were enrolled. The study was approved by the hospital Ethic's Committee for Clinical Research.
Design and Interventions
The infection control policy26,27 to prevent MRSA transmission in our ICBU was based on: 1) hand hygiene using 4% chlorhexidine (Hibiscrub, AstraZeneca); 2) isolation of patients colonized by MRSA; 3) protective clothing; 4) care of equipment; and 5) cleanliness of the environment. This infection control policy was strongly enforced during the entire study period. Staff screening was not part of the infection control policy.
Surveillance samples from nose, throat, and rectum were obtained on admission, and twice weekly thereafter to detect MRSA carriers. Diagnostic samples, such as tracheal aspirate, blood, and urine samples, were taken on clinical indication only. Burn wounds were regularly sampled on admission and twice weekly thereafter (surveillance frequency). Intravascular lines were removed weekly and the tip sent off for culture. The intravascular catheters have been considered diagnostic samples for the purpose of this study.
This prospective cohort study comprised 2 different periods. During period 1 (January 1, 1995 to January 31, 2000) only the infection control policy was implemented. In this period, a randomized clinical trial (107 patients: 54, placebo; 53, SDD) was performed to assess the impact of SDD using enteral polymyxin E, tobramycin, amphotericin B, and a 4-day course of intravenous cefotaxime on the incidence of infections and mortality.28
During period 2 (February 1, 2000 to February 29, 2004) all patients admitted to the ICBU received 4 times daily 4% vancomycin gel into the nose; 4% vancomycin paste (Eucerinum anhydric, Beiersdorf AG) into the oropharynx; and 500 mg vancomycin solution via the nasogastric tube (even in those patients with impaired motility). In patients with a tracheostomy, the vancomycin paste was also applied to the tracheostomy site. Additionally, during period 2 all patients received SDD.
The same antibiotic policy was kept throughout the study period. Systemic antibiotics (a cephalosporin plus an aminoglycoside) were administered empirically when clinical signs of infection developed and were adjusted according to the microbiologic results. Burn patients with a Gram-negative infection received a combination of a third-generation cephalosporin and an aminoglycoside. Infections due to Gram-positive bacteria were treated with a first-generation cephalosporin. Systemic vancomycin was given when the infection was caused by MRSA or ampicillin-resistant enterococci.
Endpoints
The endpoints were: 1) incidence of patients with diagnostic samples of blood, lower airways, urine, and intravascular catheters positive for MRSA acquired on the ICBU; 2) incidence of patients with wounds positive for acquired MRSA; 3) incidence of patients with nose, throat, and rectum surveillance samples positive for MRSA acquired on the ICBU; 4) incidence of patients with surveillance or diagnostic samples positive for VRE29; 5) number of patients with diagnostic samples positive for GISA30; 6) incidence of patients with overgrowth of MRSA in surveillance samples to detect an association between MRSA overgrowth in surveillance samples and diagnostic samples positive for MRSA; 7) percentages of primary endogenous, secondary endogenous, and exogenous positive diagnostic samples; and 8) consumption of parenteral vancomycin measured using the definition of defined daily dose, 2 g/d for 1000 days.31
Definitions
A new case was defined as a patient not known to be MRSA positive on admission, from whom subsequently MRSA was isolated in nose, throat, and rectum surveillance and/or diagnostic samples.
A positive diagnostic sample was defined as a diagnostic sample positive for MRSA in any concentration. Duplicate diagnostic samples were defined as diagnostic samples yielding MRSA from the same site of the same patient at different times, with no interval of negative cultures. Duplicate diagnostic samples were excluded from the analysis.
The term positive diagnostic sample rather than the term infection was used in this study to avoid the bias inherent to the definitions of some infections such as ventilator-associated pneumonia.
MRSA was considered to have been imported when: 1) admission surveillance swabs were positive for MRSA; and 2) burn or diagnostic samples positive within 72 hours of ICBU. MRSA cultured from burn or diagnostic samples after 72 hours of admission to ICBU admission was considered to be acquired.25
Overgrowth was defined as the isolation of MRSA in a concentration ≥105 cfu/mL or ≥105 cfu/g of saliva and/or feces.32–34
Burn wound and diagnostic samples were defined as primary endogenous when MRSA isolated from theses samples was previously present in the surveillance samples taken on admission to the ICBU; secondary endogenous, when MRSA was not isolated in surveillance samples taken on admission to the ICBU but acquired later on the ICBU; and exogenous, when MRSA was not present in surveillance samples, but directly isolated from burn wound, lower airways, intravascular catheters, urine, or blood.25
Microbiologic Methods
Surveillance Samples
Nose, throat, and rectum surveillance samples were processed qualitatively and semiquantitatively. These samples were not pooled as this does not allow the detection of overgrowth. A salt staphylococcal solid agar plate (MSOA, Soria Melguizo, Madrid, Spain) was inoculated using the four-quadrant method combined with an enrichment broth, thioglycolate. Each swab was streaked on to the solid medium; then the tip was broken off into 5 mL enrichment broth. The staphylococcal plate was incubated at 35°C and examined after 2 nights. Additionally, if the enrichment broth was turbid after 1 night's incubation, it was then inoculated on to the solid agar medium. Semiquantitative estimation of MRSA concentrations was made by grading the growth density on a scale of 1+ to 5+, as follows: growth only in broth = 1+ (equivalent to 1 to 10 cfu/mL), growth in the first quadrant of the solid plate = 2+ (103 cfu/mL), in the second quadrant = 3+ (105 cfu/mL), in the third quadrant = 4+ (107 cfu/mL), and on the whole plate = 5+ (>109 cfu/mL).34,35
The laboratory used production of DNase (by a DNA agar-plate method) and a slide agglutination test to detect clumping factor and protein A (Staphaurex plus, Murex Abbott) to differentiate S. aureus from other species of staphylococci. When the results were inconclusive, a tube coagulase test with the NCTC 6571 strain as positive control was undertaken and read at 4 and 24 hours. Coagulase-negative staphylococci were confirmed by a negative tube coagulation test. All the coagulase-positive isolates were identified and tested for antimicrobial susceptibility using Pasco 3 W (Dade Behring, Deerfield, IL) or Wider 094 (Soria Melguizo, Madrid, Spain).
S. aureus isolates were tested for methicillin susceptibility by E-test (AB Biodisk) onto Mueller-Hinton agar plate.36 All the isolates with a minimum inhibiting concentration [MIC] of ≥ 4 μg/mL in the E-test were confirmed as MRSA. Additionally, from August 2003, all MRSA strains were inoculated on a brain heart infusion plate with 6 μg/mL of vancomycin (agar BHI-vancomycin; Soria Melgizo, Madrid, Spain) to detect S. aureus with intermediate sensitivity to glycopeptides (GISA).37
All samples were inoculated on a colistin nalidixic, blood agar. After overnight incubation at 37°C, organisms with a colonial morphology consistent with Enterococcus species were tested for catalase and pyrrolidonylaminopeptidase (Pyrrolidonyl Aminopept; Rosco Laboratories). All the catalase-negative and pyrrolidonylaminopeptidase-positive isolates were tested for sensitivity to ampicillin by Kirby Bauer diffusion test and vancomycin.38 All enterococci that grew on this plate were studied by Pasco 3 W (Dade Behring, Deerfield, IL) or Wider 094 panels (Soria Melguizo, Madrid, Spain) for species identification and antibiotic susceptibility (microdilution in broth). The panels were examined after incubation periods of 24 and 48 hours for sensitivity to vancomycin. All enterococci with discrepancies, ie, growth in the screening test for vancomycin sensitivity and a MIC ≤4 μg/mL were tested by E-test for the detection of VRE (16 mg/L).29
Diagnostic Samples
Blood, lower airway secretions, urine, and pus were processed in a qualitative and semiquantitative way using standard microbiologic methods. Macroscopically distinct colonies were isolated in pure culture for all types of samples. Standard methods for identification, typing, and susceptibility patterns were used for all microorganisms.39 Semiquantitative estimation of bacterial concentrations was made by grading growth density from the 4-quadrant method combined with enhancement broth on a scale of 1+ to 5+.36 Intravascular lines were processed according to Maki et al.40
Statistical Analysis
Continuous variables are shown as means and standard deviations (SD). They were compared using Student t test or Wilcoxon's test where appropriate. Discrete variables were compared using χ2 or exact Fisher exact test and rates were compared by χ2 test.41,42 Statistical significance was considered P < 0.05.
The potential confusing effect of acquiring MRSA due to the imbalanced distribution of risk factors in the 2 periods was assessed by stratified analysis according to: body surface area burned (quartiles), full-thickness body surface area burned (quartiles), inhalation injury (yes/no), and mechanical ventilation (yes/no). The cumulative risk of MRSA acquisition in the 2 periods was compared using Kaplan-Meier curves and log-rank test. The relative risk of acquiring MRSA was assessed using propensity score methods.43 We have calculated the propensity score of receiving enteral vancomycin treatment using a logistic regression model. The dependent binary variable was enteral vancomycin treatment and the independent variables were: age, sex, inhalation injury, mechanical ventilation, body surface area burned, and full-thickness body surface area burned. Then we performed a stratified analysis using the quartiles of the propensity scores to estimate the overall Mantel-Haenszel relative risk.
The potential overt bias caused by the differences in the percentage of imported carriers in both periods was estimated using the incidence taking colonization pressure into account described by Eveillard et al,44 which was expressed as density of acquired cases per 100 patient-days of carriers identified at admission.
The length of period 2 was considered long enough to assess consistently the efficacy trough time and the safety of long-term administration of enteral vancomycin.45
SPSS 11.5 was used for general statistical analysis and Epi-info 6 software was used to compare density incidence rates.
RESULTS
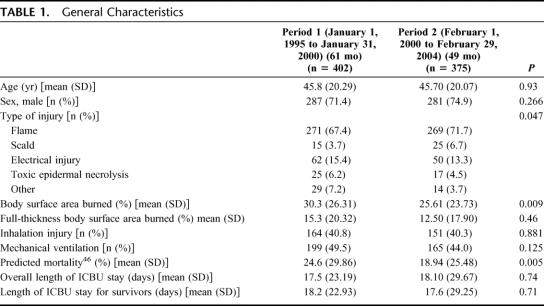
The number of patients enrolled in the study were 777: 402 in period 1 and 375 in period 2 (Table 1). There were no significant differences between the 2 patient groups, except for the total body surface area (BSA) burned, which was 4% less in the second period (P = 0.009), and for the predicted mortality,46 which was 6% lower in period 2 (P = 0.005). The observed mortality was 18.2% in period 1 compared with 10.9% in period 2 (P = 0.004).
TABLE 1. General Characteristics
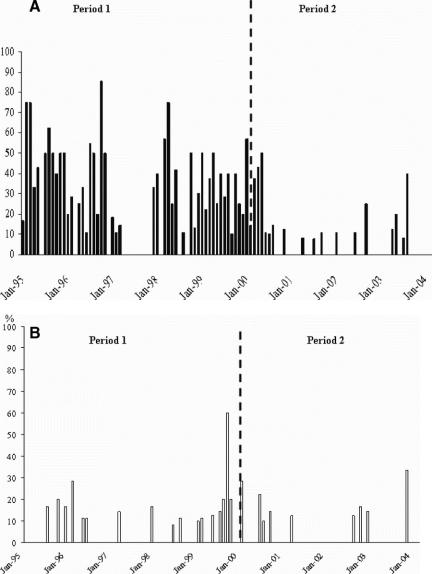
Figure 1 depicts the endemicity of MRSA in the ICBU during period 1, including 14 months (23% of the total duration of the first period) without occurrence of acquired MRSA. During the 49 months that enteral vancomycin was administered (period 2), a total of 27 months (55% of the total duration of period 2) were free from acquired MRSA. Additionally, there were no MRSA peaks in period 2.
FIGURE 1. A, Cumulative incidence rate (acquired cases) of patients positive for MRSA (surveillance, burn wound, or diagnostic samples). B, Prevalence (imported cases) of patients positive for MRSA (surveillance, burn wound, or diagnostic samples).
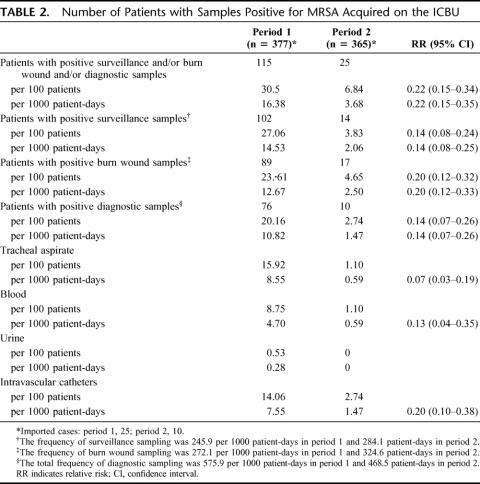
Twenty-five patients imported MRSA in period 1 and 10 in period 2, ie, 6.2% versus 2.7%, an absolute reduction of 3.5% (P = 0.03). The frequency of monthly imported cases was usually 0 or 1 case per month, except for August 1999, when there were 6 imported cases (Fig. 1). Table 2 lists the cumulative incidence and the incidence density of patients who acquired MRSA in the ICBU, as well as the relative risk of acquiring MRSA between the 2 periods. There was a significant reduction in the incidence ranging from 80% for burn wounds to 86% for surveillance and diagnostic samples.
TABLE 2. Number of Patients with Samples Positive for MRSA Acquired on the ICBU
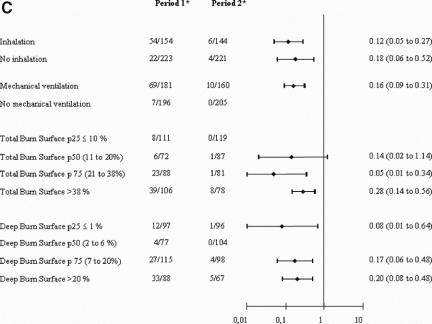
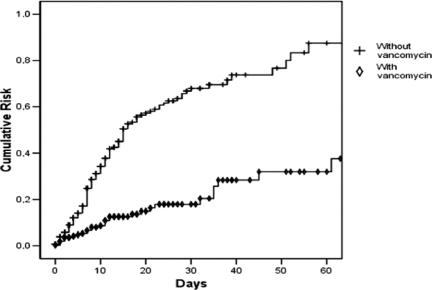
The relative risk of acquiring MRSA is consistently reduced after stratifying by inhalation injury, mechanical ventilation, and total and deep burned BSA burned (Fig. 2). The overall Mantel-Haenszel relative risk after stratifying by propensity scores quartiles was 0.25 (95% confidence interval [CI], 0.17–0.37) (P < 0.00001). Figure 3 shows that the reduction of cumulative risk of acquiring MRSA is consistent over time (P < 0.00001).
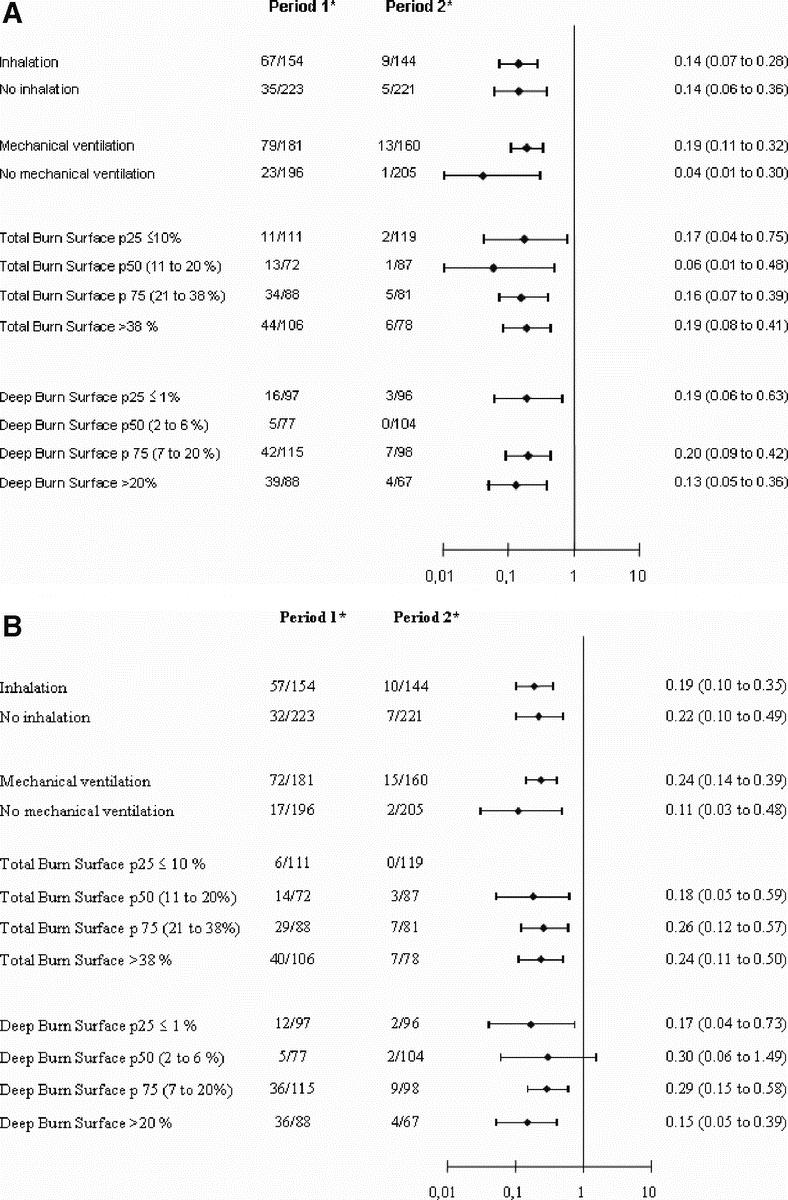
FIGURE 2. A, Patients with acquired positive surveillance samples. B, Patients with acquired positive burn samples. C, Patients with acquired positive diagnostic samples. *Patients with acquired positive surveillance samples/total acquired samples. p25, percentile 25th; p50, median; p75, percentile 75th.
FIGURE 3. Cumulative risk of acquiring MRSA (Kaplan-Meier). Log rank text, P < 0.00001.
The incidence density taking account into the colonization pressure of the MRSA carriers at admission was: 1) 18.38 in period 1 versus 4.79 in period 2 for patients with acquired positive surveillance samples (RR, 0.26; 95% CI, 0.15–0.46); 2) 16.04 versus 5.82 for patients with acquired positive burn wound samples (RR, 0.36; 95% CI, 0.22 to 0.61); and 3) 13.69 versus 3.42 for patients with acquired positive diagnostic samples (RR, 0.25; 95% CI, 0.13–0.48).
The scheduled sampling frequency for nose, throat, and gut surveillance samples was higher at 284.1 per 1000 patient-days in period 2 compared with 245.9 in period 1; similarly, the sampling frequency for burn wounds at 324.6 per 1000 patient-days was higher in the intervention period compared with the control period 1 (Table 2). In contrast, diagnostic sampling frequency decreased during the intervention period compared with the historical control period: 575.9 per 1000 patient-days in period 1 and 468.5 per 1000 patient-days in period 2. The sampling frequencies for diagnostic samples per 1000 patient-days by site, in period 1 and period 2, respectively, were: tracheal aspirate, 107.7 and 76.3; blood cultures, 144.2 and 112.3; urine 86.3 and 72.1; intravascular catheters 237.6 and 207.9. The distribution of positive diagnostic samples by site is listed in Table 2. There was a significant reduction in the incidence of positive samples for MRSA of 80% for intravascular catheters, 87% for blood samples, and 97% for tracheal aspirate, associated with the administration of enteral vancomycin
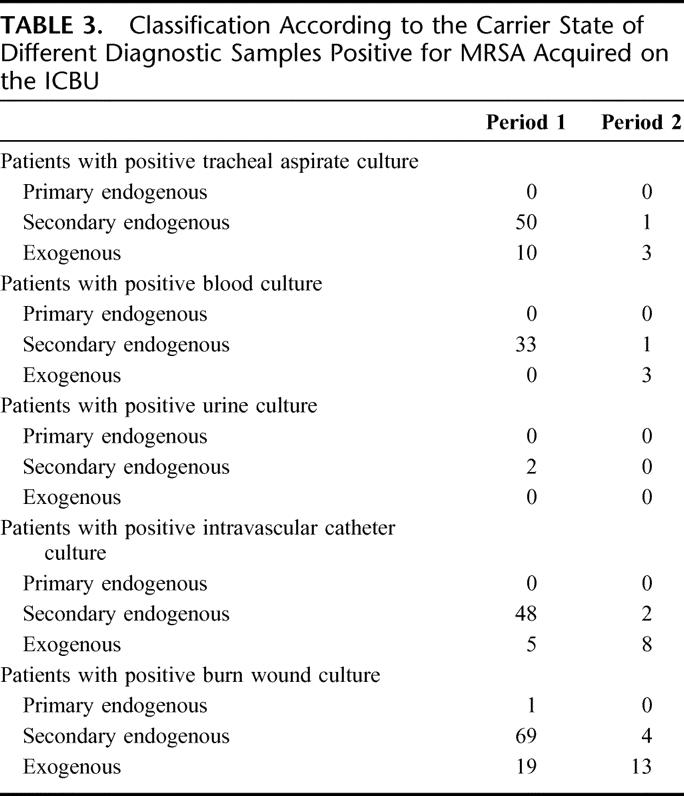
Table 3 shows the almost complete elimination of secondary endogenous MRSA samples following the introduction of enteral vancomycin. Enteral vancomycin had only a marginal impact on the exogenous MRSA burn wound samples and intravascular catheters, which were often inserted in the burn wound area.
TABLE 3. Classification According to the Carrier State of Different Diagnostic Samples Positive for MRSA Acquired on the ICBU
MRSA overgrowth was practically eradicated after the introduction of enteral vancomycin. Eighty-two patients had overgrowth without enteral vancomycin, while only 3 patients developed overgrowth following enteral vancomycin (P < 0.0001). The incidence of overgrowth was 11.7 per 1000 patient-days in period 1 and 0.44 per 1000 patient-days in period 2.
There was a relationship between overgrowth in surveillance samples and diagnostic and burn wound samples positive for MRSA, ie, the endogenous pathogenesis. Of the diagnostic samples 94% yielded MRSA once the patients developed overgrowth concentrations of MRSA in throat and/or gut. A similar high rate of 87% was observed for the burn wounds in case of overgrowth in surveillance samples. Once overgrowth was eradicated with enteral vancomycin, only one burn wound sample was found to be positive for MRSA in the endogenous samples.
There were 4 cases of VRE (1 case in 1995, 1 case in 1998, and 2 cases in 1999), all in period 1. GISA was not detected over the 9-year study period.
The use of parenteral vancomycin was 260.7 DDD per 1000 days in period 1 and 88.8 DDD per 1000 days during period 2 (RR, 0.34; 95% CI, 0.31–0.38; P < 0.0001).
DISCUSSION
Four major findings emerge from this 9-year prospective study evaluating the use of enteral vancomycin in patients with severe burns.
Significantly less burn patients acquired MRSA.
Enteral vancomycin virtually abolished MRSA overgrowth, rendering 80% of diagnostic and burn wound samples MRSA free; secondary endogenous MRSA samples disappeared with no impact on exogenous MRSA wounds and catheters.
The ICBU was free from MRSA for over half of the 5 years of the second period, while less than one fourth, ie, 14 months in the first period without enteral vancomycin; additionally, there were less acquired cases and in case of acquisition less peaks.
Neither VRE nor GISA was detected during the enteral vancomycin period in which significantly less systemic vancomycin was administered.
Illness severity is the most important independent risk factor for acquisition and subsequent carriage of abnormal flora, including MRSA.47,48 Patients who require intensive care including mechanical ventilation belong to the subset of patients who are highly susceptible for acquisition and carriage of MRSA.9 Abnormal flora is generally acquired in the oropharynx following transmission via the hands of healthcare workers. Abnormal oropharyngeal carriage of MRSA invariably leads to the abnormal gastric and intestinal carrier state of MRSA. Colonization and infection of the lower airways due to normal and abnormal flora is, in general, endogenous5,25,49 in patients who receive endotracheal ventilation. Exogenous colonization and infection, ie, bypassing the oropharynx, are significantly more frequent when ventilation is continued through a tracheostomy rather than during endotracheal ventilation.50 A similar pathogenesis of endogenous and exogenous colonization/infection applies to patients with extensive wounds, in that, potential pathogens in particular S. aureus sensitive and resistant to methicillin are directly transmitted via hands into the wounds, without previous oropharyngeal and intestinal carriage. All above-described observations of acquisition and subsequent carriage of MRSA followed by endogenous and exogenous lower airway and wound infections due to MRSA invariably apply to the severely burned (≥20%) patient requiring mechanical ventilation. Inhalation injury and the loss of the natural skin barrier promote colonization/infection of the lungs and burns due to the burn trauma-induced systemic immunoparalysis.51
The mechanism of endemicity control is based on the prevention of acquisition due to transmission, following the eradication of gut overgrowth. The concept that gut overgrowth is essential in the development of an outbreak and maintaining endemicity is in line with reported outbreaks due to extended spectrum beta-lactamase producing Klebsiella sp,52 fluconazole-resistant Candida parapsilosis,32 or MRSA.25 Reinforcement of infection control measures failed to control these outbreaks that were brought under control once enteral antimicrobials were implemented.25,32,52 In our study, enteral vancomycin was effective in clearing MRSA overgrowth as the incidence of patients with surveillance samples positive for MRSA per 1000 patients days was 2.0, compared with a 14.5 incidence during the previous period. Overgrowth eradication prevented colonization/infection of all other internal organs including lower airways and blood, and of intravascular catheter (Table 4).
The sequential design, as used in the present study, is the accepted method to assess the efficacy of an intervention in the control of outbreaks. However, the sequential design does not control for changes in the patient population, care procedures, and natural dynamics of epidemic strain transmission over a period of 9 years. While our study suggests that enteral vancomycin played a central role of decreasing MRSA incidence and infection, due to the design of this study and the natural “wax and wane” dynamics of MRSA colonization and infection, we think that there is cause-and-effect relationship. The first episode of MRSA endemicity lasted for more than 2 years and came to an end after 5 months, after rigid reinforcement of the 5 infection control maneuvers. However, not for long, the ICBU faced a second episode of MRSA endemicity (Fig. 1). Despite major efforts using the conventional infection control maneuvers, the MRSA endemicity continued until enteral vancomycin was added to the enteral component of the classic SDD protocol. After 4 months of enteral vancomycin, our ICBU was free from MRSA and until now remains so. Although we cannot provide a definitive proof for the effects of enteral vancomycin, these temporal trends are better explained by the administration of enteral vancomycin than by the natural evolution of MRSA outbreaks.
The demographics of our patient population show an imbalance in the distribution of risk factors for MRSA acquisition, in that, in period 2 patients experienced a decreased illness severity as indicated by less extensive burns and lower predicted mortality. The impact of these potential confounding factors on the results was assessed by stratified (Fig. 2) and multivariate analysis. Both analyses demonstrate that the effect of enteral vancomycin is independent from these variables. Additionally, the cumulative risk for MRSA acquisition was consistently reduced over time (Fig. 3).
The risk of acquisition of resistant flora in the ICU is in part related to the number of patients who carry resistant flora when admitted to the ICU. In our study, there was a marginal absolute reduction of 3.5% of imported MRSA cases in period 2. We do not think that this small decrease explains the magnitude of the risk reduction found in association with the administration of enteral vancomycin. Moreover, the MRSA acquisition incidence adjusted by the imported patient-days confirms a reduction in period 2, with a relative risk ranging from 0.26 in surveillance samples to 0.36 in burn wound samples.
Changes in the sampling frequency could modify the estimation of the incidence of MRSA acquisition. However, in this study the probability of identifying MRSA carriers actually increased in period 2 as the scheduled sampling frequency for both nose, throat, and gut surveillance samples and burn wound samples was higher in period 2 compared with period 1. Therefore, the reduction in the risk of acquiring MRSA in period 2 is not related to a reduction in the frequency of sampling.
During period 1, 53 (13%) patients received SDD, whereas during period 2 all patients received SDD with enteral vancomycin added. One can argue that the observed decrease in MRSA infection and colonization is due to the more generalized use of SDD in the second period rather than to vancomycin itself. By design, SDD using enteral polymyxin and tobramycin is active against aerobic Gram-negative bacteria but not against MRSA. Indeed, it has been suggested that SDD increases the risk of colonization and infection by MRSA. Of the 55 RCTs assessing SDD, 6 report a nonsignificant trend toward an increased incidence of MRSA infections.28,53–57 These RCTs were conducted in units where MRSA was endemic at the time of the trial. Therefore, the expected effect of SDD administration in period 2 in our study, in a setting with MRSA endemicity, would be an increase in the risk of MRSA acquisition. Hence, the decrease in MRSA acquisition is not explained by the use of SDD with polymyxin and tobramycin, but by the addition of vancomycin.
Although the compliance with the barrier maneuvers was not assessed in our study, it is very unlikely that a change in the level of compliance during a 9-year period can explain the reduction in MRSA acquisition. Exogenous samples are samples positive for MRSA without previous carriage and reflect the standard of hygiene. The changes in this type of sample between period 1 and 2 were marginal supporting no major changes in hygiene practices. Moreover, the virtual absence of secondary endogenous samples suggests that MRSA transmission was abolished from the ICBU only when enteral vancomycin was introduced.
VRE and GISA were not encountered during the 4 year period 2, supporting the safety of the addition of enteral vancomycin to SDD. The absence of VRE in this study is in keeping with previous investigations, which fail to show an increased incidence of VRE associated with the administration of enteral vancomycin as part of the SDD regimen.24,25,58–63 This observation is also in line with recent work that shows that systemic broad spectrum antibiotics that disregard the gut ecology, rather than high doses of enteral vancomycin, or even parenteral vancomycin, promote VRE.64–66
CONCLUSION
Enteral vancomycin is useful and safe when MRSA endemicity is not controlled with other measures and when SDD is used in a critical care setting with MRSA endemicity. This intervention requires further testing in hospitals and ICBUs with endemic VRE.
ACKNOWLEDGMENTS
The authors thank Mr. Christian Duncan and Ms. Nia Taylor for carefully reviewing the manuscript.
Footnotes
Supported by two grants from Fondo de Investigación Sanitaria: FIS 02/1883 and Red Respira C 03/11. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Reprints will not be available from the authors.
Correspondence: Dr. Miguel A. de la Cal, MD, Department of Critical Care Medicine, Hospital Universitario de Getafe, Carretera de Toledo Km 12,5, Madrid, Spain 28905. E-mail: mcal@ucigetafe.com.
REFERENCES
- 1.Deitch EA. The management of burns. N Engl J Med. 1990;323:1249–1253. [DOI] [PubMed] [Google Scholar]
- 2.Pruitt BJ, McManus AT. The changing epidemiology of infection in burn patients. World J Surg. 1992;16:57–67. [DOI] [PubMed] [Google Scholar]
- 3.O'Sullivan ST, O'Connor TPF. Immunosuppression following thermal injury: the pathogenesis of immunodysfunction. Br J Plast Surg. 1997;50:615–623. [DOI] [PubMed] [Google Scholar]
- 4.Rue LW III, Cioffi WG, Mason AD Jr, et al. The risk of pneumonia in thermally injured patients requiring ventilatory support. J Burn Care Rehabil. 1995;16:262–268. [DOI] [PubMed] [Google Scholar]
- 5.de la Cal MA, Cerda E, Garcia-Hierro P, et al. Pneumonia in patients with severe burns: a classification according to the concept of the carrier state. Chest. 2001;119:1160–1165. [DOI] [PubMed] [Google Scholar]
- 6.Mayhall CG. The epidemiology of burn wound infections: then and now. Clin Infect Dis. 2003;37:543–550. [DOI] [PubMed] [Google Scholar]
- 7.Cerdá E, de la Cal MA, García Hierro P. Infection in burn patients. In: Rello J, Valles J, Kollef M, eds. Critical Care Infection Diseases Textbook. Boston: Kluwer Academic; 2001:845–859. [Google Scholar]
- 8.Edwards-Jones V, Greenwood JE. On behalf of the Manchester Burns Research Group: what's new in burn microbiology. Burns. 2003;29:15–24. [DOI] [PubMed] [Google Scholar]
- 9.Hardy KJ, Hawkey PM, Gao F, et al. Methicillin-resistant Staphylococcus aureus in the critically ill. Br J Anaesthesia. 2004;92:121–130. [DOI] [PubMed] [Google Scholar]
- 10.Phillips LG, Heggers JP, Robson MC. Burn and trauma units as sources of methicillin-resistant Staphylococcus aureus. J Burn Care Rehabil. 1992;13:293–297. [DOI] [PubMed] [Google Scholar]
- 11.Taylor GD, Kibsey P, Kirkland T, et al. Predominance of Staphylococcal organisms in infections occurring in a burns intensive care unit. Burns. 1992;18:332–335. [DOI] [PubMed] [Google Scholar]
- 12.Oncul O, Yuksel F, Altunay H, et al. The evaluation of nosocomial infection during 1-year-period in the burn unit of a training hospital in Istanbul, Turkey. Burns. 2002;28:738–744. [DOI] [PubMed] [Google Scholar]
- 13.Reardon CM, Brown TP, Stephenson AJ, et al. Methicillin-resistant Staphylococcus aureus in burn patients: why all the fuss? Burns. 1998;24:393–397. [DOI] [PubMed] [Google Scholar]
- 14.Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect Control Hosp Epidemiol. 2003;24:362–386. [DOI] [PubMed] [Google Scholar]
- 15.Farrington M, Ling J, Ling T, et al. Outbreaks of infection with methicillin-resistant Staphylococcus aureus on neonatal and burns units of a new hospital. Epidemiol Infect. 1990;105:215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danchivijitr S, Chokloikaew S, Chantrasakul C, et al. An outbreak of methicillin-resistant Staphylococcus aureus (MRSA) in a burn unit. J Med Assoc Thai. 1995;78(suppl 1):11–14. [PubMed] [Google Scholar]
- 17.Meier PA, Carter CD, Wallace SE, et al. A prolonged outbreak of methicillin-resistant Staphylococcus aureus in the burn unit of a tertiary medical center. Infect Control Hosp Epidemiol. 1996;17:798–802. [DOI] [PubMed] [Google Scholar]
- 18.Bang RL, Sharma PN, Sanyal SC, et al. Burn septicaemia in Kuwait: associated demographic and clinical factors. Med Princ Pract. 2004;13:136–141. [DOI] [PubMed] [Google Scholar]
- 19.Silvestri L, Petros AJ, Sarginson RE, et al. Handwashing in the intensive care unit: a big measure with modest effects. J Hosp Infect. 2005;59:172–179. [DOI] [PubMed] [Google Scholar]
- 20.Cooper BS, Stone SP, Kibbler CC, et al. Isolation measures in the hospital management of methicillin resistant Staphylococcus aureus (MRSA): systematic review of the literature. BMJ. 2004;329:533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cepeda JA, Whitehouse T, Cooper B, et al. Isolation of patients in single rooms or cohorts to reduce spread of MRSA in intensive-care units: prospective two-centre study. Lancet. 2005;365:295–304. [DOI] [PubMed] [Google Scholar]
- 22.Matsumura H, Yoshizawa N, Narumi A, et al. Effective control of methicillin-resistant Staphylococcus aureus in a burn unit. Burns. 1996;22:283–286. [DOI] [PubMed] [Google Scholar]
- 23.Loeb M, Main C, Walker-Dilks C, Eady A. Antimicrobial drugs for treating methicillin-resistant Staphylococcus aureus colonization. Cochrane Database Syst Rev. 2003. [DOI] [PubMed] [Google Scholar]
- 24.Silvestri L, Milanese M, Oblach L, et al. Enteral vancomycin to control methicillin-resistant Staphylococcus aureus outbreak in mechanically ventilated patients. Am J Infect Control. 2002;30:391–399. [DOI] [PubMed] [Google Scholar]
- 25.de la Cal MA, Cerda E, van Saene HK, et al. Effectiveness and safety of enteral vancomycin to control endemicity of methicillin-resistant Staphylococcus aureus in a medical/surgical intensive care unit. J Hosp Infect. 2004;56:175–183. [DOI] [PubMed] [Google Scholar]
- 26.Hospital Infection Control Practices Advisory Committee. Garner JS, Guideline for isolation precautions in hospitals. Infect Control Hosp Epidemiol. 1996;17:53–80. [DOI] [PubMed] [Google Scholar]
- 27.British Society for Antimicrobial Chemotherapy, Hospital Infection Society and the Infection Control Nurses Association. Revised guidelines for the control of methicillin-resistant Staphylococcus aureus infection in hospitals. J Hosp Infect. 1998;39:253–290. [DOI] [PubMed] [Google Scholar]
- 28.de la Cal MA, Cerdá E, García-Hierro P, et al. Survival benefit in critically ill burned patients receiving selective decontamination of the digestive tract: a randomized, placebo controlled, double blind trial. Ann Surg. 2005;241:424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendrix CW, Hammond JM, Swoboda SM, et al. Surveillance strategies and impact of vancomycin-resistant enterococcal colonization and infection in critically ill patients. Ann Surg. 2001;233:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Department of Health and Human Services. Centers for Disease Control and Prevention. Vancomycin-intermediate/resistant Staphylococcus aureus laboratory testing algorithm. Available at: http://www.cdc.gov/ncidod/dhqp/ar_visavrsa_algo.html. Accessed February 7, 2006.
- 31.Anonymous. Intensive care antimicrobial resistance epidemiology (ICARE) surveillance report, data summary from January 1996 through December 1997. Am J Infect Control. 1999;27:279–284. [DOI] [PubMed] [Google Scholar]
- 32.Damjanovic V, Connolly CM, van Saene HK, et al. Selective decontamination with nystatin for control of a Candida outbreak in a neonatal intensive care unit. J Hosp Infect. 1993;24:245–259. [DOI] [PubMed] [Google Scholar]
- 33.van Uffelen R, van Saene HKF, Fidler V, et al. Oropharyngeal flora as a source of bacteria colonizing the lower airways in patients on artificial ventilation. Intensive Care Med. 1984;10:233–237. [DOI] [PubMed] [Google Scholar]
- 34.Sarginson RE, Taylor N, van Saene HKF. Glossary of terms and definitions. Curr Anaesth Crit Care. 2001;12:2–5. [Google Scholar]
- 35.Leonard EM, van Saene HKF, Shears P, et al. Pathogenesis of colonization and infection in a neonatal surgical unit. Crit Care Med. 1990;18:264–269. [DOI] [PubMed] [Google Scholar]
- 36.Performance standards for antimicrobial susceptibility testing; eighth informational supplement. NCCLS. 1998;18:M100–S8. [Google Scholar]
- 37.Performance standards for antimicrobial susceptibility testing; twelfth informational supplement. NCCLS. 2002;22:M100–S12. [Google Scholar]
- 38.Performance standards for antimicrobial susceptibility testing. NCCLS. 1999:19: M100-S9. [Google Scholar]
- 39.Isenberg HD. Clinical Microbiology Procedures Handbook, 1st ed. Washington, DC: ASM Press, 1994. [Google Scholar]
- 40.Maki DG, Weise CE, Sarafin HW. A semiquantitative culture method for identifying intravenous catheter-related infections. N Engl J Med. 1977;296:1305–1309. [DOI] [PubMed] [Google Scholar]
- 41.Bernard PM, Lapointe C. Mesures statistiques en épidémiologie, Québec: Presses de l'Université du Québec, 1987. [Google Scholar]
- 42.Hennekens CH, Buring JE. Measures of disease frequency and association. Analysis of epidemiologic studies: evaluating the role of chance. In: Mayrent SL, ed. Epidemiology in Medicine, 1st ed. Boston: Little, Brown; 1987:77–96, 254–257. [Google Scholar]
- 43.Rubin DB. Estimating causal effects from large data sets using propensity scores Ann Intern Med. 1997;127:757–763. [DOI] [PubMed] [Google Scholar]
- 44.Eveillard M, Lancien E, Hidri N, et al. Estimation of methicillin-resistant Staphylococcus aureus transmission by considering colonization pressure at the time of hospital admission. J Hosp Infect. 2005;60:27–31. [DOI] [PubMed] [Google Scholar]
- 45.Cunha BA. Effective antibiotic-resistance control strategies. Lancet. 2001;357:1307–1308. [DOI] [PubMed] [Google Scholar]
- 46.Smith DL, Cairns BA, Ramadan F, et al. Effect of inhalation injury, burn size, and age on mortality: a study of 1447 consecutive burn patients J Trauma. 1994;37:655–659. [DOI] [PubMed] [Google Scholar]
- 47.Everett ED, McNitt TR, Rahm AE, et al. Epidemiologic investigation of methicillin-resistant Staphylococcus aureus in a burn unit. Mil Med. 1978;143:165–167. [PubMed] [Google Scholar]
- 48.Chang FY, Singh N, Gayowski T, et al. Staphylococcus aureus nasal colonization in patients with cirrhosis: prospective assessment of association with infection. Infect Control Hosp Epidemiol. 1998;19:328–332. [DOI] [PubMed] [Google Scholar]
- 49.Cook DJ, Walter SD, Cook RJ, et al. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med. 1998;129:433–440. [DOI] [PubMed] [Google Scholar]
- 50.Morar P, Singh V, Makura Z, Jones A, et al. Differing pathways of lower airway colonization and infection according to mode of ventilation [endotracheal vs tracheostomy]. Arch Otolaryngol Head Neck Surg. 2002;128:1061–1066. [DOI] [PubMed] [Google Scholar]
- 51.Wassermann D. Systemic complications of extended burns. Ann Chir Plast Esthet. 2001;46:196–209. [DOI] [PubMed] [Google Scholar]
- 52.Brun-Buisson C, Legrand P, Rauss A, et al. Intestinal decontamination for control of nosocomial multiresistant gram-negative bacilli: study of an outbreak in an intensive care unit. Ann Intern Med. 1989;110:873–881. [DOI] [PubMed] [Google Scholar]
- 53.Gastinne H, Wolff M, Delatour F, et al. A controlled trial in intensive care units of selective decontamination of the digestive tract with non-absorbable antibiotics. N Engl J Med. 1992;326:594–599. [DOI] [PubMed] [Google Scholar]
- 54.Hammond JM, Potgieter PD, Saunders GL, et al. Double-blind study of selective decontamination of the digestive tract in intensive care. Lancet. 1992;340:5–9. [DOI] [PubMed] [Google Scholar]
- 55.Ferrer M, Torres A, Gonzalez J, et al. Utility of selective digestive decontamination in mechanically ventilated patients. Ann Intern Med. 1994;120:389–385. [DOI] [PubMed] [Google Scholar]
- 56.Lingnau W, Berger J, Javorsky F, et al. Selective intestinal decontamination in multiple trauma patients: prospective, controlled trial. J Trauma. 1997;42:687–694. [DOI] [PubMed] [Google Scholar]
- 57.Verwaest C, Verhaegen J, Ferdinande P, et al. Randomized controlled trial of selective digestive decontamination in 600 mechanically ventilated patients in a multi-disciplinary intensive care unit. Crit Care Med. 1997;25:63–71. [DOI] [PubMed] [Google Scholar]
- 58.Pugin J, Auckenthaler R, Lew DP, et al. Oropharyngeal decontamination decreases incidence of ventilator- associated pneumonia: a randomized, placebo-controlled, double-blind clinical trial. JAMA. 1991;265:2704–2710. [PubMed] [Google Scholar]
- 59.Gaussorgues P, Salord F, Sirodot M, et al. Efficacité de la décontamination digestive sur la survenue des bactériémies nosocomiales chez les patients sous ventilation méchanique et recevant des betamimétiques. Réanimation Soins Intensifs Médecin d'Urgence. 1991;7:169–174. [Google Scholar]
- 60.Korinek AM, Laisne MJ, Nicolas MH, et al. Selective decontamination of the digestive tract in neurosurgical intensive care unit patients: a double-blind, randomized, placebo-controlled study. Crit Care Med. 1993;21:1466–1473. [DOI] [PubMed] [Google Scholar]
- 61.Schardey HM, Joosten U, Finke U, et al. The prevention of anastomotic leakage after total gastrectomy with local decontamination: a prospective, randomized, double-blind, placebo-controlled multicenter trial. Ann Surg. 1997;225:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bergmans DC, Bonten MJ, Gaillard CA, et al. Prevention of ventilator-associated pneumonia by oral decontamination: a prospective, randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med. 2001;164:382–388. [DOI] [PubMed] [Google Scholar]
- 63.Krueger WA, Lenhart FP, Neeser G, et al. Influence of combined intravenous and topical antibiotic prophylaxis on the incidence of infections, organ dysfunctions, and mortality in critically ill surgical patients: a prospective, stratified, randomized, double-blind, placebo-controlled clinical trial. Am J Respir Crit Care Med. 2002;166:1029–1037. [DOI] [PubMed] [Google Scholar]
- 64.McManus AT, Goodwin CW, Pruitt BA. Observation on the risk of resistance with extended use of vancomycin. Arch Surg. 1998;133:1207–1211. [DOI] [PubMed] [Google Scholar]
- 65.Stiefel U, Paterson DL, Pultz NJ, et al. Effect of the increasing use of piperacillin/tazobactam on the incidence of vancomycin-resistant enterococci in four academic medical centers. Infect Control Hosp Epidemiol. 2004;25:380–383. [DOI] [PubMed] [Google Scholar]
- 66.Salgado CD, Giannetta ET, Farr BM. Failure to develop vancomycin-resistant Enterococcus with oral vancomycin treatment of Clostridium difficile. Infect Control Hosp Epidemiol. 2004;25:413–417. [DOI] [PubMed] [Google Scholar]