Abstract
Objective:
To explore the role of surgery of residual disease following a period of therapy with imatinib mesylate in advanced gastrointestinal stromal tumors (GIST).
Methods:
From January 2001 to June 2005, 159 patients with advanced/metastatic GIST were treated with imatinib mesylate at a single institution. As of June 2002, 38 patients were selected for surgery following a variable period of imatinib therapy. Twenty-seven patients were operated on while they were in response, 8 in progression, 3 for localized disease. Clinical, pathologic, and molecular features were assessed and are reported.
Results:
Postsurgery PFS was 96% at 12 months and 69% at 24 months for responding patients, while it was nil at 12 months for progressing ones. Disease-specific survival at 12 months was 100% for responding patients and 60% for progressing ones. In responding cases, secondary progression was mainly related to postsurgical imatinib discontinuation, irrespective of pathologic or molecular variables. In progressing patients, secondary resistance was mainly related to acquired mutations.
Conclusion:
In advanced GIST patients who are responding to imatinib mesylate, the role of surgery is not formally demonstrated at the moment, but this option may well be considered investigational, or suitable for an individualized decision-making in the lack of evidence. In our series, patients progressing on imatinib mesylate did not seem to have any major benefit from surgery, although their number is low.
In this series of 38 patients affected by advanced gastrointestinal stromal tumors treated by surgery of residual disease after a period of therapy with imatinib mesylate, progression-free survival was 69% at 2 years for patients responding to the medical therapy and 0% at 1 year for those operated on with a focal/generalized progression.
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal malignancies of the gastrointestinal tract. In most series before the imatinib era, some advanced patients were offered surgical resection of their liver or peritoneal disease, with reportedly poor results.1 Currently, imatinib mesylate (IM) has become the standard therapy for recurrent/metastatic disease.2–4 Two large, randomized, phase III trials have reported the activity and efficacy of IM in advanced GIST patients, both in terms of progression-free and overall survival.5,6
The major limitation of such a highly effective therapy has been the development of secondary resistance. Primary resistance refers to patients who do not achieve any response, or stable disease. There is clear evidence that tumors with KIT mutations other than to exon 11, such as mutations to exon 9, 13, and 17, or no detectable kinase mutation (wild-type kit), are overrepresented in this group of nonresponders.7,8 Secondary progression is often related to acquired mutations, which differ in type from the primary ones.9,10
Progressing patients have undergone surgery of evolving disease as from the earliest cases observed, even because progression often seemed to affect only a portion of the disease. Then, the idea was to anticipate surgery of residual disease at a time in which progression has not developed yet, under the assumption that it might prevent, or delay, the occurrence of resistant clones. Surgery of residual disease has therefore been progressively more and more used as from 2002. This retrospective analysis provides data about the outcome of patients undergoing surgery of residual disease at our institution.
MATERIALS AND METHODS
Patients
A total of 159 patients with advanced and/or metastatic GIST were referred at the Istituto Nazionale per lo Studio e la Cura dei Tumori (Milan, Italy) between January 2001 and June 2005, and treated with IM within an EORTC-STBSG led intergroup trial,5 and then within a Southern European Phase II study,11 or according to the standard of care. In all cases, the diagnosis of GIST was confirmed in terms of morphology and immunophenotyping.
Since June 2002, all patients on IM were considered for surgical treatment of residual disease by the multidisciplinary sarcoma board, which eventually selected the following categories of patients.
Patients on medical therapy for at least 12 months, achieving stable disease, partial response or complete response, if a complete resection could be foreseen. For the purpose of this analysis, these patients will be identified as group A (“patients in response”).
Patients on medical therapy with either primary or secondary resistance, documented by at least 2 consecutive follow-up imaging procedures. For the purpose of this analysis, these patients will be identified as group B (“patients in progression”).
Patients with bulky primary GIST candidated to demolitive major surgery, if surgical resection could have been modified by a major tumor shrinkage. For the purpose of this analysis, these patients will be identified as group C (“cytoreductive treatment”).
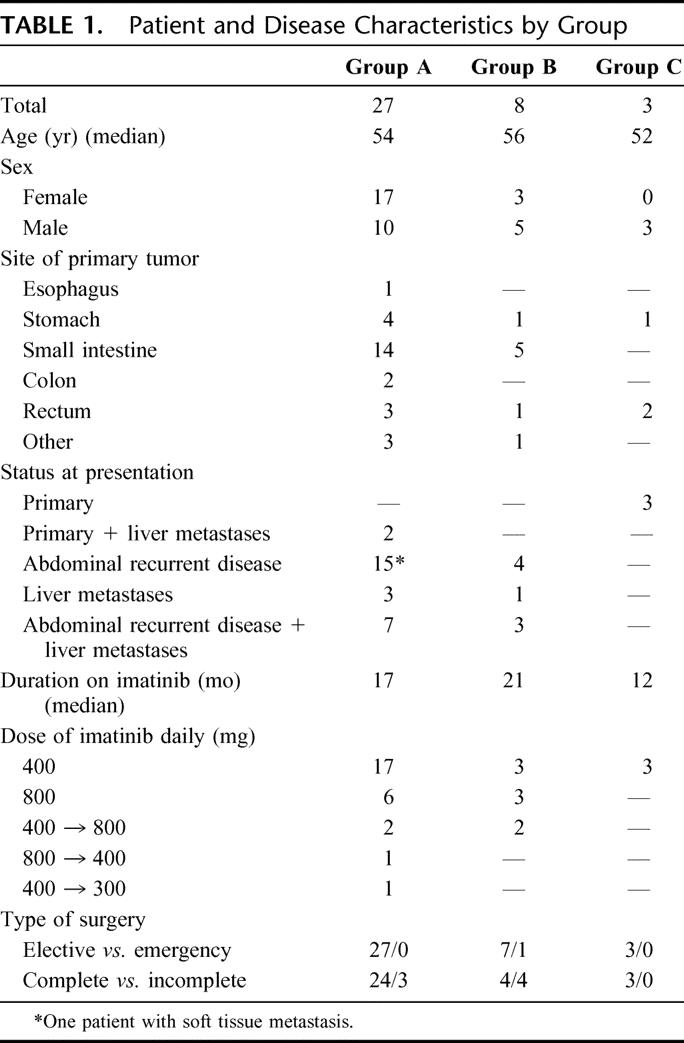
Clinical features of this series, encompassing the above groups, are detailed in Table 1.
TABLE 1. Patient and Disease Characteristics by Group
Standard surgical approach consisted in a midline laparotomy. Hepatic staging by intraoperative ultrasound was performed in all cases. Gross disease was completely removed with as limited visceral resections as possible. Complete omentectomy was routinely performed in all cases, with evidence of peritoneal disease. Hepatic lesions were resected whenever feasible with limited extra-anatomic metastasectomy. Radiofrequency ablation was performed on deep-seated liver metastases to avoid major hepatectomies.
Patients were prospectively followed up, with complete staging every 3 months.
Clinical follow-up of the patients was updated to October 2005, with a median follow-up of 29 months for group A, 12 months for group B, and 21 months for group C.
Disease-specific survival and progression-free survival were estimated by Kaplan-Meier method and calculated both from the time of IM onset and from the time of surgery.
Preliminary data from this series were presented during ASCO Annual Meeting 2005.12
Pathologic Classification of the Response
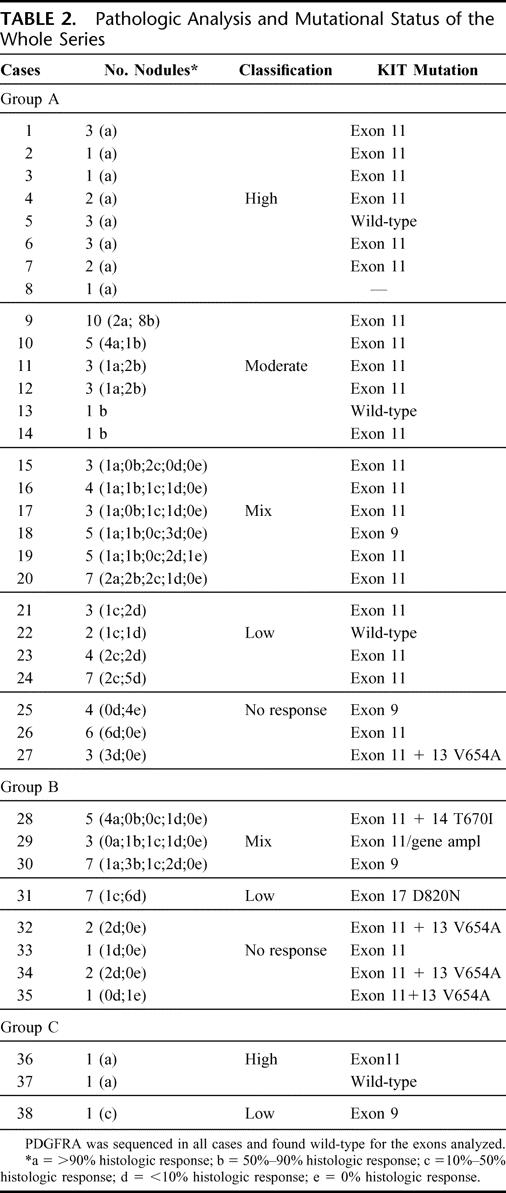
Assessment of pathologic responses was performed on post-IM surgical specimens and was based on microscopic findings. Response was codified for each excised tumor nodule according to previously reported criteria,10 while adding one class of response (identified as mixed). In brief, the response was classified as follows (the percentage of nonvital tissue is put in parentheses): high (>90%), moderate (50%–90%), low (10%–50%), no response (0%–10%), and mixed (from 0% up to >90%). Scores of response are detailed in Table 2.
TABLE 2. Pathologic Analysis and Mutational Status of the Whole Series
Molecular Analysis
The most representative areas of responding and/or progressing lesions of post-IM tumor specimens were selected.
DNA was extracted from formalin-fixed, paraffin-embedded tumors as previously described and amplified for KIT and for PDGFRA, as reported elsewhere.13,14 DNA sequencing was performed following standard procedures with an automated 377 DNA sequencer ABIPRISM-PE, Applied Biosystem (Foster City, CA).
Fifty-one samples from 37 patients (2 different nodules were analyzed in 14 patients) were molecularly characterized by performing DNA sequencing of exons 9, 11, 13, 14, and 17 of c-KIT gene, and of exons 12, 14, and 18 of PDGFRA. One case in group A was excluded since there were not enough viable tumoral cells throughout all tumor specimens (patient 8 in Table 2).
FISH Analysis on Cytologic Specimens: Touch Imprints
All cases from group B were analyzed for KIT and PDGFRA gene amplification. Slides were placed under running water for few seconds, air dried, and then fixed, as previously reported.15 BAC clones RP11-231C18 (PDGFRα gene, 4q12) and RP11-586A2 (cKIT gene, 4q12) were used as FISH probes. All BAC clones were kindly provided by Dr M. Rocchi (Resources for Molecular Cytogenetics, University of Bari, Bari, Italy).
FISH on Tissue Sections
All probe mixtures were used on 5-μm thin section, as previously reported.15
RESULTS
As of June 2002, 38 patients were considered eligible for surgery and operated on at our institution, and they constitute the basis of the present study. Twenty-seven patients were considered in group A, 8 in group B, and 3 in group C.
Overall Survival and Progression-Free Survival
Group A (27 Patients)
Median preoperative IM duration was 17 months (range, 7–39 months). Best clinical response at the time of surgery was CR in 7 patients, PR in 18 patients, SD in 1 patient, and liver CR along with peritoneal PR in 1 patient.
On surgery, all patients were found to have remnants of disease in the abdominal cavity/liver, irrespective of any radiologic PR or CR. Intraoperative staging revealed multiple peritoneal implants in 15 cases (55%), with subcentimetric nodules, which were underestimated by radiologic assessments.
In 3 cases (11%), complete surgery could not be performed because of diffuse peritoneal seeding in 2 patients and inoperable liver metastasis in 1 case. All other 24 patients had a macroscopically complete cytoreduction, which encompassed removal of peritoneal implants in 8 cases, removal of peritoneal implants + intestinal resections in 7 cases (small bowel in 5 cases, colon in 2 cases), 5 hepatic resections (liver metastases were single in 2 cases and multiple in 3), removal of peritoneal implants + intestinal resections (small bowel) and hepatic resections (single nodule) in 2 cases, removal of peritoneal implants and radiofrequency ablation of one liver nodule in 1 case and removal of peritoneal implants + resection of 1 liver nodule and radiofrequency ablation of one other liver nodule in 1 case.
After a median follow-up of 29 months (range, 6–36 months) from the time of surgery, DSS was 100% at 2 years, and PFS was 96% and 69% at 1 and 2 years, respectively (Figs. 1, 2). When considering the same variables from the time of onset of IM therapy, after a median follow-up of 47 months (range, 22–58 months), DSS was 100% at 4 years and PFS was 85%, and 72% at 3 and 4 years, respectively (Figs. 3, 4).
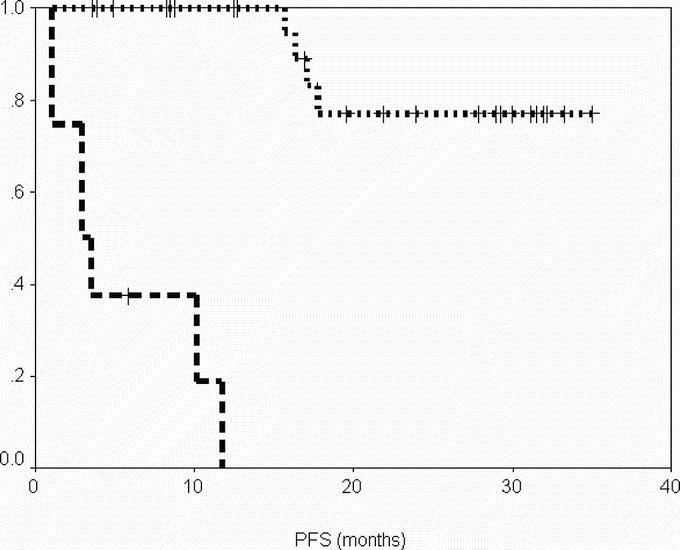
FIGURE 1. Progression-free survival from date of surgery according to group. Group A (patients operated in response), dotted line; group B (patients operated in progression), dashed line.
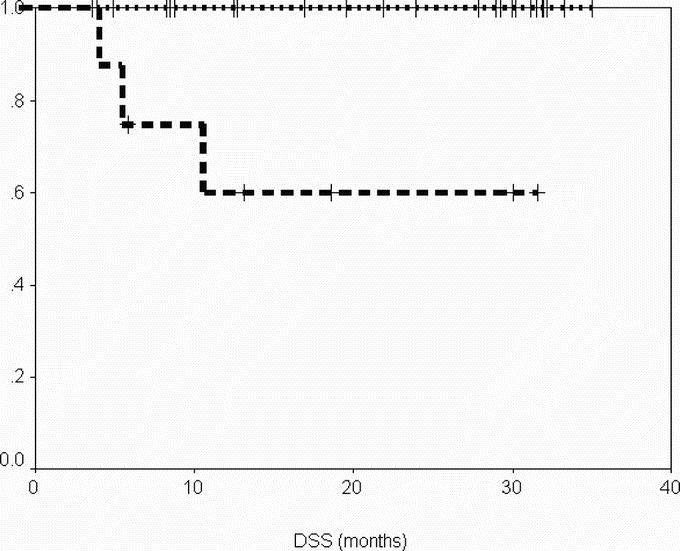
FIGURE 2. Disease-specific survival from date of surgery according to group. Group A (patients operated in response), dotted line; group B (patients operated in progression), dashed line.
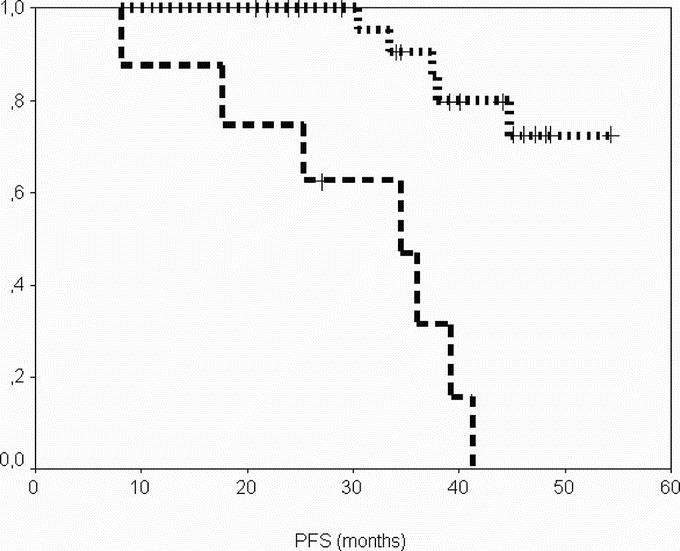
FIGURE 3. Progression-free survival from the onset of imatinib according to group. Group A (patients operated in response), dotted line; group B (patients operated in progression), dashed line.
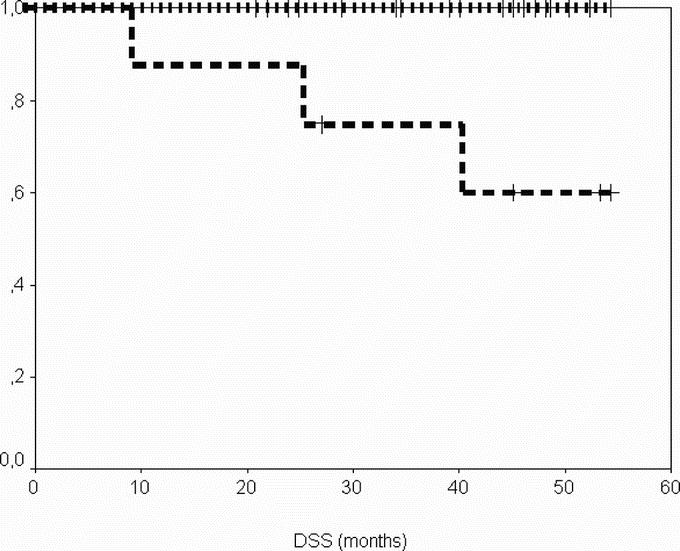
FIGURE 4. Disease-specific survival from the onset of imatinib according to group. Group A (patients operated in response), dotted line; group B (patients operated in progression), dashed line.
Six patients had progressive disease at 9, 16, 16, 17, 17, and 18 months after surgery.
Three patients discontinued IM postoperatively because of drug intolerance (2 cases) and intercurrent chemotherapy for newly diagnosed breast carcinoma (1 patient) after 5, 13, and 18 months. Two of them recurred 3 and 11 months after IM withdrawal, and could be retreated with IM on progression. They are presently alive with disease in response.
Only 4 patients progressed out of the 24 who did not discontinue IM postoperatively. All of them had to be treated with a second line targeted drug, after having increased the IM dose to 800 mg without success. Interestingly, 1 of these patients had and adjunctive mutation in exon 13, responsible for V654A amino acidic substitution. This secondary point mutation was detected only in the tumoral area with histologic/morphologic features of nonresponding disease (with <10% of response), not otherwise detected by radiologic and clinical evaluation. This patient relapsed in the abdomen 17 months after surgery, while on imatinib 400 mg daily.
Group B (8 Patients)
Median preoperative time on IM was 21 months (range, 6–39 months). One patient with primary resistance had a generalized progression and underwent surgery 6 months after the onset of IM therapy. All the remaining 7 patients with secondary resistance had isolated partial progression after evidence of response. Two patients had 2 lesions in progression. Among these patients, 1 had had a PR and 1 an SD. Five cases had only one lesion in progression (an abdominal nodule or a liver nodule in 2 cases and a local recurrence of a rectal GIST with bladder invasion in 1 case).
All patients in group B underwent elective surgery but 1, who was operated on as an emergency procedure for hemoperitoneum, which was due to a rupture of an abdominal mass not present at routine follow-up evaluation 3 months earlier.
In 4 of 8 cases (50%), surgery consisted only in the removal of progressing disease, because of diffuse peritoneal implants (2 cases) or inoperable liver metastases (2 cases). The other 4 cases had a complete cytoreduction, including both responding and progressing lesions, which encompassed the removal of peritoneal disease + colonic resections in 2 cases, hepatic resection in 1 case, and pelvic exenteratio in 1 case.
After a median follow up of 12 months (range, 4–32 months) from time of surgery, DSS was 60% at 1 and 2 years, and PFS was 0% at 1 year (Figs. 1, 2). When considering the same variables from the time of onset of IM therapy, after a median follow-up of 26 months (range, 10–56 months), DSS was 60% at 4 years, and PFS was 30% and 0% at 3 and 4 years, respectively.
Seven of 8 patients relapsed, and median time to progression was 3 months (range, 1–12 months). The only patient who did not progress had a local recurrence of a rectal GIST and is presently alive without evidence of further progression 8 months after surgery.
Group C (3 Patients)
In all 3 patients, IM was administered at the dose of 400 mg for 12 months. Two patients had evidence of clinical PR, while 1 patient had only a minor response, with symptomatic relief. There were one large gastric GIST and 2 rectal GISTs. All patients could undergo conservative surgery. The patient with a gastric mass had only a minor gastric resection, with negative margins. The other 2 patients, who had a low rectal GIST just above the anal canal, could be successfully operated conservatively. All of them continued IM for 12 months postoperatively, and they are alive and disease-free after a median follow-up of 21 months.
Pathologic Response and Molecular Analysis
Table 2 enlists some results in more details.
DISCUSSION
In this series of patients with advanced GIST undergoing surgery of residual disease after various periods of therapy with IM, PFS from the onset of imatinib therapy was 85% at 3 years, and 72% at 4 years, for patients responding to the medical therapy, whereas it was 30% at 3 years and nil at 4 years for progressing ones.
Based on these data alone, it is obviously impossible to state whether surgery of residual disease makes some difference. In principle, surgery of residual disease might delay the development of resistant clones by reducing the tumor burden, at least resulting in a prolongation of time to progression.16 However, this needs to be confirmed empirically. The ideal way to do so would be a prospective clinical trial randomizing between surgery and no surgery. No such trial is open today, and a randomized answer cannot be expected in the forthcoming future. For the moment, therefore, we must base our clinical practice on uncontrolled observations drawn from early series such as the present one.
One first observation is about progressing patients. In this series, they seem to have had a limited benefit from surgery. Macroscopic complete surgery was achievable in roughly one half of them. Median time to progression was 3 months after surgery, and 1-year PFS was nil. Among these 8 patients undergoing surgery while in progression, only 1 patient had a generalized progression (from primary resistance). Indeed, all other 7 patients were operated upon with a “focal” progression occurring after an initial response. Indeed, they showed evidence of both primary (exon 17, 1 case) and secondary (exon 13, 3 cases, and exon 14, 1 case17) IM-insensitive mutations and one KIT amplification. While surgery did not avoid a subsequent “generalized” progression in these patients, it is most likely that these cases may only benefit from new molecular-targeted agents. Although it is too premature to draw definite conclusions on surgery of progressing residual disease, as our institutional policy we have now abandoned this option, as long as clinical studies on further-line molecular-targeted therapies have been opened up. Formal evidence of efficacy of second-line molecular-targeted therapy with Sunitinib has now been provided,18 while studies are ongoing on a variety of other such agents.
In responding patients, conversely, surgery of residual disease remains a widely resorted practice all over the world as well as at our institution. Though, as said above, the basic issue of its efficacy is still unanswered, the most obvious practical question to address is its appropriate timing. Median time to secondary progression during primary treatment with IM has been shown to be approximately 2 years.1,5,19 On the other side, fully developed tumor response may be seen even after several months of therapy.5 Therefore, it seems quite reasonable to place surgery around 1 year from treatment start.4 In our series, surgery was placed after longer intervals on average. At the very least, the outcome does not seem to have been impaired by this delay. Therefore, we may empirically confirm that placing surgery after 1 year, or so, is a reasonable option.
Then, in the lack of any controlled confirmation of efficacy, one may wonder if, at least, our observations support or contradict the theoretical premises of surgery of residual disease.20–22
First, we did not observe any pathologic complete response. A wide variety of pathologic responses are present in this group (Table 2), but no correlation has been found with postsurgical progression. Which is the meaning of vital nodules in responding patients is difficult to understand. On one side, they could be related to the presence of resistant clones, not yet detectable by molecular analysis, which sooner or later will lead to secondary progression. On the other hand, one may speculate that they might be related to differences in drug delivery to the various neoplastic sites. In any case, at the end, this finding encourages surgical cytoreduction. Most of these “partial responders” carried an exon 11 mutation, and no secondary mutation was found but in 1 patient, who was apparently in clinical response before surgery. At least, the hypothesis that debulking residual disease may have prevented secondary mutations is not contradicted by these data, though of course cannot it either be substantiated.
Second, if the obvious aim of surgery is to render the patient free of visible macroscopic disease, our experience suggests that it is unlikely to reach an actual postsurgical CR in patients with multiple peritoneal or liver lesions. Indeed, macroscopic complete excisions could be made in 89% of our patients, but the value of such estimations in patients with multiple implants is really questionable.
Third, the only factor by which postsurgical progressions seemed to be significantly affected in group A was the discontinuation of IM postoperatively. This observation is in keeping with the trial by the French Sarcoma Group.23 We therefore confirm that IM should not be interrupted even following complete surgery. Theoretically, however, this might be interpreted as casting serious doubts on the relevance of surgery itself.
In regard to the exceedingly limited group of the 3 patients undergoing cytoreductive preoperative IM for localized disease, we were able to operate conservatively all. A major response was seen in 2 cases. Not surprisingly, a major response occurred in the exon 11 mutant, while the exon 9 mutant had only a minor response, despite a negative FDG-PET scan. In the third patient, an almost complete pathologic response was found to have occurred, and the mutational analysis suggested a wild-type KIT, but it was probably flawed by the absence of enough residual tumor cells. Therefore, in patients with lesions of borderline resectability (eg, lesions amenable to conservative surgery only in case of substantial shrinkage), we might only suggest to do both biomolecular analysis and baseline FDG-PET, to better predict, and monitor, tumor response.4
CONCLUSION
Whether surgery of residual disease after a period of IM has some efficacy in advanced GIST patients cannot be said on the basis of this series, as others reported so far. The prognosis of IM-responding patients looks relatively fine after surgery of residual disease, but of course the selection bias is such as to make it impossible to draw any conclusion about the efficacy of the procedure in itself. Prospective controlled trials are not ongoing at the moment. For the time being, surgery of residual disease should be held as an investigational option, although, understandably, of wide use within institutions treating these patients. At least, the indication to major surgical procedures should be carefully balanced against the lack of evidence, and other procedures, like ablations, should be considered in the individual patient. On the other hand, though in a limited number of patients, our data do not encourage surgery of residual progressing disease. New agents are currently available, and they are likely to be most useful in these patients.
Footnotes
Supported by grants from Associazione Italiana per la Ricerca sul Cancro (to S.P.) and Ministero della Sanita, Ricerca Finalizzata 2004, Italy.
Preliminary data from this paper were presented during ASCO Annual Meeting 2005 (Abstract No. 9038).
Reprints: Alessandro Gronchi, MD, Department of Surgery, Istituto Nazionale per lo studio e la cura dei Tumori, via Venezian, 1-20133, Milano, Italy. E-mail: alessandro.gronchi@istitutotumori.mi.it.
REFERENCES
- 1.De Matteo R, Lewis J, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence pattern and prognostic factors for survival. Ann Surg. 2000;231:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–1056. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–465. [DOI] [PubMed] [Google Scholar]
- 4.Blay JY, Bonvalot S, Casali P, et al. Consensus meeting for the management of gastrointestinal stromal tumors: Report of the GIST Consensus Conference of 20–21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566–578. [DOI] [PubMed] [Google Scholar]
- 5.Verveij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomized trial. Lancet. 2004;364:1127–1134. [DOI] [PubMed] [Google Scholar]
- 6.Rankin C, Von Mehren M, Blanke C, et al. Dose effect of imatinib in patients with metastatic GIST: Phase III sarcoma group study S0033. J Clin Oncol. 2004;22(suppl 14):819. [Google Scholar]
- 7.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. [DOI] [PubMed] [Google Scholar]
- 8.Debiec-Rychter M, Dumez H, Judson J, et al. Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2004;40:689–695. [DOI] [PubMed] [Google Scholar]
- 9.Tamborini E, Bonadiman L, Greco A, et al. A new mutation in the KIT ATP pocket causes acquired resistance to imatinib in a gastrointestinal stromal tumor patient. Gastroenterology. 2004;127:2294–2299. [DOI] [PubMed] [Google Scholar]
- 10.Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005;11:4182–4190. [DOI] [PubMed] [Google Scholar]
- 11.Casali PG, Fumagalli E, Messina A, et al. Tumor response to imatinib mesylate in advanced GIST. J Clin Oncol. 2004;22(suppl 4):9028. [Google Scholar]
- 12.Gronchi A, Fiore M, Bertulli R, et al. Surgery of residual disease following imatinib mesylate in advanced gastrointestinal stromal tumors (GIST). J Clin Oncol. 2005;23(suppl 16):9038. [Google Scholar]
- 13.Perrone F, Tamborini E, Dagrada GP, et al. 9p21 locus analysis in high-risk gastrointestinal stromal tumors characterized for c-kit and platelet-derived growth factor receptor alpha gene alterations. Cancer. 2005;104:159–169. [DOI] [PubMed] [Google Scholar]
- 14.Lasota J, Stachura J, Miettinen M. GISTs with PDGFRA exon 14 mutations represent subset of clinically favorable gastric tumors with epithelioid morphology. Lab Invest. 2006;86:94–100. [DOI] [PubMed] [Google Scholar]
- 15.Lagonigro MS, Tamborini E, Negri T, et al. PDGFRα, PDGFRβ and KIT expression/activation in conventional chondrosarcoma. J Pathol. 2006;208:615–623. [DOI] [PubMed] [Google Scholar]
- 16.Van Glabbeke M, Verweiy J, Casali PG, et al. Initial and late resistance to Imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: a European Organisation for Research and Treatment of Cancer (Italian Sarcoma Group) Australasian Gastrointestinal Trials Group study. J Clin Oncol. 2005;23:5795–5803. [DOI] [PubMed] [Google Scholar]
- 17.Tamborini E, Gabanti E, Lagonigro MS, et al. KIT/Val654 Ala receptor detected in one imatinib-resistant GIST patient. Cancer Res. 2005;65:1115. [PubMed] [Google Scholar]
- 18.Demetri G, Van Oosterom AT, Garrett C, et al. Improved survival and sustained clinical benefit with SU11248 (SU) in pts with GIST after failure of imatinib mesylate (IM) therapy in a phase III trial. 2006 ASCO Gastrointestinal Cancers Symposium [Abstract 8].
- 19.Blanke C, Joensuu H, Demetri G, et al. Outcome of advanced gastrointestinal stromal tumor (GIST) patients treated with imatinib mesylate: four-year follow-up of a phase II randomized trial. 2006 ASCO Gastrointestinal Cancers Symposium [Abstract 7].
- 20.Scaife CL, Hunt KK, Patel SR, et al. Is there a role for surgery in patients with ‘unresectable’ cKIT+ gastrointestinal stromal tumors treated with imatinib mesylate? Am J Surg. 2003;186:665–669. [DOI] [PubMed] [Google Scholar]
- 21.Hohenberger P, Schneider U, Pink D, et al. Resection of progressive or residual tumor after treatment with imatinib for advanced GI stromal tumors. Ann Surg Oncol. 2004;11(suppl):53. [Google Scholar]
- 22.Bauer S, Hartmann JT, de Wit M, et al. Resection of residual disease in patients with metastatic gastrointestinal stromal tumors responding to treatment with imatinib. Int J Cancer. 2005;117:316–325. [DOI] [PubMed] [Google Scholar]
- 23.Le Cesne A, Perol D, Ray-Coquard I, et al. Interruption of imatinib (IM) in GIST patients with advanced disease: updated results of the prospective French Sarcoma Group randomized phase III trial on survival and quality of life. J Clin Oncol. 2005;23(suppl 16):9031.16339756 [Google Scholar]


