Abstract
Objective:
To determine patient and injury variables that influence fluid requirements following burn injury and examine the association between fluid volume received and outcome.
Background:
Fluid resuscitation remains the cornerstone of acute burn management. Recent studies suggest that patients today are receiving more fluid per percent total body surface area (TBSA) than in the past. Therefore, there is a need to better define the factors that impact fluid requirements and to determine the effects of fluid volumes on outcome.
Methods:
This study was part of a federally funded multicenter study. Multilinear regression analyses were performed to determine the patient and injury characteristics that most influenced fluid resuscitation volumes received. To assess the association of fluid volumes on outcome, propensity scores were developed to provide a predicted volume of fluid for each patient. Logistic models were then used to assess the impact of excess fluid beyond predicted volumes on outcome.
Results:
Seventy-two patients were included in this analysis. Average patient age was 40.6 years and average TBSA was 44.5%. Average fluid volume received during the first 24 hours after injury was 5.2/mL/kg/TBSA. Significant predictors of fluid received included % TBSA, age, intubation status, and weight. Increased fluid volume received increased risk of development of pneumonia (odds ratio [OR] = 1.92), bloodstream infections (OR =2.33), adult respiratory distress syndrome (OR = 1.55), multiorgan failure (OR= 1.49), and death (OR = 1.74).
Conclusion:
TBSA, age, weight, and intubation status on admission were significant predictors of fluid received. Patients who received larger volumes of resuscitation fluid were at higher risk for injury complications and death.
The purpose of this paper is to examine the factors that impact the volume of fluid administered following burn injury and the association between fluid volume and outcome. This study is based on the clinical data collected as part of the multicenter study, “Inflammation and the Host Response to Injury”.
Fluid resuscitation remains the cornerstone of early burn management. Adequate fluid administration is critical to the prevention of burn shock and other complications of thermal injury. Eighty years after Frank Underhill’s observations on the critical importance of fluid resuscitation on survival following major burn,1 a perfect formula for predicting fluid requirements remains elusive despite decades of research and debate.2–6 In addition, controversy persists over the best method to determine fluid volume necessary to prevent the complications of hypovolemic shock.7–13
The Parkland formula has been used to estimate appropriate volumes of fluid resuscitation for over 40 years. Based on a number of studies in animal models, Baxter and Shires determined that adequate fluid resuscitation can be achieved in the majority of patients by using 3.7 to 4.3 mL/kg/% TBSA of crystalloid solution.3,14 However, more recent evidence suggests that the Parkland formula does not accurately predict fluid requirements, particularly in patients with larger burns, and that patients today are receiving more fluid per-percent TBSA than in the past.8,15–18 Cancio et al reported that the Parkland formula underestimated fluid requirements in 84% of patients.15 Friedrich et al compared the volume of fluid resuscitation delivered in the first 24 hours after injury to 10 patients in the 1970s with age and % TBSA-matched patients in the year 2000 and found the patients in 2000 received over twice the volume of resuscitation.17 In a multicenter survey, Engrav et al found that 58% of patients with large burns received more than 4.3 mL/kg/% TBSA.16 Pruitt has labeled this trend toward larger volumes of fluid administered “fluid creep.”19
Concerns over administered fluid volumes are predicated on the belief that fluid volume can be critical to the development of organ failure, infections, and death. The potential sequelae of underresuscitation involve complications of inadequate perfusion, including hypovolemic shock, renal failure, and the conversion of partial thickness wounds to full thickness wounds. There has similarly been increasing emphasis on the potentially deleterious effects of massive volumes of fluid resuscitation, including extremity, orbital and abdominal compartment syndromes, acute respiratory distress syndrome (ARDS), prolonged periods of ventilator dependence, and increased mortality.18,20–22
As awareness of the potential negative sequelae of large fluid volume administration increases, the need to better define the factors that can impact fluid requirements and the need to understand the effects of larger fluid volumes have similarly increased. Baxter initially identified 3 risk factors for fluid requirements above volumes predicted by the formula: excessively deep burns with muscle necrosis, inhalation injury, and delay in resuscitation.5,23 Several other patient and clinical variables may also impact fluid requirements.8,10,11,13
The Inflammation and Host Response to Injury is a collaborative program supported by the National Institute of General Medical Sciences designed to better define the proteomic and genomic response to injury. In the context of this large cohort study, we set out: 1) to evaluate the relationship between injury characteristics and volume of resuscitation, and 2) to examine the relationship between volumes of fluid resuscitation and adverse outcomes.
METHODS
This is a retrospective cohort study evaluating the relationship between fluid resuscitation and outcome following thermal injury. The principal exposure of interest is the receipt of excessive volumes of resuscitation fluid and the outcomes of interest are infectious and noninfectious complications and mortality.
Patient Recruitment
The 72 subjects who form the basis of this report are the initial cohort of adult patients enrolled in an ongoing multicenter study of inflammation. The participating burn centers are listed in Table 1. Permission for the conduct of the study was obtained from each individual institution’s Human Subjects Committee and patient consent for enrollment was obtained within 48 hours of admission to the burn center. The following criteria were required for adult patient enrollment: age ≥18 years, burn size ≥20% TBSA, no other concomitant trauma, and admission to the study center within 96 hours of injury. Patients who were not resuscitated and placed on comfort care were not eligible for the study.
TABLE 1. Participating Institutions
Data Collection
Clinical data were prospectively collected by trained nurse abstractors and entered into TrialDb, a web-based data collection platform specifically adapted for this program. Data integrity was evaluated centrally through an assessment of missing values, range checks, evaluation for implausible values, and internal consistency. Additionally, data were validated through an external review of a random sample of charts by a physician and an independent chart abstractor.
Diagnosis of inhalation injury was determined by the standard practice of the participating institution (either by clinical history/physical examination or bronchoscopy). The diagnosis of pneumonia was based on quantitative culture when available or sputum samples with counts >3+ of a single organism with radiographic evidence of pneumonia and leukocytosis. The diagnosis of ARDS required acute onset of bilateral infiltrates, a PaO2/FiO2 ratio of <200 regardless of PEEP, a pulmonary capillary wedge pressure of <18 if a pulmonary artery catheter was in place or absence of congestive heart failure if a pulmonary artery catheter was not in place. Bloodstream infections were based on positive blood culture. Other complications of injury, including renal failure, cardiac arrest or myocardial infarction, pulmonary embolism, gastrointestinal bleed, or pneumothorax were categorized as “events.”
Data Analysis
Fluid Volumes
Total fluid volumes (including colloid and crystalloid) administered in the first 24 hours following injury were examined in 2 ways: as a function of the predicted volume requirements based on the Parkland formula (Parkland score) and as a function of the patient’s weight (fluid-weight score) as described by Ivy et al.20 Parkland scores were calculated as total fluid volume (mL)/(4 × wt (kg) × TBSA). Fluid-weight scores were calculated for each patient as total fluid volume/wt (kg). T tests and Fisher exact test tests were used to compare injury and outcome variables between patients who received above and below 250 mL/kg of fluid.
Outcome Analysis
Incidence of ARDS, multiple organ failure and bloodstream infections, total number of infections, total number of complications, and mortality status at time of discharge were determined for each patient. Multiple organ failure was defined as having a maximum Denver Score ≥4 (Table 2). 24 The crude relative odds of adverse outcome per 5 L of fluid administered were estimated for each patient based on total volume of fluid received in the first 24 hours following injury. All adverse outcomes were considered independently, without consideration for potential interactions.
TABLE 2. Denver Multiple Organ Failure Score24
To adjust for other variables that may confound the relationship between volume resuscitation and outcome, a model was developed to predict the estimated fluid requirements based on baseline patient and injury characteristics. Inclusion in the prediction model was determined by regression of baseline and injury characteristics against total fluids administered in the first 24 hours following injury, with a P value of <0.10 used to determine inclusion. Logistic regression models were then used to study the relationship between patient outcome and deviation from predicted fluid requirement where deviation from predicted fluids was calculated as the relative percent increase (or decrease) over predicted: [(fluids received − fluids predicted)/fluids predicted] × 100. The percentage deviation from predicted was categorized as less than or equal to predicted (reference); 0% to 25% above predicted; >25% predicted.
RESULTS
Baseline Patient and Injury Characteristics
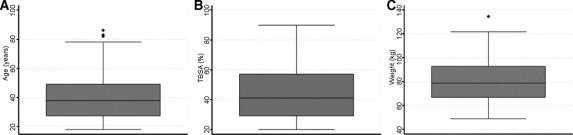
Seventy-six patients were enrolled in the study at the time of data analysis. Complete fluid and outcome data were available for 72 patients, and these form the subjects of this analysis. The 4 patients who were still hospitalized at the time of analysis were excluded. Baseline and injury characteristics of the 72 patients are summarized in Table 3 and Figure 1. The majority of burns were caused by flame or flash injuries (76% and 11%, respectively). Average patient age was 40.6 years (range, 18–86 years) and average total body surface area burned (% TBSA) was 44.5% (range, 20%–90%). Patients were admitted to the burn center 3.4 hours following injury (range, 1–12 hours) and had an admission APACHE II score of 20.1 (range, 6–36). Inhalation injury was diagnosed in 30 patients (42%). Average base deficit was 4.5 (range, −9 to 15) on admission to the burn center.
TABLE 3. Baseline Patient and Injury Characteristics
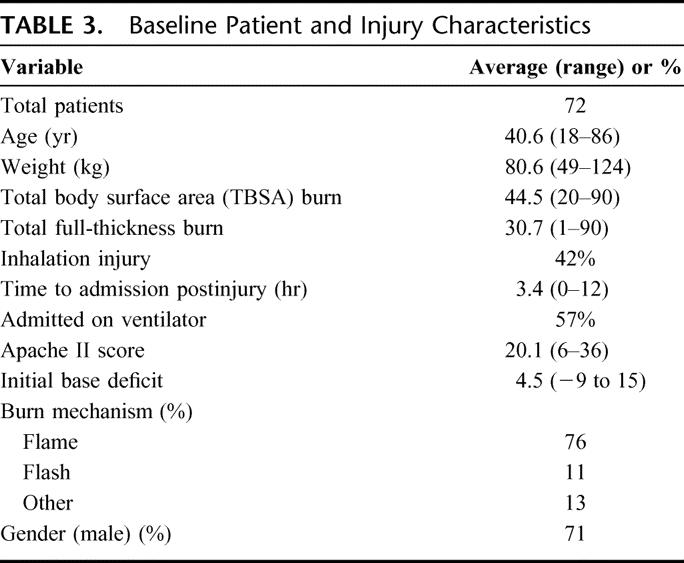
FIGURE 1. Box plots of patient age (A), burn size (% TBSA) (B), and patient weight (C).
Fluids Administered
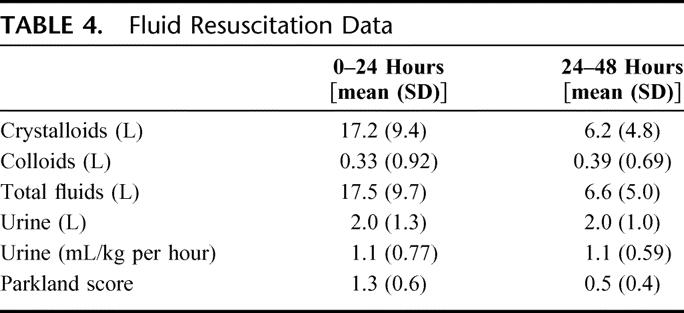
A summary of fluids administered over the first 48 hours following injury is shown in Table 4. The average total volume of fluid administered over the first 24 hours following injury was 17.2 L (±9.4 L); nearly all fluid was crystalloid. This fluid volume is equivalent to an average of 5.2 mL/kg/TBSA (Parkland score of 1.3). Average hourly urine output in the first 24 hours following injury was 1.1 mL/kg per hour.
TABLE 4. Fluid Resuscitation Data
Patient Outcomes
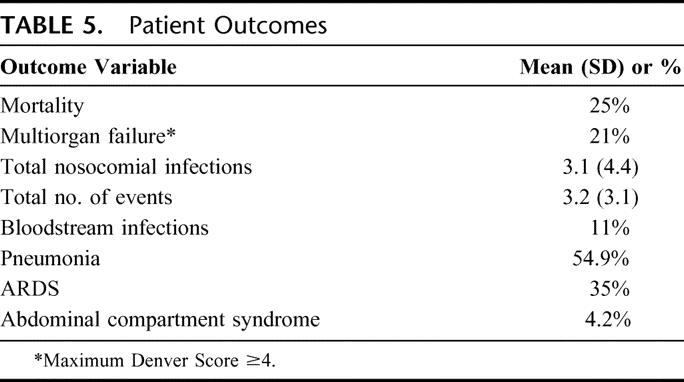
Patient outcomes are shown in Table 5. Overall mortality was 25%; 21% of patients developed multiple organ failure. Bloodstream infections occurred in 11% of patients, and 35% of patients developed ARDS. A total of 54% of patients developed pneumonia, and all but 2 of these patients had a pneumonia diagnosed by bronchoalveolar lavage. On average, each patient had 3.1 nosocomial infections and 3.2 other hospital events. Three patients were diagnosed with abdominal compartment syndrome. These 3 patients had an average TBSA burn of 47% and received an average of 18.8 L of fluid in the first 24 hours following injury.
TABLE 5. Patient Outcomes
Predictors of Fluid Requirements and Effects of Fluids on Outcome
For each 5 L increase in fluid received, there was a significant increase in the unadjusted odds of developing pneumonia (OR = 1.92; CI, 1.35–2.74), bloodstream infections (OR = 2.33; CI, 1.38–3.93), ARDS (OR =1.55; CI, 1.16–2.06), multiorgan failure (OR =1.49; CI, 1.02–2.01), and death (OR =1.74; CI, 1.26–2.42).
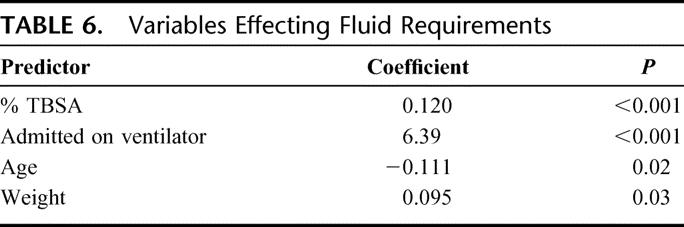
Since baseline injury characteristics are associated with both fluid requirements and outcome, we developed a multivariate prediction model to estimate fluid requirements as described in Methods. Multivariate regression identified 4 parameters strongly predictive of fluid received: % TBSA, age (inversely), weight, and intubation status on burn center admission (Table 6).
TABLE 6. Variables Effecting Fluid Requirements
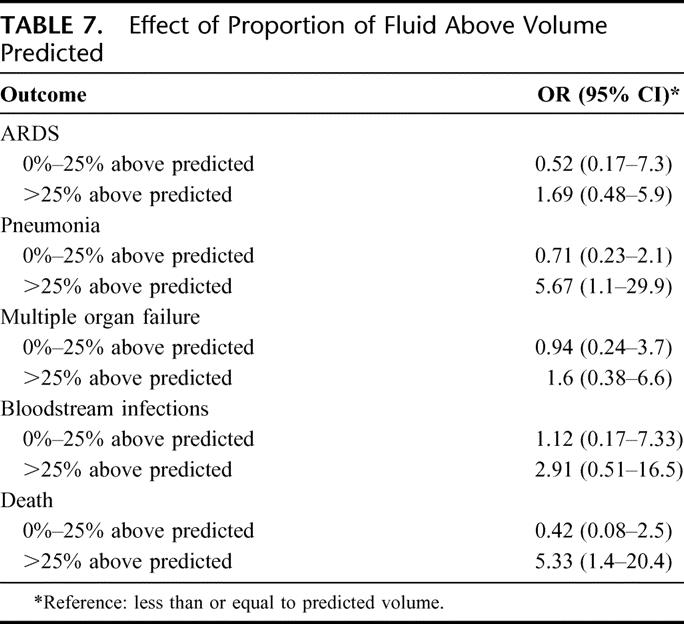
The impact of excessive fluid received in excess of predicted affects the development of complications as shown in Table 7. For fluids in excess of 25% of predicted volumes, the estimated increase in odds for adverse outcome were: ARDS (OR = 1.69; CI, 0.48–5.9), pneumonia (OR = 5.67, CI, 1.1–29.1), multiple organ failure (OR = 1.6; CI, 0.38–6.6), bloodstream infections (OR = 2.9; CI, 0.51–16.5), and death (OR = 5.33; CI, 1.4–20.4). In addition, all patients receiving ≥25% above predicted fluid volumes developed at least one of the adverse outcomes.
TABLE 7. Effect of Proportion of Fluid Above Volume Predicted
Fluid Analysis by Parkland score and Fluid/Weight Score
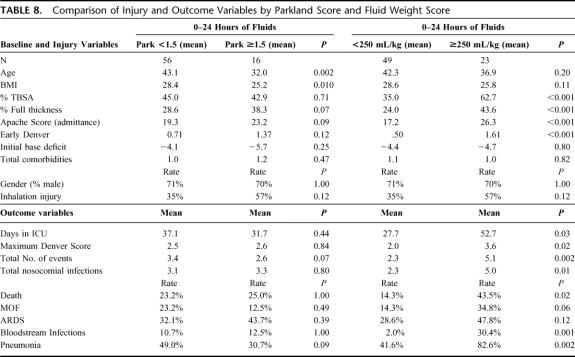
Comparison of patients stratified by Parkland score and fluid/weight score (above or below 250 mL/kg) is shown in Table 8. There was no statistically significant difference in the incidence of multiorgan failure, total number of nosocomial infections, incidence of ARDS, and mortality rate between patients who were stratified by a Parkland score above and below 1.5. Similarly, no difference in patient outcome was found when dichotomizing patients by a Parkland score of 2.0 (data not shown). However, when stratified by fluid weight score, differences in injury characteristics and outcome were significant. Patients who received over 250 mL/kg of crystalloid in the first 24 hours following injury had significantly higher incidence of multiple organ failure (34% vs. 13%), total nosocomial infections per patient (4.8 vs. 2.2), incidence of ARDS (50% vs. 27%), and mortality (42.3% vs. 15.6%). The patients who received over 250 mL/kg of fluid also had larger burn size and higher rate of inhalation injury.
TABLE 8. Comparison of Injury and Outcome Variables by Parkland Score and Fluid Weight Score
DISCUSSION
Decisions regarding fluid resuscitation comprise a critical component of the early care of the thermally injured patient. However, despite the development of simple formulae and guidelines for fluid administration, a great deal of controversy over fluid resuscitation remains.6,12,14,25 Recently, a trend toward the administration of larger fluid volumes has been noted, and the potential negative impact of this practice has similarly received a great deal of attention.8,16–19,22 The Inflammation and Host Response to Injury multicenter study provides the unique opportunity to study the patterns and sequelae of fluid resuscitation on a large cohort of patients with major burn injuries.
Traditionally, increased fluid requirements have been attributed to increased burn depth, presence of inhalation injury, and delay in initiation of resuscitation.5,23 Cancio et al recently demonstrated that burn size and need for mechanical ventilation were associated with increased fluid requirements and that weight was inversely related to fluid requirements.15 In this study, multivariate analysis showed that burn size, need for intubation prior to admission, weight, and age (inversely) were all important predictors of fluid volume administered. However, time to admission and inhalation injury were not predictors of increased fluid volumes administered. The finding that inhalation injury was not found to be a significant predictor of fluid requirements was surprising since inhalation injury has been repeatedly found to be associated with increased fluid requirements.26–28 The inclusion of both admitted on ventilator and inhalation injury in the fluid requirement regression model is the most likely explanation, as there is a strong interaction between these 2 variables. The majority of patients with inhalation injury (28 of 29) were also intubated prior to admission to the burn center.
The relationship between fluid volume received and patient outcomes was analyzed in 2 ways: using odds ratios and logistic regression on excess fluid beyond predicted. In the former analysis, there was s a significant increased risk of developing ARDS, pneumonia, bloodstream infections, multiple organ failure, and death with increasing fluid requirements. After adjustment for patient and injury characteristics that might confound the relationship between fluid and outcome, there was again a trend toward increase risk of adverse outcome, including death when fluid received exceeded predicted fluid requirements by more than 25%. These findings confirm the hypothesis that increasing volumes of fluid (or fluid creep as termed by Pruitt) may be associated with negative sequelae.16–22
Severely burned patients clearly require large volumes of fluid resuscitation to ensure adequate organ perfusion and minimize the risk of renal failure. Therefore, the increased risk of adverse outcome attributable to fluid volume may not be entirely avoidable, and may indeed be a reflection of overall severity of injury and/or an individual’s response injury. While urine output has been questioned as an adequate tool to assess systemic perfusion,8,29–32 the fact that average hourly urine output in this study was 1.1 mL/kg per hour suggests that larger volumes of fluid than needed may have been administered in some cases. However, controversy remains over the best tool to assess adequate systemic perfusion during fluid resuscitation.9,29,30,33–38
Another goal of this study was to assess the predictive value of 2 different formulae used to assess fluid volumes. The Parkland formula is the most widely used method for estimating fluid resuscitation volumes. Patients requiring volumes in excess of those predicted by the formula are often thought to be “failing” resuscitation and, accordingly, be at increased risk for development of complications of their injury. However, in this study we found that resuscitation volumes 1.5× or even 2.0× predicted levels were not statistically significantly associated with the development of infections, ARDS, multiorgan failure, or mortality. Conversely, we found that dichotomizing patients based on received fluid volumes >250 mL/kg appeared to better predict the development of infectious and noninfectious complications. This finding suggests that complications may be related more to the absolute total volume of fluid received irrespective of burn size.
The threshold of 250 mL/kg was first described by Ivy et al who reported that patients receiving >250 mL/kg of fluid are at increased risk for development of abdominal compartment syndrome.20 Based on these findings, Ivy et al suggested that patients who receive this volume of fluid warrant consideration for decompressive laparotomy. Receiving over 250 mL/kg of fluid during the first 24 hours following burn was also recently found to be predictive of the need for orbital compartment release for elevated intraocular pressure.22 However, the use of a fluid score that is not adjusted for burn size, inhalation injury, and other injury variables that impact volume requirements can limit the score’s utility as a predictor of outcome. Clearly, patients with large burns will be likely to receive volumes in excess of 250 mL/kg simply by the extent of their injury, and accordingly, would be the patients who one would predict to be at increased risk for adverse outcome. As shown in Table 8, patients who received larger fluid volumes had larger burn size, and worse APACHE II scores and initial Denver scores. Further investigation is required to better elucidate the significance of fluid volume thresholds in predicting outcome and their utility in guiding clinical management.
While the multicenter design of this study offered the benefit of a large cohort of with extensive burn injuries, there are a number of potential limitations. Whereas practice guidelines were developed for subjects in the trial, individual surgeon and institution preferences in terms of both critical care management and surgical management may vary slightly between institutions. Combining the patients from different centers with different practices could influence the data and bias the results toward a center with more patients enrolled. In addition, this study includes only adults, which may limit the study’s generalizability to pediatric patients. Accordingly, a separate study of the pediatric burn patients in this study is warranted and is currently underway.
Finally, despite the use of statistical models to adjust for the injury and patient characteristics that confound the relationship between fluid and outcome, it is still difficult to fully account for the complex interactions between injury and outcome, as well as the potential interactions of one outcome on another. Many of the outcome variables studied are competing risks; clearly, a patient who dies can no longer develop ARDS or multiorgan failure. This complicates the ability to directly examine the effects of fluids received on individual outcome measures. However, a separate analysis that did account for death as a competing risk yielded evidence that excess fluid volumes are associated with some negative outcomes (data not shown). Furthermore, there may be a physician-driven component to fluids received that is independent of injury factors. For example, the average hourly urine output was 80 mL, which is above the typical target of 0.5 mL/kg per hour. This suggests that excessive fluid may have been administered as a result of a decision by the treating physician or emergency department staff not captured in the data collected.
CONCLUSION
The % TBSA burn, patient age, weight, and intubation status prior to admission were found to significantly influence fluid requirements in the first 24 hours following burn injury. This study also confirmed that large volumes of resuscitation fluid are associated with increased risk of infectious complications, ARDS, and death, supporting the long-held hypothesis that overresuscitation may negatively impact patient outcome. Clearly, additional studies utilizing prediction models to better define a critical threshold for fluid administration may increase our understanding of this impact of fluid on outcome. Finally, in addition to the patient and injury characteristics analyzed in this study, one must consider the potential contribution of the genomic component of an individual’s response to injury to the development of complications and mortality. Understanding the role of these genetic factors may better elucidate fluid volume needs and the subsequent risk for complications. These analyses are currently underway as part of the larger goal of this collaborative study.
Footnotes
Supported by funding from the National Institutes of Health (No. NIH NIGMS U54 GM062119-1) and the National Institutes of Health Roadmap/National Institute of Child Health and Human Development (No. K12 HD049100).
Reprints: Matthew B. Klein, MD, University of Washington Burn Center, Harborview Medical Center, 325 9th Avenue, Box 359796, Seattle, WA 98121. E-mail: mbklein@u.washington.edu.
REFERENCES
- 1.Underhill F. The significance of anhydremia in extensive surface burn. JAMA. 1930;95:852–857. [Google Scholar]
- 2.Evans EI, Purnell OJ, Robinett PW, et al. Fluid and electrolyte requirements in severe burns. Ann Surg. 1952;135:804–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter C. Fluid volume and electrolyte changes of the early postburn period. Clin Plast Surg. 1974;1:693–709. [PubMed] [Google Scholar]
- 4.Baxter C. Fluid resuscitation, burn percentage, and physiologic age. J Trauma. 1979;19(suppl 11):864–865. [PubMed] [Google Scholar]
- 5.Baxter C, Shires T. Physiologic response to crystalloid resuscitation in severe burns. Ann NY Acad Sci. 1968;150:874–893. [DOI] [PubMed] [Google Scholar]
- 6.Warden GD. Burn shock resuscitation. World J Surg. 1992;16:16–23. [DOI] [PubMed] [Google Scholar]
- 7.Baxter C. Guidelines for fluid resuscitation. J Trauma. 1981;21(suppl 8):687–689. [Google Scholar]
- 8.Cartotto RC, Innes M, Musgrave MA, et al. How well does the Parkland formula estimate actual fluid resuscitation volumes? J Burn Care Rehabil. 2002;23:258–265. [DOI] [PubMed] [Google Scholar]
- 9.Holm C. Resuscitation in shock associated with burns: tradition or evidence-based medicine? Resuscitation. 2000;44:157–164. [DOI] [PubMed] [Google Scholar]
- 10.Jeng JC, Lee K, Jablonski K, et al. Serum lactate and base deficit suggest inadequate resuscitation of patients with burn injuries: application of a point-of-care laboratory instrument. J Burn Care Rehabil. 1997;18:402–405. [DOI] [PubMed] [Google Scholar]
- 11.Kaups KL, Davis JW, Dominic WJ. Base deficit as an indicator or resuscitation needs in patients with burn injuries. J Burn Care Rehabil. 1998;19:346–348. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz SI. Supportive therapy in burn care: consensus summary on fluid resuscitation. J Trauma. 1979;19(suppl 11):876–877. [PubMed] [Google Scholar]
- 13.Wolf SE, Rose JK, Desai MH, et al. Mortality determinants in massive pediatric burns: an analysis of 103 children with > or = 80% TBSA burns (> or = 70% full-thickness). Ann Surg. 1997;225:554–565; discussion 565–569. [DOI] [PMC free article] [PubMed]
- 14.Baxter CR, Shires T. Physiological response to crystalloid resuscitation of severe burns. Ann NY Acad Sci. 1968;150:874–894. [DOI] [PubMed] [Google Scholar]
- 15.Cancio LC, Chavez S, Alvarado-Ortega M, et al. Predicting increased fluid requirements during the resuscitation of thermally injured patients. J Trauma. 2004;56:404–413; discussion 413–414. [DOI] [PubMed]
- 16.Engrav LH, Colescott PL, Kemalyan N, et al. A biopsy of the use of the Baxter formula to resuscitate burns or do we do it like Charlie did it? J Burn Care Rehabil. 2000;21:91–95. [DOI] [PubMed] [Google Scholar]
- 17.Friedrich JB, Sullivan SR, Engrav LH, et al. Is supra-Baxter resuscitation in burn patients a new phenomenon? Burns. 2004;30:464–466. [DOI] [PubMed] [Google Scholar]
- 18.O’Mara MS, Slater H, Goldfarb IW, et al. A prospective, randomized evaluation of intra-abdominal pressures with crystalloid and colloid resuscitation in burn patients. J Trauma. 2005;58:1011–1018. [DOI] [PubMed] [Google Scholar]
- 19.Pruitt BA Jr. Protection from excessive resuscitation: pushing the pendulum back. J Trauma. 2000;49:567–568. [DOI] [PubMed] [Google Scholar]
- 20.Ivy ME, Atweh NA, Palmer J, et al. Intra-abdominal hypertension and abdominal compartment syndrome in burn patients. J Trauma. 2000;49:387–391. [DOI] [PubMed] [Google Scholar]
- 21.Ivy ME, Possenti PP, Kepros J, et al. Abdominal compartment syndrome in patients with burns. J Burn Care Rehabil. 1999;20:351–353. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan SR, Ahmadi AJ, Singh CN, et al. Elevated orbital pressure: another untoward effect of massive resuscitation after burn injury. J Trauma. 2006;60:72–76. [DOI] [PubMed] [Google Scholar]
- 23.Baxter CR, Marvin JA, Curreri PW. Early management of thermal burns. Postgrad Med. 1974;55:131–138. [DOI] [PubMed] [Google Scholar]
- 24.Ciesla DJ, Moore EE, Johnson JL, et al. The role of the lung in post injury organ failure. Surgery. 2005;138:749–757. [DOI] [PubMed] [Google Scholar]
- 25.Pruitt BA. Fluid resuscitation of the extensively burned patients. Ann Chir Plast. 1979;24:268–272. [PubMed] [Google Scholar]
- 26.Dai NT, Chen TM, Cheng TY, et al. The comparison of early fluid therapy in extensive flame burns between inhalation and noninhalation injuries. Burns. 1998;24:671–675. [DOI] [PubMed] [Google Scholar]
- 27.Herndon DN, Barrow RE, Linares HA, et al. Inhalation injury in burned patients: effects and treatment. Burns Incl Therm Inj. 1988;14:349–356. [DOI] [PubMed] [Google Scholar]
- 28.Navar PD, Saffle JR, Warden GD. Effect of inhalation injury on fluid resuscitation requirements after thermal injury. Am J Surg. 1985;150:716–720. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal N, Petro J, Salisbury RE. Physiologic profile monitoring in burned patients. J Trauma. 1983;23:577–583. [DOI] [PubMed] [Google Scholar]
- 30.Dries DJ, Waxman K. Adequate resuscitation of burn patients may not be measured by urine output and vital signs. Crit Care Med. 1991;19:327–329. [DOI] [PubMed] [Google Scholar]
- 31.Elliott DC. An evaluation of the end points of resuscitation. J Am Coll Surg. 1998;187:536–547. [DOI] [PubMed] [Google Scholar]
- 32.Mizock BA, Falk JL. Lactic acidosis in critical illness. Crit Care Med. 1992;20:80–93. [DOI] [PubMed] [Google Scholar]
- 33.Bernard R, Gueugniaud P-Y, Bertin-Maghit M, et al. Prognostic significance of early cardiac index measurements in severely burned patients. Burns. 1994;20:529–531. [DOI] [PubMed] [Google Scholar]
- 34.Holm C, Mayr M, Tegler J, et al. A clinical randomized study on the effects of invasive monitoring on burn shock resuscitation. Burns. 2004;30:798–807. [DOI] [PubMed] [Google Scholar]
- 35.Aikawa N, Ishibiki K, Naito C, et al. Individualized fluid resuscitation based on haemodynamic monitoring in the management of extensive burns. Burns Incl Therm Inj. 1982;8:249–255. [DOI] [PubMed] [Google Scholar]
- 36.Aikawa N, Martyn JA, Burke JF. Pulmonary artery catheterization and thermodilution cardiac output determination in the management of critically burned patients. Am J Surg. 1978;135:811–817. [DOI] [PubMed] [Google Scholar]
- 37.Barton RG, Saffle JR, Morris SE, et al. Resuscitation of thermally injured patients with oxygen transport criteria as goals of therapy. J Burn Care Rehabil. 1997;18(1 Pt 1):1–9. [DOI] [PubMed]
- 38.Miller JG, Bunting P, Burd DA, et al. Early cardiorespiratory patterns in patients with major burns and pulmonary insufficiency. Burns. 1994;20:542–546. [DOI] [PubMed] [Google Scholar]