Abstract
Objective:
Almost 50% of traumatic brain-injured (TBI) patients are alcohol intoxicated. The Glasgow Coma Scale (GCS) is frequently used to direct diagnostic and therapeutic decisions in these patients. It is commonly assumed that alcohol intoxication reduces GCS, thus limiting its utility in intoxicated patients. The purpose of this study was to test the hypothesis that the presence of blood alcohol has a clinically significant impact on GCS in TBI patients.
Methods:
The National Trauma Data Bank of the American College of Surgeons was queried (1994–2003). Patients 18 to 45 years of age with blunt injury mechanism, whose GCS in the emergency department, survival status, anatomic severity of TBI (Head Abbreviated Injury Score [AIS]), and blood alcohol testing status were known, were included. GCS of patients who tested positive for alcohol (n = 55,732) was compared with GCS of patients who tested negative (n = 53,197), stratified by head AIS.
Results:
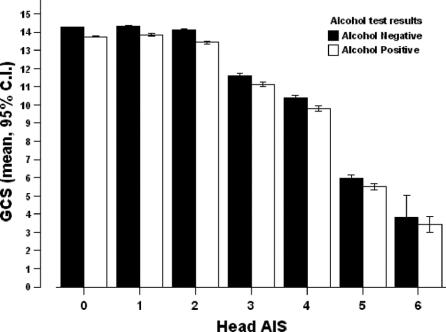
Groups were similar in age (31 ± 8 vs. 30 ± 8 years), Injury Severity Score (ISS; 12 ± 11 vs. 12 ± 11), systolic blood pressure in the ED (131 ± 25 vs. 134 ± 25 mm Hg), TRISS (Trauma Injury Severity Score; probability of survival (94% ± 16% vs. 95% ± 15%), and actual survival (96% vs. 96%). When stratified by anatomic severity of TBI, the presence of alcohol did not lower GCS by more than 1 point in any head AIS group (GCS in alcohol-positive vs. alcohol-negative patients; AIS 1 = 13.9 ± 2.8 vs. 14.3 ± 2.3; AIS 2 = 13.4 ± 3.2 vs. 14.1 ± 2.4; AIS 3 = 11.1 ± 4.7 vs. 11.6 ± 4.6; AIS 4 = 9.8 ± 4.9 vs. 10.4 ± 4.9; AIS 5 = 5.5 ± 3.8 vs. 5.9 ± 4.1, AIS 6: 3.4 ± 1.1 vs. 3.8 ± 2.8).
Conclusion:
Alcohol use does not result in a clinically significant reduction in GCS in trauma patients. Attributing low GCS to alcohol intoxication in TBI patients may delay necessary diagnostic and therapeutic interventions.
To determine if alcohol intoxication alters Glasgow Coma Scale in patients with traumatic brain injuries, 55,732 blood alcohol-positive trauma patients were compared with 53,197 alcohol-negative patients. The presence of alcohol did not lower Glasgow Coma Scale by more than 1 point, when patients were stratified by severity of brain injuries.
Alcohol plays a causative role in up to 50% of injuries requiring trauma center admission.1,2 As a potent central nervous system (CNS) depressant, alcohol may reduce level of consciousness and result in stupor, coma, and even death due to respiratory depression.3 The Glasgow Coma Scale (GCS) is the most commonly used means of quantifying level of consciousness in patients with traumatic brain injury (TBI).4 It is widely used for clinical decision-making and triage in prehospital settings and to guide diagnosis and management of TBI patients in emergency departments and trauma centers. For example, intracranial pressure (ICP) monitoring is recommended in all patients with a GCS 8 or less and an abnormal head computed tomography (CT) scan.5 The Advanced Trauma Life Support protocols recommend endotracheal intubation for patients with GCS of 8 or less.6 GCS is also used to identify patients for participation in TBI research trials.
If alcohol intoxication results in overestimation of the severity of TBI, unnecessary diagnostic studies, overly aggressive monitoring and treatments, and unwarranted Intensive Care Unit (ICU) admissions may occur. Alternatively, if a depressed level of consciousness in a TBI patient is mistakenly attributed to alcohol intoxication, clinicians may decide to delay urgently needed diagnostic and therapeutic interventions such as ICP monitoring or craniotomy, expecting the GCS to improve as alcohol is metabolized. It is important to determine the effect of alcohol on GCS, as an increasing number of trauma centers are testing patients for the presence of blood alcohol.7
Prior studies assessing the impact of alcohol intoxication on GCS in trauma patients have shown conflicting results, likely due to small numbers.8,9 Earlier, we undertook a retrospective review of over 1000 blunt TBI patients admitted to our institution, a major urban, level I trauma center, and found that alcohol intoxication did not result in clinically significant changes in GCS.10 However, the results represented a single institution experience and hence may reflect local patient demographics and alcohol use patterns. The purpose of the current study was to determine if alcohol intoxication alters GCS in trauma patients in a nationwide sample. The specific hypothesis of the study was that intoxicated patients have a lower GCS compared with patients with similar brain injury severity who do not have alcohol in their bloodstream.
METHODS
Data were acquired from the National Trauma Data Bank (version 4.0, 1994–2003) of the American College of Surgeons, which collects data on patients from trauma centers around the country in a standardized format. Patients in the 18- to 45-year age group who sustained blunt injuries were included. Age criteria were selected to minimize the effects of extremes of ages and comorbidities on study variables. Patients with incomplete information on the severity of brain injury, GCS in the emergency department, and survival were excluded. These criteria identified 243,854 patients. Of these, alcohol testing was carried out in 108,929 (45%) and constituted the study group. Patients who were not tested for alcohol (134,925, 55%) were excluded.
The severity of head injuries was graded using the abbreviated injury score (AIS), a strictly anatomic measure of injury severity. We assigned a value of AIS = 0 to those without TBI. The first GCS recorded in the emergency department (ED) was used in the analysis to minimize the time for metabolism of alcohol. Patients who tested positive for blood alcohol (n = 55,732, 51%) were compared with those with a negative BAC (n = 53,197, 49%). The 2 groups were compared using Student t test for continuous variables, and χ2 (or Fisher exact test) for categorical variables. A P value <0.05 was considered statistically significant. Because of the large sample size, even small differences in GCS were likely to be statistically significant. Therefore, we defined a difference of at least 1 point in GCS as clinically significant. Data were analyzed using SPSS 13.0 for windows (SPSS Inc., Chicago, IL).
RESULTS
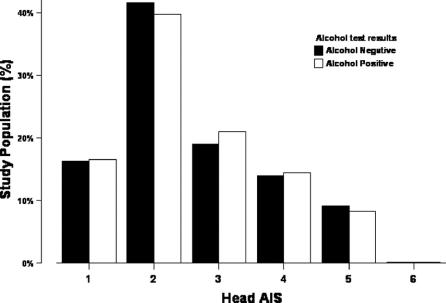
Demographic features, injuries, injury severity, comorbidities, and complications are listed in Table 1. Several differences achieved statistical significance but were not clinically significant. Alcohol-positive and -negative patients were similar in age, injury severity, and survival. There were significantly more males in the alcohol-positive group, but we did not find any impact of gender on relationship between alcohol and GCS (mean GCS in females 13.5 ± 3.4 vs. males 13.1 ± 3.8). Alcohol-positive patients were more likely to have sustained a head injury (43% vs. 36%, P < 0.001), but with a similar distribution of AIS grade (Fig. 1).
TABLE 1. Characteristics of Alcohol-Positive Versus Alcohol-Negative Patients
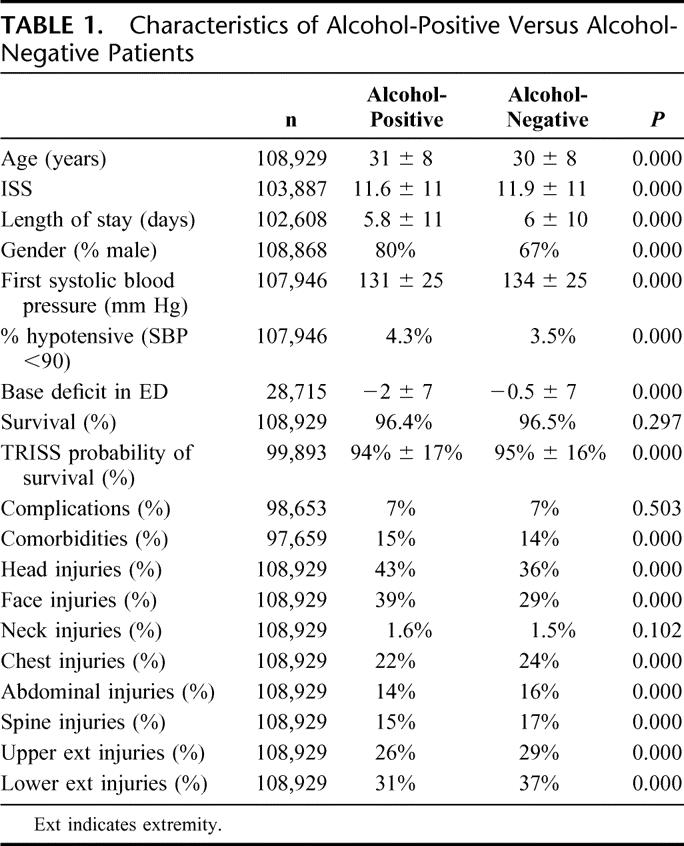
FIGURE 1. Distribution of head injuries by alcohol.
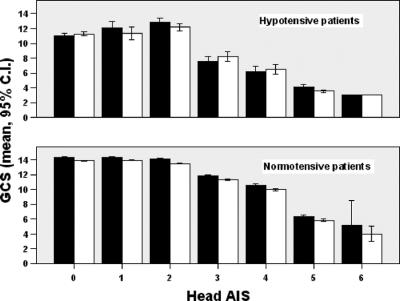
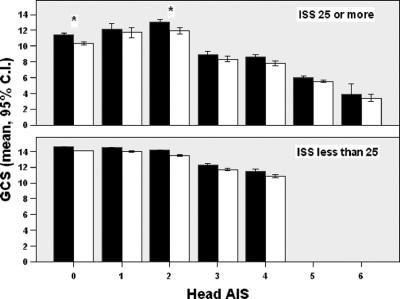
When stratified by anatomic severity of head injuries, the presence of alcohol did not reduce total GCS or any of its components (motor, speech, eye-opening), by more than 1 point in any group (Figs. 2 and 3). To ascertain the effects of shock and severe extracranial injuries on GCS measurement, we stratified patients by presence or absence of systemic hypotension, and by Injury Severity Score (ISS), and did not find a difference between alcohol-positive and -negative patients in any sub group except in the most severely injured patients with a head AIS of 0 or 2, where the difference in total GCS between alcohol-positive and -negative patients was slightly over 1 point (10.3 ± 5 vs. 11.4 ± 5 and 11.9 ± 4 vs. 13 ± 4, respectively, Figs. 4 and 5).
FIGURE 2. GCS by head AIS.
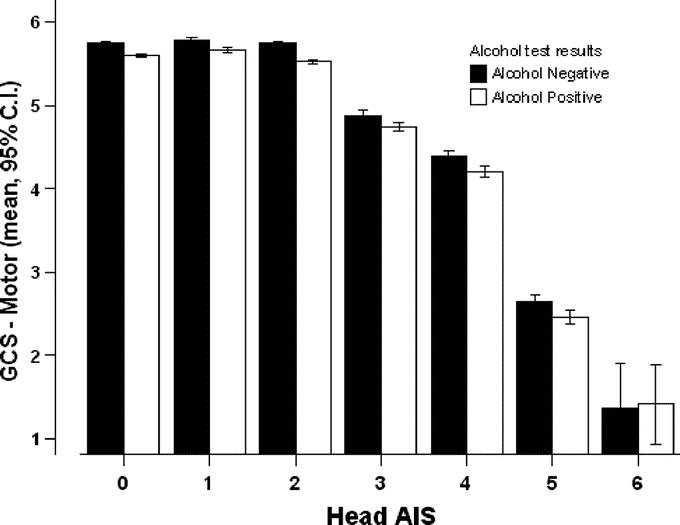
FIGURE 3. GCS motor component by head AIS.
FIGURE 4. GCS by head AIS in normotensive (systolic blood pressure >90 mm Hg) and hypotensive patients (systolic blood pressure ≤90 mm Hg). Black bars = alcohol-negative; white bars = alcohol-positive.
FIGURE 5. GCS by head AIS in severely injured (Injury Severity Score ≥25) and mild to moderately injured (Injury Severity Score <25). Black bars = alcohol-negative; white bars = alcohol-positive. *Clinically and statistically significant difference.
DISCUSSION
The results of this study suggest that alcohol intoxication does not alter GCS to a clinically significant extent in young to middle-age patients with blunt TBI. The lack of effect of alcohol on GCS persisted when stratified by severity of TBI. The only exception was noted in severely injured patients (ISS ≥25) without TBI or with minor TBI identified by computed tomography, where alcohol intoxication was associated with lowering of GCS by slightly more than 1 point. These findings are contrary to the study hypothesis, and validate the value of GCS as a measure of level of consciousness in TBI patients, even in the presence of alcohol intoxication. The implications of these findings are that a positive blood alcohol concentration (BAC) should not be used to delay procedures such as brain CT scanning, ICP monitoring, or to modify treatment plans.
The most likely explanation for our findings is that patients admitted to a trauma center with a positive BAC use alcohol at levels sufficient to induce tolerance. The mean BAC of injured patients admitted to a trauma center with a positive BAC is 162 mg/dL, over twice the legal limit for driving.11 It is possible that the findings of this analysis apply to the study population as a whole but cannot be applied to an individual patient who has not developed tolerance. However, there is no way to reliably determine whether or not an individual TBI patient with altered mental status is a chronic drinker with tolerance because a drinking history is unlikely to be obtainable or reliable in this clinical situation. Therefore, this study suggests that the safest strategy is to consider all mental status changes in these patients as attributable to brain injury, rather than to alcohol.
Studies indicate that even in awake, nonintubated trauma patients trauma center staff cannot reliably identify whether or not a trauma patient has alcohol in their bloodstream.12 The World Health Organization undertook a similar study and confirmed that the validity of clinical prediction of intoxication at identifying patients who had been drinking prior to injury is very poor.13 While a potentiating effect of alcohol on level of consciousness on patients with an already altered mental status due to TBI might be expected, this nationwide study found no evidence of such an effect, which is consistent with our institutional experience.10
The impact of alcohol on level of consciousness has been shown by others to be highly variable and depends upon the quantity and frequency of alcohol consumption, the rate of metabolism, the functional status of the liver, and concomitant medications.14,15 It appears that significant alterations in level of consciousness in trauma patients are predominantly a result of factors other than alcohol use. These factors may include head injuries, extracranial injuries, shock, hypothermia, or concomitant use of other CNS depressants, such as medications or illicit drugs. Another explanation of our findings is that GCS is not a sensitive measure of alterations in mental status. GCS is used extensively in clinical practices and in research protocols to assess severity of head injuries.16 However, recent evidence suggests that GCS may not be a reliable marker of TBI. Demetriades et al17 and Walder et al18 have shown that GCS correlated poorly with anatomic severity of head injuries as measured by head AIS grade.
The most important implication of our findings relates to the emergency management of intoxicated trauma patients with altered mental status. It is possible that trauma and emergency medicine physicians attribute some portion of depressed GCS in TBI patients to concomitant alcohol intoxication in patients with a positive BAC. As a result, urgent diagnostic and therapeutic interventions, such as ICP monitoring or craniotomy for head injuries, may be inappropriately withheld to determine if neurologic status will improve during a period of observation. It is important to note that the findings of this study do not suggest that intoxication does not confound clinical clearance of the cervical spine, or does not make clinical examination of the abdomen unreliable. Although we were unable to document that alcohol affects GCS, further data are needed to determine the effect of alcohol on pain perception, and the reliability of clinical examination.
The number of trauma patients who are tested for blood alcohol level is likely to increase in the near future for 2 reasons. One, the Uniform Accident and Sickness Policy Provision Law, which currently exists in most states and allows insurers to refuse payment for treatment of injuries in patients with a positive alcohol or drug test, is increasingly being repealed by state governments.7 Second, the American College of Surgeons, Committee on Trauma, has added a requirement that level 1 and 2 Trauma Centers must have a mechanism in place to screen injured patients for an alcohol problem.19 Measurement of a BAC is the most commonly used means of determining if a trauma patient has an alcohol use disorder, or is a candidate for a brief intervention.7,20 Increased BAC testing is likely to lead to an increase in the detection of alcohol intoxication in brain-injured patients. This study indicates that alcohol use does not alter GCS, and hence interventions indicated by a patient's GCS should not be withheld or postponed in BAC-positive patients.
There are 2 important limitations of this study. First, the database used for this study does not report the actual blood alcohol concentration. It is possible that patients who tested positive for alcohol did not have a high enough blood alcohol level to suppress the GCS. However, this is unlikely as prior studies have shown that trauma patients with any alcohol in their blood have a mean BAC greater than 160 mg/dL.21 At our own institution, the mean BAC of patients with alcohol in their blood is 172 ± 94 mg/dL.10 A second limitation is that alcohol testing is usually performed selectively in trauma centers, based upon clinical suspicion, or patient demographic characteristics, which may confound the analysis by means of selection bias.12 Finally, the potential confounding effects of drug use could not be determined, as toxicology testing is not well documented in the database.
CONCLUSION
This study of a large nationwide sample of trauma patients suggests that the presence of a positive blood alcohol concentration does not result in a clinically significant reduction in GCS. Diagnostic and therapeutic interventions indicated by a patient's GCS should be undertaken promptly, and not delayed due to concomitant alcohol intoxication.
Footnotes
Presented at the 58th Annual Meeting of the Southwestern Surgical Congress, Kauai, Hawaii, 2006.
Supported in part by a grant from the Robert Wood Johnson Foundation (Grant No. 046488).
Reprints: Shahid Shafi, MD, MPH, Department of Surgery, Division of Burns, Trauma and Surgical Critical Care, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Mail Code 9158, Dallas, TX 75390-9158. E-mail: shahid.shafi@utsouthwestern.edu.
REFERENCES
- 1.Soderstrom CA, Carson SL. Update: alcohol and other drug use among vehicular crash victims. Md Med J. 1988;37:541–545. [PubMed] [Google Scholar]
- 2.Soderstrom CA, Trifillis AL, Shankar BS, et al. Marijuana and alcohol use among 1023 trauma patients: a prospective study. Arch Surg. 1988;123:733–737. [DOI] [PubMed] [Google Scholar]
- 3.Johnston JJ, McGovern SJ. Alcohol related falls: an interesting pattern of injuries. Emerg Med J. 2004;21:185–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2:81–84. [DOI] [PubMed] [Google Scholar]
- 5.Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care: initial management. J Neurotrauma. 2000;17:463–469. [DOI] [PubMed] [Google Scholar]
- 6.American College of Surgeons-Committee on Trauma. Advanced Trauma Life Support, 6th ed. Chicago: American College of Surgeons, 1997. [Google Scholar]
- 7.Gentilello LM, Donato A, Nolan S, et al. Effect of the Uniform Accident and Sickness Policy Provision Law on alcohol screening and intervention in trauma centers. J Trauma. 2005;59:624–631. [PubMed] [Google Scholar]
- 8.Brickley MR, Shepherd JP. The relationship between alcohol intoxication, injury severity and Glasgow Coma Score in assault patients. Injury. 1995;26:311–314. [DOI] [PubMed] [Google Scholar]
- 9.Pories SE, Gamelli RL, Vacek P, et al. Intoxication and injury. J Trauma. 1992;32:60–64. [DOI] [PubMed] [Google Scholar]
- 10.Sperry J, Gentilello LM, Diaz-Arrastia R, et al. Waiting for the patient to ‘sober up’: effect of alcohol intoxication on Glasgow Coma Scale in brain injured patients. J Trauma. 2006;60:252. [DOI] [PubMed] [Google Scholar]
- 11.Gentilello LM, Rivara FP, Donovan DM, et al. Alcohol interventions in a trauma center as a means of reducing the risk of injury recurrence. Ann Surg. 1999;230:473–480; discussion 480–483. [DOI] [PMC free article] [PubMed]
- 12.Gentilello LM, Villaveces A, Ries RR, et al. Detection of acute alcohol intoxication and chronic alcohol dependence by trauma center staff. J Trauma. 1999;47:1131–1135; discussion 1135–1139. [DOI] [PubMed]
- 13.Cherpitel C, Bond J, Ye Y, et al. Clinical assessment compared with breathalyser readings in the emergency room: concordance of ICD-10 Y90 and Y91 codes. Emerg Med J. 2005;22:689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunne JR, Tracy JK, Scalea TM, et al. Lactate and base deficit in trauma: does alcohol or drug use impair their predictive accuracy? J Trauma. 2005;58:959–966. [DOI] [PubMed] [Google Scholar]
- 15.Minion GE, Slovis CM, Boutiette L. Severe alcohol intoxication: a study of 204 consecutive patients. J Toxicol Clin Toxicol. 1989;27:375–384. [DOI] [PubMed] [Google Scholar]
- 16.Valadka A. Injury to the cranium. In: Mattox K, Feliciano D, Moore E, eds. Trauma, 4th ed. New York: McGraw-Hill, 2000:377–399. [Google Scholar]
- 17.Demetriades D, Kuncir E, Murray J, et al. Mortality prediction of head Abbreviated Injury Score and Glasgow Coma Scale: analysis of 7,764 head injuries. J Am Coll Surg. 2004;199:216–222. [DOI] [PubMed] [Google Scholar]
- 18.Walder AD, Yeoman PM, Turnbull A. The abbreviated injury scale as a predictor of outcome of severe head injury. Intensive Care Med. 1995;21:606–609. [DOI] [PubMed] [Google Scholar]
- 19.American College of Surgeons-Committee on Trauma. Resources for Optimal Care of the Injured Patient. Chicago: American College of Surgeons, 2006. [Google Scholar]
- 20.Schermer CR, Gentilello LM, Hoyt DB, et al. National survey of trauma surgeons’ use of alcohol screening and brief intervention. J Trauma. 2003;55:849–856. [DOI] [PubMed] [Google Scholar]
- 21.Galbraith S, Murray WR, Patel AR, et al. The relationship between alcohol and head injury and its effect on the conscious level. Br J Surg. 1976;63:128–130. [PubMed] [Google Scholar]