Abstract
Objective:
This study evaluates the outcome of patients who underwent surgery for recurrent pancreatic cancer.
Summary Background Data:
Recurrence of pancreatic ductal adenocarcinoma occurs in up to 80% of pancreatic cancer patients within 2 years of a potential curative resection because, in most cases, occult (local and/or distant) micrometastases are present at the time of the initial resection.
Methods:
Thirty patients were operated for recurrent pancreatic cancer between October 2001 and April 2005. Median time between the initial resection and recurrence was 12.0 months. While 15 patients were resected, 15 patients either underwent palliative bypass or only exploration. Prospectively recorded data were analyzed retrospectively. Survival analysis was performed using Kaplan-Meier estimation and log-rank test.
Results:
The overall median survival of patients with recurrent disease was 29.0 months. After the first reresection/exploration for recurrent disease, the median survival was 11.4 months. There was a tendency of increased median survival in the group of patients undergoing resection (17.0 months) compared with the bypass/exploration group (9.4 months), although this difference was not significant (P = 0.084). In addition, patients with a prolonged interval (>9 months) from resection to recurrence were more likely to benefit from reresection compared with patients with recurrence within 9 months (median survival 17.0 vs. 7.4 months; P = 0.004). The in-hospital morbidity and mortality rate of resected patients was 20% and 6.7% compared with 13.3% and 0% of patients who underwent only exploration/palliative bypass.
Conclusion:
Resection for recurrent pancreatic cancer can be carried out safely. Further studies are required to address the question whether a subgroup of patients might actually benefit from this procedure.
Resection for recurrent pancreatic cancer does not seem to change the natural progression of the disease in most patients. Better diagnostic modalities and further studies will help to identify the subgroup of patients that might benefit from this approach.
Pancreatic ductal adenocarcinoma remains difficult to treat with an incidence rate that almost equals its mortality rate. The American Cancer Society estimated an incidence rate of 33,730 and a mortality rate of 32,300 patients for the year 2006, which clearly underscores the dismal prognosis of this disease.1 Pancreatic cancer is now the fourth leading cause of cancer-related deaths in the United States. Because of the progress that has been made over the last 20 years in diagnostics, surgery, and perioperative care, the operative mortality rates have fallen to well below 5% for pancreaticoduodenectomies at major centers. Nevertheless, the 5-year survival rate of resected patients remains only 15% to 20%.2 This is partly because even after macroscopically curative tumor resection, malignant cells are observed on the edge of resected specimens in up to 50% of cases.3 This R1-like situation explains the high local recurrence rate.3 Another reason is the presence of systemic occult disease at the time of diagnosis in most of the patients, leading to occurrence of distant metastasis in the liver (50% of resected patients) and peritoneal metastasis (25%).3–6
Until now, the commonly used treatment options for nonresectable pancreatic cancer have been chemotherapy with 5-fluorouracil or gemcitabine, palliative surgical bypass procedures, endoscopic or radiologically placed stents for biliary obstruction, palliative radiation procedures, or other palliative measures.7 For recurrent pancreatic cancer, there are no established therapeutic strategies, although the pattern of recurrence is well known. Sperti et al8 analyzed this in 78 patients after resection for pancreatic ductal adenocarcinoma: 72% developed local recurrence and 62% had hepatic metastases. The median disease-free survival was 7 months for local recurrence versus 3 months for hepatic recurrence. Similarly, several other studies have shown that pancreatic cancer patients who develop local recurrence without distant metastasis after curative resection of the primary tumor, appeared to have a better prognosis.6,9,10 The efficacy of combined chemoradiotherapy in these patients was recently described by Wilkowski et al.11 The median progression-free survival (ie, from the start of chemoradiotherapy) was 14.7 months and this may be a promising therapeutic option. A small study from Horiuchi et al12 compared 2 groups of patients with recurrent pancreatic cancer with liver metastasis: one group receiving chemotherapy versus the other group without chemotherapy. The administration of gemcitabine seemed to prolong the survival period from a mean survival rate of 6.6 months without any therapy to 22.3 months. To the best of our knowledge, there are no series and only few cases about reresection of recurrent pancreatic cancer.13
On the background of high incidence of local recurrence and the lack of effective chemotherapy,14 this paper reports on the outcome of patients reoperated for recurrent disease. We evaluated the survival of 30 patients who underwent radical resection with curative intent (R0/R1), R2 resection, palliative bypass, or only explorative laparotomy depending on the extent of the disease.
PATIENTS AND METHODS
Patients
Thirty patients who underwent surgery for recurrent disease after initially curative (R0/R1) resection were identified from a prospectively recorded database of 586 consecutive explorations for pancreatic ductal adenocarcinoma (undertaken between October 2001 and April 2005) at the Department of Surgery, University of Heidelberg, Germany. Indications for resection/exploration in these patients were a high probability of potentially resectable local recurrence without any signs of disseminated disease. In addition, these patients were in relatively good general condition (ASA I–III). Standard preoperative evaluation included contrast-enhanced helical computed tomography and/or state-of-the-art magnetic resonance imaging, as well as tumor marker (CA 19–9) analysis. Fifteen patients were resected, of which 8 were with curative intent (R0/R1 resection). Twenty-four patients were reoperated for recurrent disease once, 5 patients twice, and 1 patient 3 times. There were 22 men and 8 women. The median age at the time of initial resection was 57 years with an interquartile range (IQR) of 53 to 64 years. As a control group, 30 patients of the same time period matched for age, stage (at the initial operation), and type of operation that developed recurrent pancreatic cancer and that did not undergo resection for recurrent disease were analyzed.
The following parameters were documented prospectively: the disease stage after initial resection (TNM classification), the site of recurrent and metastatic disease, the indication for resurgery, the duration of hospitalization after surgical intervention, details of any adjuvant therapy, short-term outcome after operation (hospital morbidity/mortality), and long-term survival after surgical resection. The data were analyzed retrospectively. The analysis was based on a median follow-up of 10.5 months after resection of the recurrent disease, with 23 deaths among 28 patients at the time of the last follow-up; 2 patients were lost to follow-up 1 month after surgery.
Statistical Analysis
SAS software (Release 9.1, SAS Institute, Inc., Cary, NC) was used for statistical analysis. The distribution of age at operation, postoperative hospital stay, and follow-up period were described as median with an IQR. The Fisher exact test was used to analyze morbidity and mortality between subgroups of patients. Overall survival from the date of initial surgery and of operation due to recurrence was estimated using the Kaplan-Meier method. The 1-, 2-, and 5-year survival rates and the median survival time with the corresponding 95% confidence intervals (CI) were presented. Patients alive at the last follow-up (or lost to follow-up) were censored and marked in the figures (1–4). The end of the follow-up period for the patients alive was in January 2006. The log-rank test was performed to compare survival time distributions between curves regarding the operative intent, ie, curative versus palliative, the time span between initial and recurrent operation, and the age at recurrent operation. Two-sided P values were always computed, and an effect at a P value ≤0.05 was considered statistically significant.
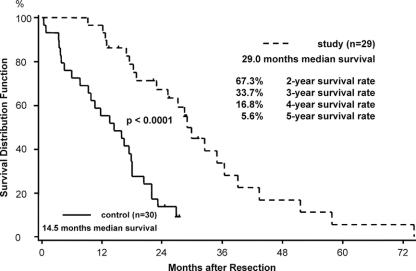
FIGURE 1. Median survival and actuarial 2-, 3-, 4-, and 5-year survival rate after primary tumor resection of 29 patients with pancreatic ductal adenocarcinoma (in 1 patient, the exact date of the primary operation was not known). Median survival of 30 patients matched for age, stage, and type of operation that did not undergo exploration for recurrent disease. Patients alive at follow-up or lost to follow-up were censored (∣).
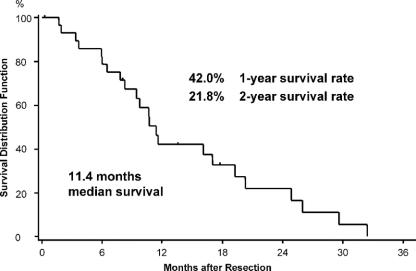
FIGURE 2. Median survival and actuarial 1- and 2-year survival rate after the first operation for recurrent cancer of 30 patients with pancreatic ductal adenocarcinoma. Patients alive at follow-up or lost to follow-up were censored (∣).
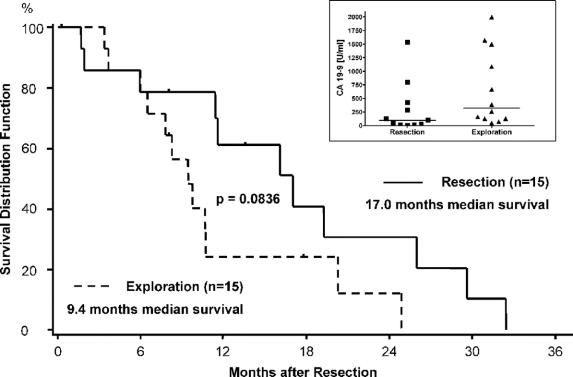
FIGURE 3. Median survival for of 30 pancreatic ductal adenocarcinoma patients after the first operation for recurrent cancer: R0/R1/R2 resection versus exploration/bypass. Inset: CA 19-9 levels (in U/mL) in the indicated groups (horizontal lines median values). Patients alive at follow-up or lost to follow-up were censored (∣).
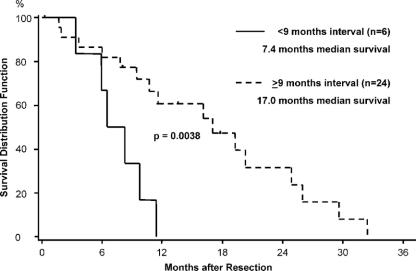
FIGURE 4. Median survival of 30 pancreatic ductal adenocarcinoma patients after the first operation for recurrent cancer with a disease-free interval of more than 9 months and less than 9 months. Patients alive at follow-up or lost to follow-up were censored (∣).
RESULTS
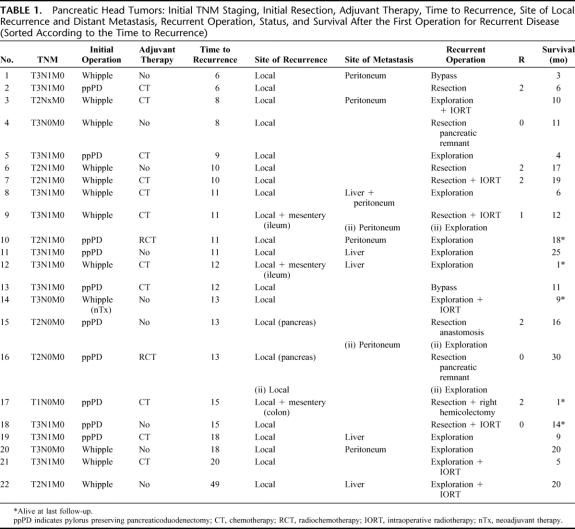
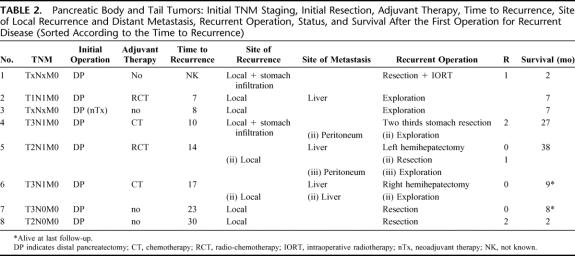
The outcome of 30 patients who underwent operation for recurrent pancreatic cancer after initially curative (R0/R1) resection was analyzed. All patients had histologically proven pancreatic ductal adenocarcinoma. Tumors were localized in the pancreatic head (n = 22), body (n = 4), and in the tail (n = 4). There were 12 classic Whipple procedures, 10 pylorus-preserving pancreaticoduodenectomies, and 8 distal pancreatectomies. When diagnosed, most of the tumors were already in an advanced stage: 57% were T3 tumors and 63% had N1 status. None of the patients harbored distant metastases (M1) at the time of the initial resection. In Tables 1 and 2, the details of primary tumor, initial TNM staging, initial resection, adjuvant therapy, time to recurrence, site of local recurrence, distant metastasis, surgery for recurrent disease, and survival are summarized. Thirteen patients received adjuvant chemotherapy (6 patients received gemcitabine, 5 patients 5-fluorouracil, including one combined with carboplatin; in 2 patients, details of adjuvant treatment were not known), 4 patients received adjuvant radiochemotherapy (three times combined with gemcitabine, once combined with 5-fluorouracil), and 13 patients received no adjuvant treatment. Two patients additionally received neoadjuvant therapy with gemcitabine and radiotherapy (45 Gy) before the initial resection.
TABLE 1. Pancreatic Head Tumors: Initial TNM Staging, Initial Resection, Adjuvant Therapy, Time to Recurrence, Site of Local Recurrence and Distant Metastasis, Recurrent Operation, Status, and Survival After the First Operation for Recurrent Disease (Sorted According to the Time to Recurrence)
TABLE 2. Pancreatic Body and Tail Tumors: Initial TNM Staging, Initial Resection, Adjuvant Therapy, Time to Recurrence, Site of Local Recurrence and Distant Metastasis, Recurrent Operation, Status, and Survival After the First Operation for Recurrent Disease (Sorted According to the Time to Recurrence)
The median disease-free time (time span between the primary tumor resection and the operation for the recurrent tumor) was 12.0 months with an IQR of 9.5 months and 16.0 months. The longest time to progression (4 years) was observed in a 69-year-old patient with a pancreatic head tumor, classified initially pT2 pN1 pM0 who underwent a classic Whipple procedure.
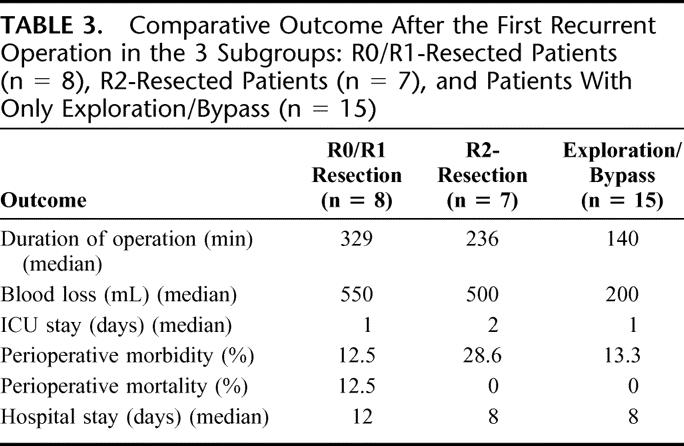
At the time of first surgery for recurrent disease, the median age was 60 years, with an IQR of 54 to 66 years. Eight patients (27%) were resected with curative intent (R0/R1), 7 patients (23%) underwent palliative (R2) resection, and 15 patients (50%) underwent explorative laparotomy (n = 13) or palliative bypass (n = 2). Twelve of these patients harbored distant metastasis at the time of the first operation for recurrence: in the liver in 8 cases, peritoneal seedlings in 5 cases (1 patient harbored liver as well as peritoneal metastasis). Eight patients received intraoperative radiotherapy (10 Gy). An overview of the outcome after the first surgery for recurrent disease is given in Table 3. There was no significant difference in morbidity and mortality between these 3 groups (P = 0.677 and P = 0.500, respectively). The median hospital stay was 8 days with an IQR of 3 to 16 days. The status of adjuvant/palliative therapy after operation for recurrent disease was known in 21 patients. Seventeen patients received radiochemotherapy (n = 7), radiotherapy (n = 1), or chemotherapy (n = 9), and 4 patients received no adjuvant treatment.
TABLE 3. Comparative Outcome After the First Recurrent Operation in the 3 Subgroups: R0/R1-Resected Patients (n = 8), R2-Resected Patients (n = 7), and Patients With Only Exploration/Bypass (n = 15)
At the time of last follow-up in January 2006, only 5 patients were still alive (2–18 months after the first surgery for recurrent disease). The majority of the patients (24 of the group) were operated once for recurrent disease, 5 patients were operated twice for recurrent cancer and 1 patient was operated 3 times.
The patients were sorted according to the first recurrent operation into 3 groups: 1) patients resected with curative intention (n = 8), 2) patients resected with tumor debulking (R2) with gross residual disease (n = 7), and 3) patients with only exploration or bypass (n = 15). The overall median survival after resection of the primary tumor was 29 months (95% CI, 22.9–36.5 months). The Kaplan-Meier curve shown in Figure 1 refers to only 29 patients because the exact date of the primary operation of one patient could not be retrieved. The overall 2-year survival rate was 67.3% (95% CI, 46.4%–81.5%) and the 5-year survival rate was 5.6% (95% CI, 0.4%–22.4%). The survival curves were compared with 30 patients matched for age, stage, and type of operation that developed recurrent pancreatic cancer and that did not undergo surgical exploration for their recurrence (Fig. 1). In this group, there was a significantly shorter median survival of 14.5 months (95% CI, 9.3–18.0 months) (P < 0.0001).
The overall median survival after the first surgery for recurrent disease was 11.4 months (95% CI, 8.2–19.3 months) (Fig. 2). The 1-year survival rate was 42.0% (95% CI, 22.8%–60.1%) and the 2-year survival rate 21.8% (95% CI, 7.5%–40.8%). Comparing the median survival of the patients with R0/R1 resection (16.1 months) with the other 2 groups (ie, 17 months for R2 resections and 9.4 months for those who underwent only exploration or palliative bypass), no significant difference could be observed (P = 0.15) (not shown). However, there was a tendency for improved survival when of patients with resection (median survival, 17 months) were compared with those who underwent only exploration/palliative bypass (median survival, 9.4 months) (P = 0.0836) (Fig. 3). Although there was a tendency for higher CA 19–9 levels in patients with disseminated disease (Fig. 3; inset), this difference was not significant. Patients with late onset of recurrence, ie, later than 9 months after initial resection, were more likely to benefit from reresection with a median survival of 17.0 months compared with 7.4 months of patients with recurrence within 9 months (P = 0.0038) (Fig. 4). Furthermore, in patients younger than 65 years, there was a tendency to benefit from operation for recurrence with a median survival of 11.6 months versus 7.8 months in patients older than 65 years (P = 0.16; data not shown).
DISCUSSION
Because of an overall median survival of less than 6 months after diagnosis15,16 and a disease recurrence in up to 80% of resected tumors within 2 years,4 pancreatic cancer remains difficult to treat. With surgery being the only option with a potential to cure, it appears logical that reresection for localized recurrence might open a small window of opportunity for some patients. This philosophy was the indication for operating on these 30 patients in our high-volume center.
The 30 patients chosen for first reresection were in good general condition and in none of them multiple sites of recurrent cancer were suspected preoperatively. However, intraoperatively 12 were discovered to harbor distant metastasis in addition to their local recurrence (7 liver, 4 peritoneal, and 1 with metastases at both sites). Furthermore, in 7 patients, the local recurrence could not be resected radically (R2 resection). Interestingly, the lack of significant difference in survival between our 3 groups indicates that, contrary to established oncologic perceptions, localized recurrence of pancreatic cancer seems to match systemic disseminated disease in many cases and this holds true even for the radically resected (R0/R1) patients.
In the study by Sperti et al,8 78 patients lived with a median disease-free interval of 8 months after initial resection. Without surgical reintervention the median survival of patients with local recurrence alone was 7 months, and of patients with hepatic or combined recurrence was 3 months. On this background, the median disease-free interval of 12.0 months in our patients and the overall median survival of 11.4 months after reresection for recurrence gains significance. Similarly, Wilkowski et al11 observed in 18 patients a median interval of 10.4 months between initial resection of pancreatic cancer and diagnosis of isolated local recurrence. Lending further credence to our philosophy of attempting to offer treatment of localized recurrence, the median overall survival from the start of combined chemoradiotherapy was 17.5 months.
The 5-year survival rate of resected pancreatic cancer patients in other major series such as from Richter et al in 200317 was 25%. Yeo et al in 199518 reported a 5-year survival rate of 26% and Wagner et al in 200419 described a 5-year survival rate of 24% for R0 resected adenocarcinoma. Cameron et al20 reported a series of 1000 consecutive pancreaticoduodenectomies, confirming again that this operation has become safe and effective, particular for pancreatic cancer patients with negative margin and negative lymph nodes. The 5-year survival in this highly selected subgroup was 41% set against an overall 5-year survival of 18%. Compared with these figures, the median survival of 29.0 months in our selected series of resection for recurrent cancer with a shorter long-term survival (5-year survival rate of 5.6%) would appear to suggest that the tumor biology in our selected group was not more benign as it could have been speculated. In contrast, it seems that, although the median survival is somewhat longer, 5-year survival rates are comparable lower. Thus, one could conclude that resection for recurrent pancreatic cancer does not influence the natural progression of the disease. There was, however, a difference between the 30 patients explored for pancreatic cancer (median survival, 29.0 months) and 30 matched patients with recurrent disease that were not treated surgically (median survival, 14.5 months; P < 0.0001). Although patient selection likely contributed to this difference, our findings nonetheless suggest that: 1) there might indeed be a difference in tumor biology between these 2 groups of patients and 2) there is some benefit from resection for recurrent cancer. Most likely, the true explanation is a combination of both. To what extent resection (vs. patient selection and tumor biology) contributed to this effect remains speculative. Clearly, a randomized trial would be necessary to clarify this question.
The overall median survival rate after initial resection of pancreatic cancer is determined by the radicality of resection as shown by Wagner et al19 in a series of 366 patients. Patients who were resected curatively (R0) and without lymph node involvement survived longer: the overall 5-year survival was 19.8%, after curative resection it was 24.2%, and in lymph-node negative patients it was 31.6%. In the present study, there was a tendency to survival benefit for all patients who were reresected, but independent from the extent of resection (R0/R1 and R2) (17 months median survival compared with 9.4 months for patients who underwent only exploration). It is thus tempting to speculate that resection (even partial) for recurrent pancreatic cancer offers a survival benefit (17 vs. 9.4 months median survival). However, one has to take into account that these data are heavily biased. Thus, in the first group the tumor recurrence was localized and at least partially removed compared with a group with metastasized recurrence. Therefore, without any surgical intervention, one would expect a survival advantage for the first group. Second, there were patients in the resection group who were resected for recurrence more than once. As mentioned, randomized controlled trials are needed to definitively answer this question. However, looking at the overall data from 30 patients and taking into account the survival rate following disease recurrence, it seems unlikely that resection for recurrent disease offers a substantial overall survival advantage.
Nevertheless, some individual patients may benefit from reresection. To cite an example, a 60-year-old patient with pancreatic head cancer underwent a pylorus-preserving pancreaticoduodenectomy and was initially staged pT2 pN0 pM0 (R1). He lived disease free for 13 months and later developed local recurrence that was resected with curative intent. The patient survived another 30 months following this reresection. Clearly, this patient might have benefited from the reresection. Thus, there could be a selected group, which may benefit from resection of recurrent pancreatic cancer, although it needs further study. Our results suggest that, if there is a group that potentially benefits, it would be younger (<65 years) patients in whom recurrence appeared comparably later (>9 months). More advanced imaging (eg, PET CT scan)21 might also help to better identify potential candidates for reresection. Although not significant in our study, CA 19–9 levels might further aid to identify this subgroup of patients. In addition, combination therapies including intraoperative radiotherapy might further improve the outcome. However, the number of patients that received IORT in our study (8 of 30) was too small to comment on the efficacy of this modality in surgery for recurrent pancreatic cancer.
CONCLUSION
Resection for recurrent pancreatic cancer does not seem to substantially prolong overall survival. However, there might be a subgroup of patients that benefits from these procedures, and this has to be analyzed in randomized controlled trials. Regarding the outcome of patients with primary and recurrent pancreatic cancer after initially curative resection, development of better multimodality treatment remains the major challenge for all involved specialists.
ACKNOWLEDGMENTS
The authors thank Holger Zentgraf for his support in patient documentation and data analysis.
Footnotes
Reprints: Markus W. Büchler, MD, Department of General Surgery, University of Heidelberg, Im Neuenheimer Feld 110, 69120 Heidelberg, Germany. E-mail: markus.buechler@med.uni-heidelberg.de.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. [DOI] [PubMed] [Google Scholar]
- 2.Yeo C, Abrams R, Grochow L, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma. Postoperative adjuvant chemoradiation improves survival: a prospective, single institution experience. Ann Surg. 1997;225:621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smeenk HG, Incrocci L, Kazemier G, et al. Adjuvant 5-FU-based chemoradiotherapy for patients undergoing R-1/R-2 resections for pancreatic cancer. Dig Surg. 2005;22:321–328. [DOI] [PubMed] [Google Scholar]
- 4.Smeenk HG, Tran TC, Erdmann J, et al. Survival after surgical management of pancreatic adenocarcinoma: does curative and radical surgery truly exist? Langenbecks Arch Surg. 2005;390:94–103. [DOI] [PubMed] [Google Scholar]
- 5.Tepper J, Nardi G, Sutt H. Carcinoma of the pancreas: review of MGH experience from 1963 to 1973: analysis of surgical failure and implications for radiation therapy. Cancer. 1976;37:1519–1524. [DOI] [PubMed] [Google Scholar]
- 6.Westerdahl J, Andren-Sandberg A, Ihse I. Recurrence of exocrine pancreatic cancer: local or hepatic? Hepatogastroenterology. 1993;40:384–387. [PubMed] [Google Scholar]
- 7.Yeo CJ, Yeo TP, Hruban RH. Cancer in the Pancreas. 2005:945–982.
- 8.Sperti C, Pasquali C, Piccoli A, et al. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21:195–200. [DOI] [PubMed] [Google Scholar]
- 9.Menke-Pluymers MB, Klinkenbijl JH, Tjioe M, et al. Treatment of locoregional recurrence after intentional curative resection of pancreatic cancer. Hepatogastroenterology. 1992;39:429–432. [PubMed] [Google Scholar]
- 10.Sunamura M, Egawa S, Shibuya K, et al. Therapeutic strategy for the recurrence of pancreatic cancer following pancreatectomy. Nippon Geka Gakkai Zasshi. 1999;100:200–205. [PubMed] [Google Scholar]
- 11.Wilkowski R, Thoma M, Bruns C, et al. Combined chemoradiotherapy for isolated local recurrence after primary resection of pancreatic cancer. Jop. 2006;7:34–40. [PubMed] [Google Scholar]
- 12.Horiuchi H, Uchida S, Hisaka T, et al. A study of recurrent pancreatic cancer with metastatic liver tumors after pancreatectomy. Gan To Kagaku Ryoho. 2005;32:1685–1687. [PubMed] [Google Scholar]
- 13.Inoue K, Kosuge T, Shimada K, et al. Repeated radical resection and intraoperative irradiation for recurrent pancreatic ductal adenocarcinoma after pancreatoduodenectomy. Surgery. 1995;118:909–911. [DOI] [PubMed] [Google Scholar]
- 14.Lockhart AC, Rothenberg ML, Berlin JD. Treatment for pancreatic cancer: current therapy and continued progress. Gastroenterology. 2005;128:1642–1654. [DOI] [PubMed] [Google Scholar]
- 15.Bramhall S, Dunn J, Neoptolemos JP. Epidemiology of Pancreatic Cancer. 1998.
- 16.Eibl G, Reber HA. A xenograft nude mouse model for perineural invasion and recurrence in pancreatic cancer. Pancreas. 2005;31:258–262. [DOI] [PubMed] [Google Scholar]
- 17.Richter A, Niedergethmann M, Sturm JW, et al. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg. 2003;27:324–329. [DOI] [PubMed] [Google Scholar]
- 18.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas: 201 patients. Ann Surg. 1995;221:721–731; discussion 731–733. [DOI] [PMC free article] [PubMed]
- 19.Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–594. [DOI] [PubMed] [Google Scholar]
- 20.Cameron JL, Riall TS, Coleman J, et al. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruf J, Lopez Hanninen E, Oettle H, et al. Detection of recurrent pancreatic cancer: comparison of FDG-PET with CT/MRI. Pancreatology. 2005;5:266–272. [DOI] [PubMed] [Google Scholar]