Abstract
Objective:
To investigate whether preoperative hepatic and regional arterial chemotherapy is able to prevent liver metastasis and improve overall survival in patients receiving curative colorectal cancer resection.
Methods:
Patients with stage II or stage III colorectal cancer (CRC) were randomly assigned to receive preoperative hepatic and regional arterial chemotherapy (PHRAC group, n = 110) or surgery alone (control group, n = 112). The primary endpoint was disease-free survival, whereas the secondary endpoints included liver metastasis-free survival and overall survival.
Results:
There were no significant differences in overall morbidity between PHRAC and Control groups. During the follow-up period (median, 36 months), the median liver metastasis time for patients with stage III CRC was significantly longer in the PHRAC group (16 ± 3 months vs. 8 ± 1 months, P = 0.01). In stage III patients, there was also significant difference between the 2 groups with regard to the incidence of liver metastasis (20.6% vs. 28.3%, P = 0.03), 3-year disease-free survival (74.6% vs. 58.1%, P = 0.0096), 3-year overall survival (87.7% vs. 75.7%, P = 0.020), and the median survival time (40.1 ± 4.6 months vs. 36.3 ± 3.2 months, P = 0.03). In the PHRAC arm, the risk ratio of recurrence was 0.61 (95% CI, 0.51–0.79, P = 0.0002), of death was 0.51 (95% CI, 0.32–0.67; P = 0.009), and of liver metastasis was 0.73 (95% CI, 0.52–0.86; P = 0.02). In contrast, PHRAC seemed to be no benefit for stage II patients. Toxicities, such as hepatic toxicity and leukocyte decreasing, were mild and could be cured with medicine.
Conclusions:
Preoperative hepatic and regional arterial chemotherapy, in combination with surgical resection, could be able to reduce and delay the occurrence of liver metastasis and therefore improve survival rate in patients with stage III colorectal cancer.
This is a randomized controlled trial that investigates whether preoperative hepatic and regional arterial chemotherapy (PHRAC) can prevent liver metastasis and improve overall survival in stage II and III colorectal cancer (CRC) patients receiving curative resection.
In the case of advanced colon carcinoma, approximately 45% of the patients die of the malignancy1 and 83% of patients with recurrent disease develop a liver metastasis.2 Therefore, prevention of liver recurrence can be expected to improve the prognosis of patients with advanced colon carcinoma. Taylor et al reported that infusion of 5-fluorouracil (5-FU) into the portal vein during the perioperative period resulted in the significant prevention of liver metastasis in patients with colorectal carcinoma without liver metastasis and improved their prognosis.3 Among several follow-up studies, only the report by Wereldsma et al4 reported a decrease in the frequency of liver metastasis after the administration of 5-FU via the portal vein, and to our knowledge, none of these studies demonstrated an improvement in survival.5,6
Conversely, several randomized control trials (RCTs) published to date have demonstrated that hepatic arterial infusion (HAI) of 5-FU or fluorodeoxyuridine (FUDR) produces a significantly better response rate in patients with colon carcinoma metastatic to the liver compared with systemic chemotherapy,7 but fails to significantly improve survival rates. In year 2004, Goldberg et al8 reported a chemotherapy effect of oxaliplatin and irinotecan on liver metastasis of colorectal cancer (LMCC). As to effective rate, median survival time and median progressive time, FOLFOX plan with oxaliplatin is better than IFL plan with Irinotecan. Because of these data, oxaliplatin is now used as the first-line chemotherapy drug of MLCC.
We administered FUDR and oxaliplatin as preoperative hepatic and regional arterial chemotherapy (PHRAC) to patients with stage II or stage III colorectal cancer without apparent liver metastasis based on a thorough preoperative evaluation. The study endpoints were disease-free survival, overall survival, and liver metastasis-free survival as evaluated by intent-to-treat analysis.
MATERIALS AND METHODS
Eligibility Criteria and Pretreatment Evaluation
The inclusion criteria were age <75 years with histologically proven adenocarcinoma of the colon or rectum, no severe major organ dysfunction, a World Health Organization (WHO) performance status of 0 or 1, no prior cancer therapy, and Stage II (T3–4, N0, M0) or Stage III (T0–4, N1–2, M0) disease (according to the 1997 revision of the International Union Against Cancer TNM staging system) as determined by a preoperative evaluation that included colonoscopy and an abdominal computed tomography (CT) scan.
Eligible patients were randomly assigned to receive either PHARC or surgery alone. Randomization was performed a week before surgery. To minimize the potential selection bias, random numbers were generated by one of the authors, who was not involved in enrollment of the patients. Because of the invasive nature of the treatment, randomization was not masked.
Written informed consent was provided by all patients. This RCT protocol was approved by the Institutional Review Board of Fudan University School of Medicine.
Chemotherapy
In the patients assigned to the PHRAC arm, PHRAC, which included of 2 parts (common hepatic artery chemotherapy and main tumor supplying artery chemotherapy), was performed 7 days before surgery.
Common hepatic artery chemotherapy: Under local anesthesia, the celiac artery and the superior mesenteric artery (SMA) were imaged using a 4 Fr Rosch hepatic catheter (Wilson-Cook Medical Inc., Winston-Salem, NC) to confirm the anatomy of the abdominal vessels. The tip of the catheter then was fixed in the gastroduodenal artery using a coil. FUDR 500 mg, oxaliplatin 50 mg, and dexamethasone 2.5 mg were infused from the side hole of the catheter, which was located within the common hepatic artery. Malperfusion was prevented by angiographic embolization of aberrant vasculature.
Main tumor supplying artery chemotherapy: The above standard method of infusion was then used in the primary cancers. The artery chosen for infusion was based upon the location of the cancer. The SMA, inferior mesenteric artery, and superior rectal artery were used for cancers of the cecum and ascending colon, descending or sigmoid colon, and rectum, respectively.
The control arm did not receive chemotherapy preoperatively. For both arms, adjuvant chemotherapy under the standard FOLFOX4 plan was given to patients 3 weeks after surgery.
Surgery and Pathology
Patients underwent surgery approximately 7 days after PHRAC. All patients received a standard bowel regimen. Antibiotics were administered for 1 to 4 days postoperatively. Information regarding the intra-abdominal findings with special reference to regional and distant metastases was obtained from the surgical reports.
Data regarding tumor size, histologic type, tumor penetration, lymph node metastasis, and pathologic TNM disease stage were obtained from the pathologic records.
Follow-up
The follow-up schedule after surgery for asymptomatic patients was a chest x-ray, abdominal ultrasound, or abdominal CT scan, and blood tests including carcinoembryonic antigen every 3 to 4 months for 2 years after surgery. These examinations and tests were continued every 4 to 6 months for 3 to 4 years after surgery. Colonoscopy was conducted every 6 months in the first year after surgery, and every year for 2 to 4 years after surgery. For symptomatic patients, the above examinations and tests were conducted more frequently as clinically indicated.
Statistical Analysis
The primary endpoint of the current study was disease-free survival, and the secondary endpoints were overall survival and liver metastasis-free survival by intent-to-treat analysis. It was estimated that approximately 40% of patients with advanced colon carcinoma develop recurrent disease, among which approximately 60% (or 25% of total patients) would develop a liver metastasis. Based on the hypothesis that PHRAC would reduce liver recurrence from 15% to 5% of all patients, a sample size of 100 patients per arm was calculated to be able to have an 80% chance of detecting a 5% of reduction in the primary end point.
Overall survival curves and disease-free survival curves were generated by the Kaplan-Meier method9 and compared by the log-rank test. The Student t test and χ2 test also were used for the analysis.
RESULTS
Clinical Characterization of Patients
A total of 240 patients were enrolled in the study during a 2-year period (June 2001 to June 2003) and randomized to 1 of the 2 arms prior to surgery. Eighteen patients (10 patients from PHRAC arms and 8 patients from the control) were excluded due to discovery of liver or peritoneal metastasis at the time of surgery, which had not been detected in the preoperative evaluation (Table 1).
TABLE 1. Clinical Characteristics of Patients in This Study
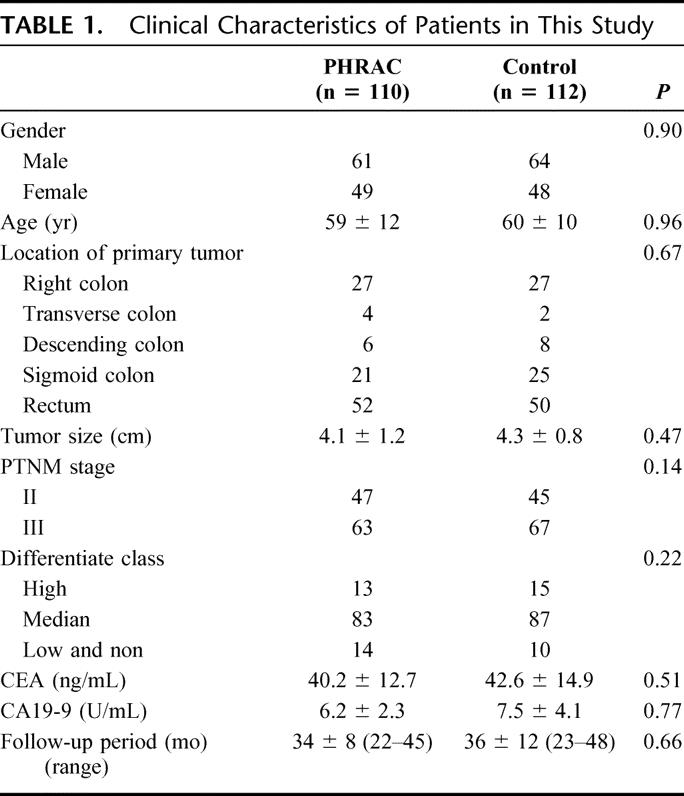
There were no significant differences between the PHRAC arm and the control arm with regard to gender, age, performance status (WHO), location of the primary tumor, tumor size, histopathologic stage of disease, prior cancer therapy, postoperative adjuvant chemotherapy, or duration of follow-up (Table 1). As of the last follow-up on December 30, 2005, no patient has been lost to follow-up. The total follow-up period was 36 ± 5 months (range, 22–48 months; median, 35 months).
Histopathologic Change After PHRAC
Histopathologic evaluation after tumor resection showed obvious necrosis in the middle of the tumor lesions as well as involved lymph nodes.
Toxicity and Complications
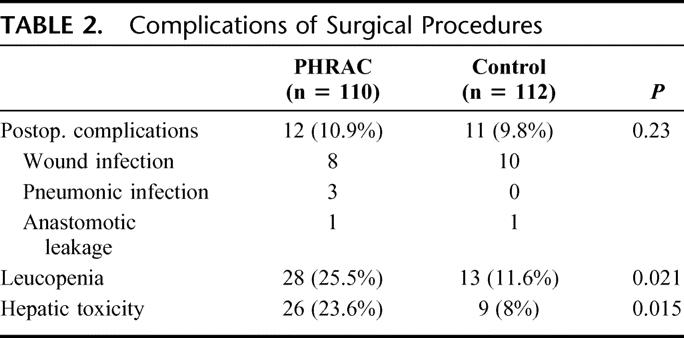
Although elevation of serum aspartate aminotransferase or alanine aminotransferase was observed in 23.6% of patients in the PHRAC arm compared with 8% in the control group (P < 0.05), Grade 3 hepatic toxicity was observed in only 2% of patients in the PHRAC and could be lightened before surgery. No other toxicities were observed. There were no differences noted between 2 arms with respect to postoperative complications, including complications of femoral artery catheterization (Table 2).
TABLE 2. Complications of Surgical Procedures
Sites of First Recurrence
As seen in Table 3, recurrences in the liver were reduced significantly in the PHRAC arm (P = 0.04). Reduction of metastasis to other organs (bone, lung, and peritoneum) was also found in treatment arm compared with the control arm.
TABLE 3. First Sites of Recurrence
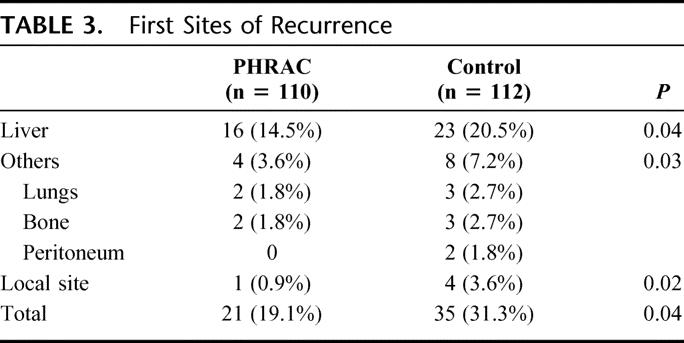
The liver recurrence rates by stage of disease were 3 of the 47 patients of the PHRAC group (6.3%), and 4 of the 45 patients of the control group (8.9%) with stage II disease (P = 0.08), whereas the corresponding rates with stage III disease were 13 of 63 patients of the PHAC group (20.6%), and 19 of 67 patients of the control group (28.4%) (P = 0.02). The same could be seen with attention to the others and local site by stage of disease (Table 4).
TABLE 4. First Site of Recurrence in Stage II and III of CRC
Overall Disease-Free Survival
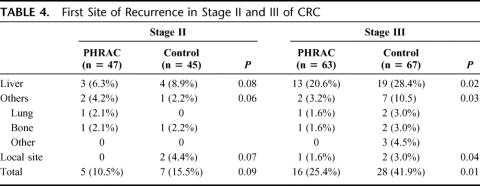
The perioperative mortality rate was 0%. Using intent-to-treat analysis, the PHRAC arm had a significantly better disease-free survival compared with the control arm (P = 0.04) (Fig. 1A). The overall disease-free survival rate was 80.9% in the PHRAC arm and 68.7% in the control arm within a 3-year follow-up. When those patients with stage II and stage III disease were analyzed and compared separately, there were no significant differences between the 2 treatment arms in the stage II patients (this is almost significant, P = 0.09) (Fig. 1B), but there was a significant difference noted in the stage III patients (P = 0.0096) (Fig. 1C). The risk ratio for disease-free survival was 0.61 (95% CI, 0.51–0.79, P = 0.0002) in the PHRAC arm, indicating that the prophylactic therapy reduced the risk of disease recurrence and death by 39% of stage III disease.
FIGURE 1. Disease-free survival. A, Overall disease-free survival rate was 80.9% in the PHRAC arm and 68.7% in the control arm at 3 years (P = 0.04). B, In stage II disease, the overall disease-free survival rate was 89.5% in the PHRAC arm and 84.5% in the control arm at 3 years (P = 0.09). C, In stage III disease, the overall disease-free survival rate was 74.6% in the PHRAC arm and 58.1% in the control arm at 3 years (P = 0.0096).
Overall Survival and Median Survival Time
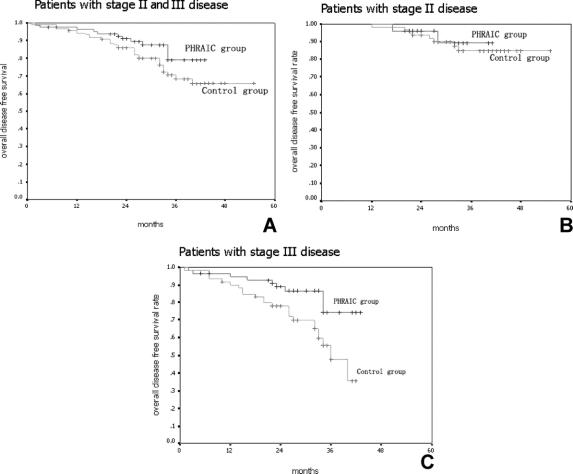
Using intent-to-treat analysis, the PHRAC arm demonstrated a significantly better survival compared with the control arm (P = 0.03) (Fig. 2A). The overall survival was 91.3% in the PHRAC arm and 83.3% in the control arm at 3 years, respectively. When the stage II and stage III patients were analyzed and compared separately, there were no significant differences noted between the 2 treatment arms in the stage II patients (P = 0.07) (Fig. 2B), but there was a significant difference in the stage III patients (P = 0.02) (Fig. 2C). In the PHRAC arm, the risk ratio of overall survival was 0.51 (95% CI, 0.32–0.67; P = 0.009), indicating that the prophylactic therapy reduced the risk of death by 49% of stage III disease.
FIGURE 2. Overall survival. A, Overall survival rate was 91.3% in the PHRAC arm and 83.3% in the control arm at 3 years (P = 0.03). B, In stage II disease, the overall survival rate was 93.9% in the PHRAC arm and 90.0% in the control arm at 3 years (P = 0.07). C, In stage II disease, the overall survival rate was 87.7% in the PHRAC arm and 75.7% in the control arm at 3 years (P = 0.02).
The median survival time was 46.5 ± 5.2 months in PHRAC group and 40.3 ± 7.2 months in the control group, respectively (P = 0.04). There were also no significant differences noted between the 2 treatment arms in the stage II patients (48.4 ± 6.2 months vs. 46.3 ± 5.1 months, P = 0.06), but there was a significant difference in the stage III patients (40.1 ± 4.6 months vs. 36.3 ± 3.2 months, P = 0.03).
Liver Metastasis-Free Survival and Median Liver Metastasis Time
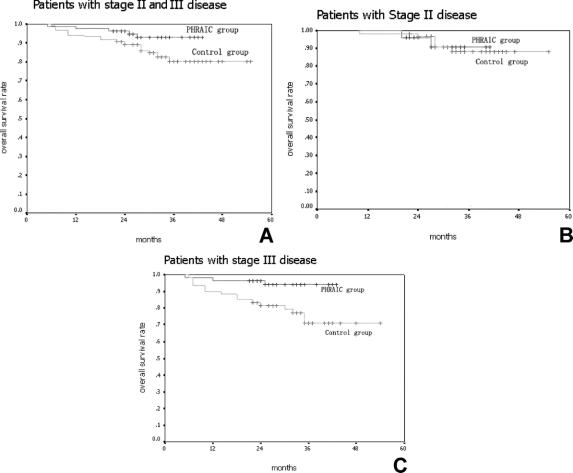
Using intent-to-treat analysis, the PHRAC arm demonstrated a significantly better liver metastasis-free survival compared with the control arm (P = 0.04) (Fig. 3A). The liver metastasis-free survival rate was 85.5% in the PHRAC arm and 79.5% in the control arm at 3 years, respectively. When the stage II and stage III patients were analyzed and compared separately, there were no significant differences noted between the 2 treatment arms in the stage II patients (P = 0.08) (Fig. 3B), but there was a significant difference noted in the stage III patients (P = 0.02). In the PHRAC arm, the risk ratio for liver metastasis-free survival was 0.73 (95% CI, 0.52–0.86, P = 0.02) (Fig. 3C), indicating that the prophylactic therapy reduced the risk of liver metastasis and death by 27% with stage III disease.
FIGURE 3. Liver metastasis-free survival. A, Liver metastasis-free survival rate was 85.5% in the PHRAC arm and 79.5% in the control arm at 3 years (P = 0.04). B, In stage II disease, liver metastasis-free survival rate was 93.7% in the PHRAC arm and 91.1% in the control arm at 3 years (P = 0.08). C, In stage III disease, the overall survival rate was 79.4% in the PHRAC arm and 71.6% in the control arm at 3 years (P = 0.01).
The median liver metastasis detection time was 19 ± 3 months in PHRAC group and 10 ± 2 months in the control group, respectively (P = 0.025).
Furthermore, there were also no significant differences noted between the 2 treatment arms in the stage II patients (22 ± 2 months vs. 18 ± 2 months, P = 0.08), but there was a significant difference in the stage III patients (16 ± 3 months vs. 8 ± 1 months, P = 0.01).
DISCUSSION
It has been estimated that in patients with colon carcinoma but no apparent liver metastasis at the time of surgery who subsequently developed liver metastases, the primary liver metastasis is considered to have developed about 2 years prior to the surgery, based on calculations from the tumor doubling time.10 Thomas et al studied liver tissues removed from cancer patients and found that ultrasonography performed ex vivo was able to detect 95% of tumors with a greatest dimension of 10 mm, but detected none of the tumors measuring 5 mm in greatest dimension.11 Therefore, in patients with no apparent liver metastasis at the time of surgery and judged by the surgeon to have been curatively resected, metachronous liver metastases occurring postoperatively are thought to originate from micrometastases already present at the time of surgery. The liver, accounting for a total of 83% of metastatic recurrence in stage II or stage III colorectal cancer after surgery,12 is the most frequent site of disease recurrence in patients with colon carcinoma. A reduction in the incidence of liver metastases may be the most effective way to reduce the likelihood of recurrence and to improve the overall prognosis. Neoadjuvant chemotherapy is one of the methods being explored to prevent liver metastases after surgery.
PHRAC, which consists of regional arterial chemotherapy and hepatic arterial chemotherapy, is one of the neoadjuvant chemotherapy methods. The killing effect of chemotherapeutic drugs to tumor cells depends on the local concentration in the tumor. This effect can be improved by 10 times as the local drug concentration is doubled. For this reason, regional arterial infusion chemotherapy is a reasonable modality to improve the local concentration of the drugs. In PHRAC, the chemotherapeutic agents were locally infused into major tumor supply arteries (ie, SMA for ascending colon cancer, inferior mesenteric artery for descending colon, and rectal cancer) so that approximately two thirds of the drug acts locally in the tumor, which not only reduced the systemic toxicity and adverse effect of the chemical drug but also obviously improved the killing effect to tumor cells. Besides, the arterial locally infused chemotherapeutic agents could also be able to suppress clinical or subclinical metastatic lesions via systemic circulation, which serve as a “second time” chemotherapy.13
Infusion of the chemical drugs into hepatic artery in PHRAC was based on 2 theories: 1) according to the tumor doubling time: the patients, who developed delayed liver metastases after curative surgery had “micrometastases,” which could not be detected by current imaging technology. Removing the primary tumor who excreted the vessel inhibition factors to inhibit the proliferation of subclinical metastatic foci, would activate the growth of the previous silent micrometastases, resulting in distant metastases.9,14 2) The liver micrometastases (0.5–3 mm in diameter) received abundant blood supply from the hepatic artery, which are the major targets in the treatment of PHRAC.15
Our study performed a neoadjuvant therapy for stage II or stage III colorectal cancer using PHRAC, which has never been reported. In previous reports,16,17 5-FU was always administrated alone, while in our study FUDR (fluorodeoxyuridine, first-pass extraction >90%, short half-time), which is better for local use was administrated combined with oxaliplatin which has certain effect to colorectal carcinoma, especially for liver metastasis of colorectal cancer.8,18 The result showed no significant difference between the PHRAC and the control group in the incidence of postoperative complications. The incidence of postoperative hepatic toxicity and leukopenia was higher in the PHRAC group, but these could be cured with conservation treatment without severe clinical complications (Table 2).
The possible mechanisms of PHRAC may include: induction of apoptosis, inhibition of proliferation, promotion of tumor necrosis, and inhibition of angiogenesis.19–23
As for the treatment after PHRAC, the time for curative surgery was determined by when the chemotherapy drugs began to act. As reported, before the fourth day after the artery infusion chemotherapy, the tumor necroses were inconspicuous as observed through microscopy, while neoformative tumor vessels and tumor tissues could be observed on the surface of necroses tumor after the 10th day. Between the fifth day and the ninth day, the tumor necroses and the embolisms of microvessels were most prominent. Based upon this rationale, we chose the seventh day after PHRAC to perform curative surgeries. The appropriateness of this treatment was supported in our research by the necroses in the middle of the tumor and involved lymph nodes observed by histopathologic examination after surgery.24,25
It is a usual concern that preoperative chemotherapy (neo-adjuvant) could increase the incidence of postoperative complications. In our study, during the 7-day period between chemotherapy and surgery, drug toxicity was closely monitored by measuring blood count, liver function, and body weight. For any patient with evidence of drug toxicity, surgery was postponed and the patient was excluded from our study. Our data did not show surgical complications significantly associated with chemotherapy prior to surgery.
The follow-up result of our study showed that the median liver metastasis time after curative surgery in patients with stage III disease was prolonged in the PHRAC group as compared with the control group, with markedly improved 3-year liver metastasis rate and disease-free survival, overall survival, and median survival time. Thus, PHRAC could prolong the median liver metastasis time and reduce liver and other distant metastases and local recurrence in patients with stage III colorectal carcinoma after surgery, but no benefit was noted in patients with stage II disease. Because of this, an accurate preoperative staging (determined by evaluations including endoscopic ultrasonography, CT, and MRI) is necessary to improve the pertinence of PHRAC and avoid unnecessary extra therapies.
CONCLUSION
PHRAC is a safe and effective method to reduce liver metastasis rate and prolong the median liver metastases time after curative surgery in patients with stage III colorectal carcinoma. It is associated with an improved survival time, but more cases and longer follow-up are required for more reliable assessments. In addition, further trials were needed to determine whether PHRAC in our study was better than the single 5-FU protocol or not.
Footnotes
Reprints: Jianmin Xu, MD, PhD, Department of General Surgery, Zhongshan Hospital, Fudan University Medical Center, Shanghai, 200032, P. R. China. E-mail: xujmin@yahoo.com.cn.
REFERENCES
- 1.Jemal A, Thomas A, Murray T, et al. Cancer statistics, 2002 CA. Cancer J Clin. 2002;52:23–47. [DOI] [PubMed] [Google Scholar]
- 2.Weiss L, Grundmann E, Torhorst J, et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986;150:195–203. [DOI] [PubMed] [Google Scholar]
- 3.Taylor I, Machin D, Mullee M, et al. A randomized controlled trial of adjuvant portal vein cytotoxic perfusion in colorectal cancer. Br J Surg. 1985;72:359–363. [DOI] [PubMed] [Google Scholar]
- 4.Wereldsma JCJ, Bruggink EDM, Meijer WS, et al. Adjuvant portal liver infusion in colorectal cancer with 5-fluorouracil/heparin versus urokinase versus control. Cancer. 1990;65:425–432. [DOI] [PubMed] [Google Scholar]
- 5.Wolmark N, Rockette H, Wickerham DL, et al. Adjuvant therapy of Dukes’ A, B, and C adenocarcinoma of the colon with portal-vein fluorouracil hepatic infusion: preliminary results of National Surgical Adjuvant Breast and Bowel Project protocol C-02. J Clin Oncol. 1990;8:1466–1475. [DOI] [PubMed] [Google Scholar]
- 6.Beart RW, Moertel CG, Wieand HS, et al. Adjuvant therapy for resectable colorectal carcinoma with fluorouracil administered by portal vein infusion: a study of the Mayo Clinic and the North Central Cancer Treatment Group. Arch Surg. 1990;125:897–901. [DOI] [PubMed] [Google Scholar]
- 7.Meta-Analysis Group in Cancer. Reappraisal of hepatic arterial infusion in the treatment of nonresectable liver metastases from colorectal cancer. J Natl Cancer Inst. 1996;88:252–258. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. [DOI] [PubMed] [Google Scholar]
- 9.Finlay IG, Meek D, Brunton F, et al. Growth rate of hepatic metastases in colorectal carcinoma. Br J Surg. 1988;75:641–644. [DOI] [PubMed] [Google Scholar]
- 10.Thomas WM, Morris DL, Hardcastle JD. Contact ultrasonography in the detection of liver metastases from colorectal cancer: an in vitro study. Br J Surg. 1987;74:955–956. [DOI] [PubMed] [Google Scholar]
- 11.Hu W, Yu DH. Operation and local therapy of liver metastasis of colon cancer: how to improve prognosis [in Chinese]. Da Chang Gang Men Wai Ke Za Zhi. 2003;9:265. [Google Scholar]
- 12.Bonnen M, Crane C, Vauthey JN, et al. Long-term results using local excision after preoperative chemoradiation among selected T3 rectal cancer patients. Int J Radiat Oncol Biol Phys. 2004;60:1098–1105. [DOI] [PubMed] [Google Scholar]
- 13.Kemeny MM, Adak SA, Gray B, et al. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver. Surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy: an intergroup study. J Clin Oncol. 2002;20:1499–1505. [DOI] [PubMed] [Google Scholar]
- 14.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–2048. [DOI] [PubMed] [Google Scholar]
- 15.Archer SG, Gray BN. Vascularization of small liver metastases. Br J Surg. 1989;76:545–548. [DOI] [PubMed] [Google Scholar]
- 16.Xu JM, Zhong YS, Niu WX, et al. Preoperative hepatic and regional arterial infusion chemotherapy in the prevention of liver metastasis after colorectal cancer surgery [in Chinese]. Natl Med J. 2006;86:88–92. [PubMed] [Google Scholar]
- 17.Sadahiro S, Suzuki T, Ishikawa K, et al. Prophylactic hepatic arterial infusion chemotherapy for the prevention of liver metastasis in patients with colon carcinoma: a randomized control trial. Cancer. 2004;100:590–597. [DOI] [PubMed] [Google Scholar]
- 18.Xin YQ, Xu JM, Liu FL, et al. Evaluation of Preoperative therapy of advanced digestive cancer [in Chinese]. Zhong Guo Shi Yong Wai Ke Za Zhi. 2005;25:231–233. [Google Scholar]
- 19.Li WS, Liu FK, Chen ZH, et al. Effect of intervention to colon cancer cell proliferation, apoptosis and vascular regeneration [in Chinese]. Chin J Gen Surg, 2002;17:245–246. [Google Scholar]
- 20.Kimura H, Konishi K, Kaji M, et al. Apoptosis, cell proliferation and expression of oncogenes in gastric carcinomas induced by preoperative administration of 5-fluorouracil. Oncol Rep. 2000;7:971–976. [DOI] [PubMed] [Google Scholar]
- 21.Huang W, Zhao Y, Luo L. Effect of preoperative regional artery chemotherapy on gastric carcinoma [in Chinese]. Acta Acad Med Mil Tert. 2003;25:1659–1661. [Google Scholar]
- 22.Ishii HH, Gobe GC, Pan W, et al. Apoptosis and cell proliferation in the development of gastric carcinomas: associations with c-myc and p53 protein expression. J Gastroenterol Hepatol. 2002;17:966–972. [DOI] [PubMed] [Google Scholar]
- 23.Xiao EH, Li JQ, Huang JF. Effects of p53 on apoptosis and proliferation of hepatocellular carcinoma cells treated with transcatheter arterial chemoembolization. World J Gastroenterol. 2004;10:190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Z, Li JS, Liu FK. Therapy opportunity of surgery for rectal cancer after preoperative adjuvant chemotherapy [in Chinese]. Chin J Surg. 1999;37:174–176. [PubMed] [Google Scholar]
- 25.Chau I, Chan S, Cunningham D. Overview of preoperative and postoperative therapy for colorectal cancer: the European and United States perspectives. Clin Colorectal Cancer. 2003;3:19–33. [DOI] [PubMed] [Google Scholar]