Abstract
Background:
Most reports on postoperative (OP) morbidity and mortality following breast cancer surgery (BCS) are limited by relatively small sample size resulting in a lack of national benchmarks for quality of care. This paper reports the 30-day morbidity and mortality following BCS in women using a large prospective multi-institutional database.
Methods:
The National Surgical Quality Improvement Program Patient Safety in Surgery, prospectively collected inpatient and outpatient 30 day postoperative morbidity and mortality data on patients undergoing surgery at 14 university and 4 community centers. Using the procedure CPT code, the database was queried for all women undergoing mastectomy (MT) or lumpectomy with an axillary procedure (L-ANP). Morbidity and mortality were categorized as mortality, wound, cardiac, renal, pulmonary, and central nervous system. Logistic regression models for the prediction of wound complications were developed. Preoperative variables having bivariate relationships with postoperative wound complications with P ≤ 0.20 were submitted for consideration.
Results:
We identified 1660 and 1447 women who underwent MT and l-ANP, respectively. The mean age was 55.9 years. The majority of procedures were under general anesthesia. The 30-day postoperative mortality for MT and l-ALNP were 0.24% and 0%, respectively. The most frequent morbid complication was wound infection, more commonly occurring in the mastectomy (4.34%) group versus the lumpectomy group (1.97%). Cardiac and pulmonary complications occurred infrequently in the mastectomy group (cardiac: MT, 0.12%; and pulmonary: MT, 0.66%). There were no cardiac or pulmonary complications in the lumpectomy group. CNS morbidities were rare in both surgical groups (MT, 0.12%; and l-ALNP, 0.07%). Development of a UTI was more common in women who underwent a mastectomy (0.66%) when compared with women that had a lumpectomy (0.14%). The only significant predictors of a wound complication were morbid obesity (BMI >30), having had a MT, low preoperative albumin and hematocrit greater than 45%.
Conclusion:
Morbidity and mortality rates following BCS in women are low, limiting their value in assessing quality of care. Mastectomy carries higher complication rate than l-ANP with wound infection being the most common.
Determination of the 30-day morbidity and mortality of breast cancer surgery using the multi-institutional prospectively collected private sector data set from the National Surgical Quality Improvement Program (NSQIP). Mastectomy was more frequently associated with complications, however, mortality from breast cancer surgery overall was rare with wound infections being the most common morbidity. The standard NSQIP measurement of quality of care, risk-adjusted morbidity and mortality, may be difficult to apply to breast cancer surgery.
The cornerstone of breast cancer management is surgical. The complication rate following breast surgical procedures is considered to be low. The mortality of breast surgical procedures is reportedly less than 1%.1 Surprisingly, the literature is sparse concerning the perioperative mortality and morbidity following breast cancer surgery. The few available reports are retrospective in nature and limited by small sample size.2–6
Complications, more frequently associated with general surgical procedures and prolonged hospital stays, such as cardiopulmonary and cerebrovascular accidents, have infrequently been investigated in patients following breast surgery, albeit secondary to their rare occurrence. The most frequently cited complications are related to wound infection and seroma formation. Incidence rates for postoperative wound infections are variable and range from 3% to 19%.3 Surgical wound infections following breast surgery are not uncommon and can result in further medical and surgical interventions, which may warrant prolonged hospitalization and outpatient follow-up. Moreover, compromise of the cosmetic outcome and psychologic burden of a surgical site infection after breast surgery are important considerations, which support the need for further clinical inquiry.
The objective of the current study was to investigate the 30-day morbidity and mortality rates following breast cancer surgery in women and define significant predictors of adverse outcomes and their relative importance.
METHODS
To evaluate the morbidity and mortality following breast cancer surgery in women, we used data from the Patient Safety in Surgery Study based on the National Surgical Quality Improvement Program (NSQIP). A detailed description of the NSQIP study methods has been previously published.7 The Patient Safety in Surgery Study was designed to validate the NSQIP in the private sector. Under a grant from the Agency for Healthcare Research and Quality through the American College of Surgeons, 14 university centers and 4 community hospitals joined the existing 123 VA hospitals and contributed data to the NSQIP between 2001 and 2004. A surgical risk-assessment nurse was assigned at each center to collect the data. These nurses underwent comprehensive training on conducting the study protocol for appropriate data collection. Periodic conferences, telephone calls, and annual meetings were held to maintain data reliability. A total of 40 preoperative and 9 intra operative data elements were collected on each surgical case. We also collected height and weight information to allow the calculation of body mass index (BMI). BMI was calculated as weight in kilograms divided by the squared height in meters. A patient was considered obese in this study if his/her BMI is greater than 30. BMI was included as a potential preoperative risk factor. Preoperative, intraoperative, and postoperative variables were chosen based on clinical relevance, reliability of data collection, and availability of data. The patients were followed up in-hospital and as outpatients for 30 days after surgery. Thirty-day operative mortality was defined as death from any cause during hospitalization or after discharge occurring within 30 days of surgery. Postoperative morbidity included 21 selected postoperative adverse events recorded within 30 days. Outcome follow-up information was obtained by chart review, communication with care providers, reports from morbidity and mortality conferences, and communication with patients on the 30th postoperative day via letter or telephone. General and vascular surgical cases were systematically sampled at each participating site. The first 40 consecutive eligible cases were entered in each 8-day cycle, with each cycle starting on a different day of the week.
Using procedure CPT code, the database was queried for all women undergoing mastectomy for cancer (19180, 19220, 19240) or lumpectomy (19120, 19125, 19160) with an axillary node procedure (L-ANP), either an axillary node dissection or a sentinel node biopsy (19162, 38745, 38525). All postoperative events were categorized as mortality, wound infection, cardiac, renal, pulmonary, and central nervous system. We limited the search to the private sector only, which included the 14 university centers and 4 community hospitals. A listing of the hospitals is provided in Appendix 1.
Initial analyses included bivariate comparisons using χ2 for categorical variables and t tests for continuous variables. The low number of nonwound complications precluded reliable analysis of risk factors for these complications. Therefore, we limited our regression analyses to only wound complications. The analysis consisted of logistic regression models for the prediction of 1 or more wound complications. The wound complication outcome is coded “1” if there is 1 or more of the wound events listed (dehiscence, deep wound infection, superficial infection) and “0” if none of the wound events took place. All yes/no predictors are coded “0” for no and “1” for yes. The association of each preoperative risk factor with wound complication was first tested by χ2 analysis. Preoperative predictor variables with P values ≤0.20 were submitted to the logistic regression analysis. The c-index is reported for each model. The c-index is a measure of the predictability of the model. It generally ranges between 0.5 and 1.0. The closer the c-index is to 1.0, the more predictable the model.8 Missing laboratory data were estimated using a regression method that used all of the other known patient characteristics.
Wound complications were classified using a modification of the Centers for Disease Control definitions.
A superficial infection is an infection that occurs within 30 days after the operation and infection involves only skin or subcutaneous tissue of the incision and at least one of the following:
Purulent drainage, with or without laboratory confirmation, from the superficial incision.
Organisms isolated from an aseptically obtained culture of fluid or tissue from the superficial incision.
At least one of the following signs or symptoms of infection: pain or tenderness, localized swelling, redness, or heat and superficial incision is deliberately opened by the surgeon, unless incision is cultured-negative.
Diagnosis of SI by the surgeon or attending physician.
All suture abscesses and infected burns were excluded.
Deep infection is an infection that occurs within 30 days after the operation and the infection appears to be related to the operation and infection involved deep soft tissues (eg, fascial and muscle layers) of the incision and at least one of the following:
Purulent drainage from the deep incision but not from the organ/space component of the surgical site.
A deep incision spontaneously dehisces or is deliberately opened by a surgeon when the patient has at least one of the following signs or symptoms: fever (>38°C), localized pain, or tenderness, unless site is culture-negative.
An abscess or other evidence of infection involving the deep incision is found on direct examination, during reoperation, or by histopathologic or radiologic examination.
Diagnosis of a deep incision infection by a surgeon or attending physician.
Wound infections that involved both the deep and superficial spaces were reported as deep as well as organ/space infections that drained through the incision.
Dehiscence is the separation of the layers of a surgical wound, which may be partial or complete, with disruption of the fascia.
Preoperative wound infection was defined as “Evidence of an open wound that communicates to the air by direct exposure, with or without cellulites or purulent exudates. This does not include osteomyelitis or localized abscess. The wound must communicate to the air by direct exposure.”
RESULTS
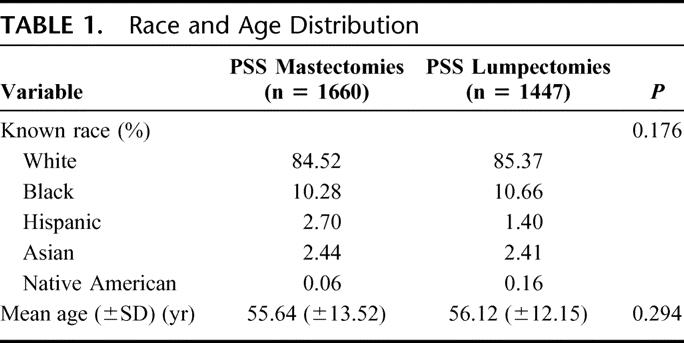
We identified 3107 patients: 1660 (53.4%) who underwent mastectomy and 1447 (46.6%) who underwent l-ANP between October 1, 2001 and September 30, 2004. The racial distribution and mean age are listed in Table 1. The mean operative time for mastectomy and l-ANP was 2.91 and 1.65 hours, respectively.
TABLE 1. Race and Age Distribution
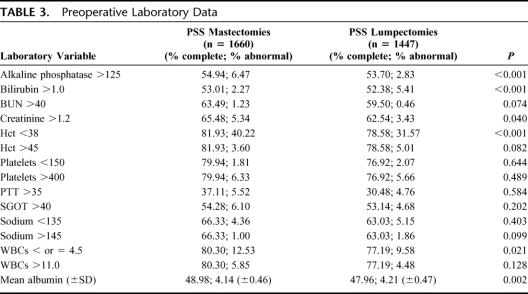
The patients undergoing mastectomy had a higher incidence of congestive heart failure, dependent functional status, diabetes, and a preoperative open wound (Table 2). They were also more likely to be ASA class 3 or 4. Higher proportions of patients undergoing mastectomy had an elevated serum alkaline phosphatase and hematocrits lower than 38% (Table 3).
TABLE 2. Preoperative Comorbidities
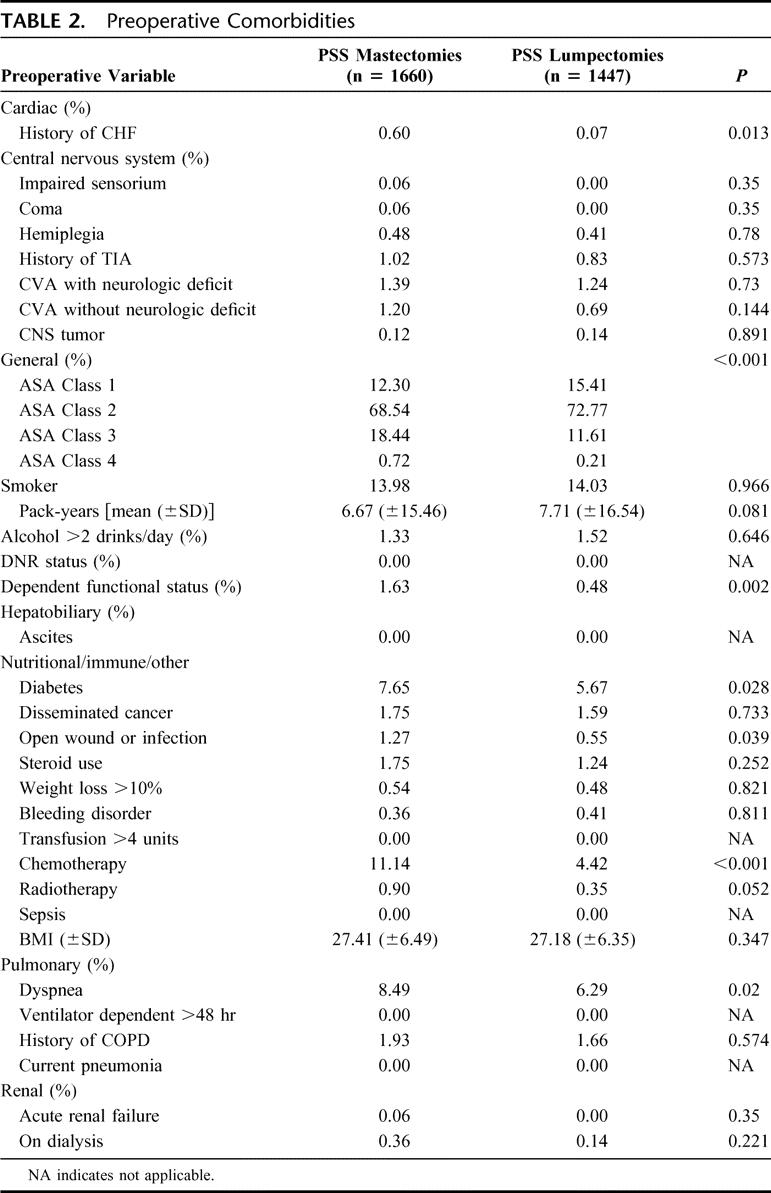
TABLE 3. Preoperative Laboratory Data
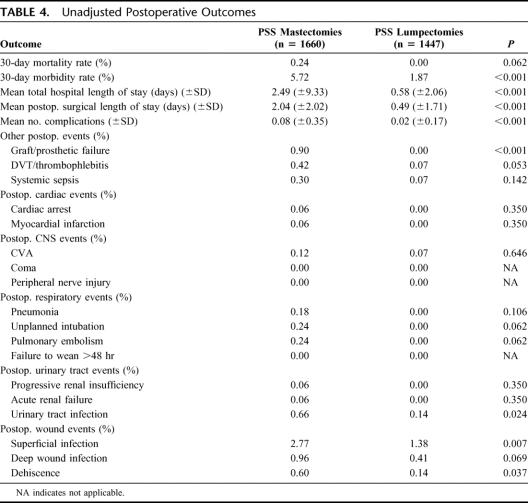
The mortality rates for mastectomy and l-ANP rate were 0.24% and 0.00%, respectively (P = 0.062), with an overall mortality for all cases of 0.128%. The 30-day morbidity rates for mastectomy and l-ANP were 5.72% and 1.87%, respectively (P < 0.001). The most frequent morbidity was wound related with an overall incidence of 3.63% (Table 4).
TABLE 4. Unadjusted Postoperative Outcomes
Cardiac morbidity following breast surgery was extremely low, as was the prevalence of preoperative cardiac risk factors in the current patient population (Table 2). One patient developed a postoperative myocardial infarction and another suffered from cardiac arrest in the mastectomy group (0.06%).
Postoperative pulmonary events were minimal despite most patients undergoing general anesthesia. Of note, there were no pulmonary complications for the l-ANP group, despite 94.6% of patients undergoing general anesthesia. All of the pulmonary events occurred in patients undergoing a mastectomy. Approximately 1.8% of patients had a preoperative diagnosis of chronic obstructive pulmonary disease.
The most frequent postoperative renal event was infection with an overall rate of 0.42%. The incidence of urinary tract infection was higher for mastectomy (0.66%) when compared with l-ANP (0.14%) (P = 0.024). Renal insufficiency and failure postoperatively were rare events. There were no cases of renal insufficiency or failure in the l-ANP group. In the mastectomy group, 1 patient (of 1660) developed progressive renal insufficiency and another patient (of 1660) developed acute renal failure. Preoperatively, the overall prevalence of renal risk factors, such as acute renal failure and patients on hemodialysis, was 0.06% and 0.26%, respectively.
The occurrence of postoperative stroke was extremely low (0.1%) in both surgical groups. No patient in either group went into coma postoperatively. No peripheral nerve injuries were recorded. Overall preoperative CNS history of transient ischemic attacks, CVA with neurologic deficit, and CVA without neurologic deficit accounted for 0.93%, 1.31%, and 1%, respectively.
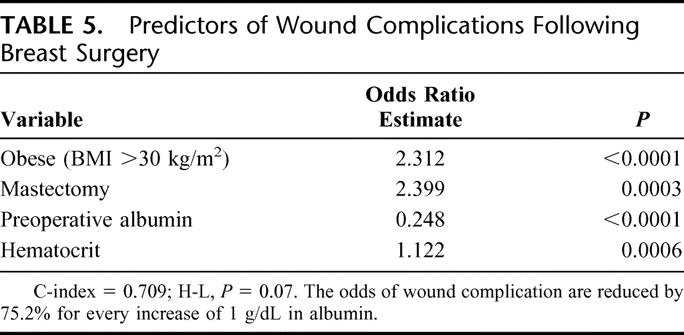
The wound infection rate for mastectomy and l-ANP were 4.34% and 1.97%, respectively, with a total of 100 events in the 3107 patients. Detailed analysis of postoperative wound events demonstrated that most infections were superficial (2.12%); mastectomy was associated with a higher rate compared with l-ANP (2.77% vs. 1.38, P = 0.007). Performance of a mastectomy, compared with l-ANP, was also associated with more significant wound issues, such as deep infection and dehiscence. Variables submitted for stepwise selection in association with wound complications were ASA class, bleeding disorder, diabetes, disseminated cancer, dyspnea, functional status, history of congestive heart failure, history of chronic obstructive pulmonary disease, inpatient/outpatient surgery, smoking, preoperative wound infection, wound class, hematocrit >45, platelets <150, mastectomy, obesity (BMI >30), and preoperative albumin. The only significant independent predictors of wound complications were obesity, undergoing a mastectomy, preoperative albumin, and hematocrit (Table 5). The c-index of 0.709 and a nonsignificant Hosmer-Lemeshow (H-L) test indicate that the model is able to predict wound events with good calibration and discrimination.
TABLE 5. Predictors of Wound Complications Following Breast Surgery
DISCUSSION
Surgical management is a fundamental treatment of breast cancer and in the majority of cases is the first mode of therapeutic intervention. Complications from breast surgical procedures could be costly and may delay subsequent adjuvant therapies. The current study used a prospective multi-institutional database from the NSQIP to determine the 30-day morbidity and mortality of breast surgical procedures. Although many studies have examined the most frequently reported postoperative morbidity of wound complication following breast surgery, the present study is the only study to investigate a broad spectrum of predictive preoperative risk factors in relation to postoperative wound complications.
The National Veteran's Affairs (VA) Surgical Risk Study was started in 1991 with the objective of developing an outcome reporting system and to evaluate risk-adjusted models to facilitate the prediction of surgical outcomes for comparative analysis of the quality of surgical care between various facilities. The database provides opportunities to analyze perioperative outcomes from multiple surgical procedures.9,10
As anticipated, we found the mortality of breast surgical procedures to be extremely low. Of the 1660 patients who underwent a mastectomy, 4 (0.24%) died, but none of the 1447 patients who underwent l-ANP died. A few factors may account for the very low mortality rate for breast surgery. First, surgical management of breast cancer is performed on an elective basis, which allows preoperative evaluation and optimization, particularly for the higher-risk patient. Given that only 1% of breast cancers are diagnosed in men, women overwhelmingly constitute the patient population undergoing surgery for the breast. The male gender has been fairly well secured as a risk factor for the sequelae of atherosclerotic disease; thus, women undergoing surgery may be less vulnerable to postoperative cardiac life-threatening complications. Yanik et al reported that high severity heart disease in women with breast cancer increased with age, affecting <6% of patients between 55 and 59 years, increasing to 16.4% in the 70- to 74-year age group.5 The patients included in our study were largely (>85%) classified as ASA 1 or 2, further confirmation that breast cancer surgery is performed in a relatively healthy population. In addition, surgery of the breast, an extracorporal gland, avoids the physiologic stresses typically associated with violation of the abdomen or chest, which certainly contributes to the low mortality rate of these procedures. Lastly, the handling of a subcutaneous structure, such as the breast, limits exposure to virulent organisms. The predominant organisms reported in the literature from wound infection following breast surgery are Staphylococcus aureus and Staphylococcus epidermis.2,3,11,12
Cardiac complications are frequently cited as the primary cause of postoperative surgical mortality.6 Eagle et al reported that patients undergoing low-risk surgical procedures including breast operations had a very low risk of perioperative myocardial infarction or death that were not significantly influenced by preoperative optimization of bypass surgery.6 As would be anticipated, the patients in this study had few preexisting cardiac comorbidities, and performance of a breast surgical procedure was associated with a very low risk of myocardial infarction or cardiac arrest (mastectomy, 0.06%; and l-ANP, 0%). Additionally, the incidence of preexisting cerebrovascular comorbidities was low, and the 30-day rate of CNS complications following breast surgery was insignificant.
Lucci et al found that patients who underwent a mastectomy, when compared with l-ANP, required significantly greater hospital stays and postoperative narcotics for pain control, longer time to return to work, and a higher rate of postoperative complications.13 Our data are consistent with the observation of Lucci et al13 that mastectomy carries a significantly higher morbidity. The trend toward increased mortality between mastectomy and breast preservation (P = 0.062) in our series further support the preference of breast preservation to mastectomy when both options are available.
The results of the current investigation demonstrated wound complications to be the most common morbidity of breast cancer surgical procedures. We found an overall wound infection rate of 3.16%, which is in accord with the incidence of clean wound infections (1%–5%).2 The wound infection rate for mastectomy was 4.34% and for l-ANP was 1.97%. These wound infection rates are lower than what has been previously reported using the VA experience in the NSQIP data set. The previously reported superficial and deep wound infection rates for breast cancer surgery in women were 6.4% and 2.5%, respectively.1 The literature to date regarding the incidence of clean wound infections in breast surgery is variable with reported rates between 3% and 19%.3 Although breast surgery is considered a clean surgical procedure wound complications are not uncommon.2–4 Wound infections, seromas, hematomas, and epidermolysis are the most frequently observed complications. A study by Vinton et al reported a wound complication rate of 35% for Lumpectomy and axillary node dissection (L-AND) and 49% for modified radical mastectomy (MRM) with the most common complication of seroma formation (L-AND, 18%; and MRM, 29%).2 Vinton et al have reported a wound infection rate of 13% for l-AND and 15% for mastectomy.2 Another study reported wound infection rates of 6.6% for lumpectomy and 19% for mastectomy.3 Although these incidence rates are considerably higher than those reported from our study, the finding of mastectomy being associated with a greater number of wound infections is comparable.2,3 Factors that may contribute to the higher rate of wound infections following a mastectomy include extensive tissue dissection, drain placement, seroma formation, and prolonged operative time. A lower rate of wound complications was also reported by Siegal et al when drains were not used following l-AND.14 A weakness of the present study is the lack of data on preoperative antibiotic, drain insertions, and postoperative seromas and hematomas. Although some studies have shown that prophylactic antibiotics reduce postoperative infection rates, a consensus has not been solidified.
Patient variables have also been investigated and shown to be influential for the development of postoperative wound infections. Age, obesity, and diabetes have consistently been associated with increased morbidity throughout the literature.2–4 Additional modifiable factors such as smoking and alcohol intake have also been associated with a higher incidence of postoperative complications. The current study evaluated a host of comorbid variables that potentially could impact wound complications. Factors that have been previously reported to be contributory such as age, smoking, and alcohol use were not significantly associated with postoperative wound complications. In the current study, the only predictors of wound complication were BMI, albumin level, hematocrit, and whether the patient underwent a mastectomy.
Prior results from the NSQIP demonstrated that hypoalbuminemia negatively impacts surgical outcomes.15 Preoperative albumin level was closely associated with major infectious complications (systemic sepsis, pneumonia, and deep wound infections). Wattanakit et al reported that only low albumin and elevated creatinine remained independent markers of risk of cardiovascular disease events.16 Proper wound healing requires recruitment of immunologic cellular activity, protein synthesis, and adequate nutritional status. Thus, in the current study, it is not surprising that preoperative albumin level was found to be a predictor of wound complications. The mean overall albumin level was 4.08 and was shown to be protective against the development of postoperative wound complications.
With approximately half of all women diagnosed with invasive breast cancer aged 65 or older, performance of breast surgery on an expanding geriatric population may potentially impact mortality and morbidity rates. Age has consistently been demonstrated to be associated with postoperative wound infections following breast surgery.2–4 However, the current study did not reveal chronologic age as a significant predictor of wound complication when included with other factors. Albumin level, hematocrit, and BMI are more important for wound healing and immune defense mechanism; these factors may also be a surrogate to comorbid conditions that in a multivariate analysis outweigh the factor of chronologic age.
Our study is consistent with others, demonstrating that mastectomy carries a higher morbidity particularly wound complications, and possibly mortality when compared with breast preservation. Similarly, our study suggests that patient's overall health status (ie, albumin, hematocrit, BMI) can place a patient at higher risk for wound infections, thereby providing an opportunity to consider prophylactic antibiotics in this select group Even so, breast cancer surgery carries a very low mortality and morbidity rate. Hence, the standard NSQIP measurement of quality of care in general surgery, risk-adjusted morbidity and mortality, may be difficult to apply to breast cancer surgery when comparing performance between different centers due to the very low number of events. Quality of care in breast cancer surgery perhaps ought to be measured with survival and local control of the disease as well as timeliness and appropriateness of care as preoperative diagnosis, frequency of breast preservation, false-negative rate of sentinel nodes, appropriate delivery of chemotherapy, radiation, and endocrine therapy.
APPENDIX
Hospitals contributing cases include the following: Barnes-Jewish Hospital, Brigham & Women's Hospital, Emory University Medical Center, Massachusetts General Hospital, New York Presbyterian Hospital-Columbia University, New York Presbyterian Hospital-Cornell Weil Medical College, St. Louis University, University of California San Francisco, University of Florida Gainesville, University of Kentucky Chandler Medical Center, University of Maryland, University of Michigan Health System, University of Utah Health Science Center, University of Virginia Health System, Community hospitals from the Partners Health Care System: Newton-Wellesley Hospital, Faulkner Hospital, Salem Hospital, and Union Hospital.
Footnotes
Supported by Grant No. 1U18HS11913-03 from the Agency for Healthcare Research and Quality.
Reprints: Mahmoud El-Tamer, MD, FACS, Atchley Pavilion, 10th Floor, 161 Fort Washington Avenue, New York, NY 10032. E-mail: me180@columbia.edu.
REFERENCES
- 1.Hynes DM, Weaver F, Morrow M, et al. Breast cancer trends and outcomes: results from a National Department of Veterans Affairs Study. J Am Coll Surg. 2004;198:707–716. [DOI] [PubMed] [Google Scholar]
- 2.Vinton AL, Traverso LW, Jolly PC. Wound complications after modified radical mastectomy compared with tylectomy with axillary lymph node dissection. Am J Surg. 1991;161:584–587. [DOI] [PubMed] [Google Scholar]
- 3.Roststein C, Ferguson R, Cumming KM, et al. Determinants of clean surgical wound infections for breast procedures at an oncology center. Infect Control Hosp Epidemiol. 1992;13:207–214. [DOI] [PubMed] [Google Scholar]
- 4.Nieto A, Lozano M, Moro MT, et al. Determinants of wound infections after surgery for breast cancer. Zentralbl Gynakol. 2002;124:429–433. [DOI] [PubMed] [Google Scholar]
- 5.Yanik R, Wesley MN, Ries LAG, et al. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. [DOI] [PubMed] [Google Scholar]
- 6.Eagle KA, Rihal CS, Mickel M, et al. Cardiac risk on noncardiac surgery: influence of coronary disease and type of surgery in 3368 operations. Circulation. 1997;96:1882–1887. [DOI] [PubMed] [Google Scholar]
- 7.Khuri SF, Daley J, Henderson W, et al. The National Veterans Affairs Surgical Risk Study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg. 1995;180:519–531. [PubMed] [Google Scholar]
- 8.Lemeshow S, Hosmer DW. A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92. [DOI] [PubMed] [Google Scholar]
- 9.Webster C, Neumayer L, Smout R, et al. Prognostic models of abdominal wound dehiscence after laparotomy. J Surg Res. 2003;109:130–137. [DOI] [PubMed] [Google Scholar]
- 10.Longo WE, Virgo KS, Johnson FE, et al. Mortality after colectomy for colon cancer. Dis Colon Rectum. 2000;43:83–91. [DOI] [PubMed] [Google Scholar]
- 11.Beatty D, Robinson GV, Zaia JA, et al. A prospective analysis of nosocomial wound infection after mastectomy. Arch Surg. 1983;118:1421–1424. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Gutkin Z, Bawnik J. Postoperative infections in breast surgery. J Hosp Infec. 1991;17:61–64. [DOI] [PubMed] [Google Scholar]
- 13.Lucci A, Shoher A, Sherman M, et al. Assessment of the current medicare reimbursement system for breast cancer operations. Ann Surg Oncol. 2004;11:1037–1044. [DOI] [PubMed] [Google Scholar]
- 14.Seigal BM, Mayzel KA, Love SM. Level I and II axillary dissection in the treatment of early-stage breast cancer: an analysis of 259 consecutive patients. Arch Surg. 1990;125:1144–1147. [DOI] [PubMed] [Google Scholar]
- 15.Gibbs J, Cull W, Henderson W, et al. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134:36–42. [DOI] [PubMed] [Google Scholar]
- 16.Wattanakit K, Folsom AR, Chambless LE, et al. Risk factors for cardiovascular event recurrence in the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2005;149:606–612. [DOI] [PubMed] [Google Scholar]