Abstract
Objective:
To describe risks for, and microbiology and antimicrobial resistance patterns of, war trauma associated infections from Operation Iraqi Freedom.
Background:
The invasion of Iraq resulted in casualties from high-velocity gunshot, shrapnel, and blunt trauma injuries as well as burns. Infectious complications of these unique war trauma injuries have not been described since the 1970s.
Methods:
Retrospective record review of all trauma casualties 5 to 65 years of age evacuated from the Iraqi theatre to U.S. Navy hospital ship, USNS Comfort March to May 2003.War trauma-associated infection was defined by positive culture from a wound or sterile body fluid (ie, blood, cerebrospinal fluid) and at least two of the following infection-associated signs/symptoms: fever, dehiscence, foul smell, peri-wound erythema, hypotension, and leukocytosis. A comparison of mechanisms of injury, demographics, and clinical variables was done using multivariate analysis.
Results:
Of 211 patients, 56 met criteria for infection. Infections were more common in blast injuries, soft tissue injuries, >3 wound sites, loss of limb, abdominal trauma, and higher Injury Severity Score (ISS). Wound infections accounted for 84% of cases, followed by bloodstream infections (38%). Infected were more likely to have had fever prior to arrival, and had higher probability of ICU admission and more surgical procedures. Acinetobacter species (36%) were the predominant organisms followed by Escherichia coli and Pseudomonas species (14% each).
Conclusions:
Similar to the Vietnam War experience, gram-negative rods, particularly Acinetobacter species, accounted for the majority of wound infections cared for on USNS Comfort during Operation Iraqi Freedom. Multidrug resistance was common, with the exception of the carbapenem class, limiting antibiotic therapy options.
We reviewed 211 war-wounded patients from Iraq. Infections were more common in blast injuries, soft tissue injuries, >3 wound sites, loss of limb, abdominal trauma, and higher Injury Severity Score (ISS). Gram-negatives accounted for the majority of wound infections. Multidrug resistance was common, except for the carbapenem class, limiting antibiotic therapy options.
Sir William Osler writing on the difficulties of casualty care in 1914 stated: This is an artillery war in which shrapnel do the damage, tearing flesh, breaking bones and always causing jagged irregular wounds. And here comes in the great tragedy – sepsis everywhere, unavoidable sepsis!..The surgeons are back in the pre-Listerian days and have wards filled with septic wounds. the wound of shrapnel and shell is a terrible affair, and infection is well nigh inevitable.1 Ninety years later, his quote remains pertinent. War wounds are distinct from peacetime traumatic injuries because these higher velocity projectiles and/or blast devices cause a more severe injury and accompanying wounds are frequently contaminated by clothing, soil, and environmental debris2 (Figure 1). Recent advances in aeromedical evacuation and trauma care have resulted in significantly lower mortality rates among battlefield casualties,3 but since the Vietnam War era4,5 there have been no published descriptions of risks for and morbidity of combat wound infections.
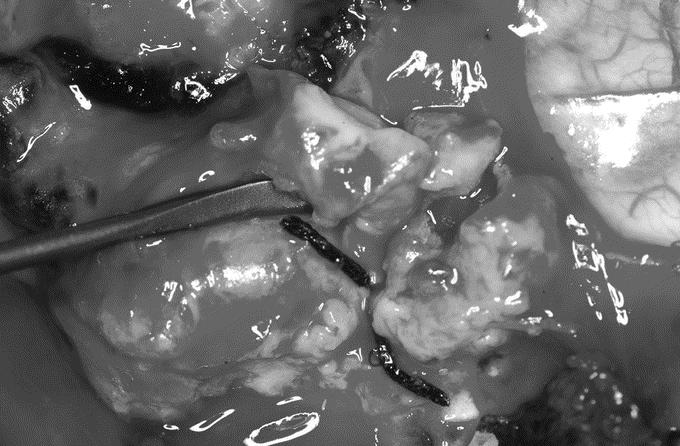
FIGURE 1. Typical war injury from Iraq offensive in 2003. Note clothing fiber and dirt and necrotic material deep in the wound bed.
Current battlefield casualty management starts at time of wounding (Figure 2). Initially, buddy/self-care is performed to include hemorrhage control and pain management. The casualty is immediately evacuated to an Echelon II facility: Forward Resuscitative Surgical System for Marines or Forward Surgical Team for Army personnel for surgical hemostasis and perioperative antibiotics. During the assault phase of Operation Iraqi Freedom, these surgical teams were embedded in front line units and therefore were immediately available to receive casualties, but they had limited ability to provide prolonged perioperative care. Once stabilized, casualties are evacuated to Echelon III facilities. These facilities are staffed with surgical subspecialists and can provide extended perioperative care due to increased bed capacity.6
FIGURE 2. Aeromedical evacuation process for casualties in initial phases of Operation Iraqi Freedom.
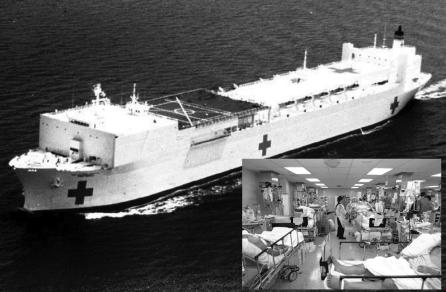
USNS Comfort (TAH-20), a 1000-bed hospital ship, was the largest Echelon III facility in theater for the initial phases of Operation Iraqi Freedom in 2003 (Figure 3). USNS Comfort initially received all wounded coalition forces from the Basrah region, but as combatants moved further inland and Medevac routes became longer, it was used more for its theater-unique neurosurgical, angiography, CT scan, and burn care capabilities, allowing for stabilization prior to Medevac to the Echelon IV facilities in Europe. In addition, it became the preferred site for receiving injured Iraqi patients, particularly enemy combatants due to its isolated location in the Persian Gulf. Military and civilian casualties were evacuated from several sources including 2 Army Combat Support Hospitals, several Forward Resuscitative Surgical System and Forward Surgical Teams, 1 Navy Fleet Hospital, and a British Hospital Ship.
FIGURE 3. USNS Comfort (TAH-20) underway. Inset: ICU #2 at height of operations, over 30 ventilated patients with multiple traumatic casualties were managed in relatively tight quarters.
During the height of casualty evacuations in April 2003, USNS Comfort experienced a cluster of multidrug resistant Acinetobacter baumanii infection on its wards and ICUs.7 A comprehensive investigation failed to locate a common environmental point source on the ship, and a frequency distribution of date of infection showed a propensity to positive culture in the first 48 hours of hospitalization. Battlefield casualties themselves were postulated to be the source of infection with acquisition occurring prior to admission to our hospital ship. In light of this, we sought to describe war trauma associated infection (WTAI), define the factors associated with acquisition of WTAI, and further characterize the causal WTAI microorganisms, including antimicrobial resistance patterns.
METHODS
During the assault phase of Operation Iraqi Freedom, the USNS Comfort admitted 300 patients, of which 211 were trauma patients classified as wounded in action (WIA). Injuries included high velocity projectile (gunshot, fragmentation/shrapnel), blunt force, or blast injuries to the skull, face, torso, abdomen, pelvis, and extremities, as well as burns. A retrospective medical record review was performed on these 211 patients. Information was abstracted by 5 investigators using a pretested structured form for pertinent information including, but not limited to, injury and hospitalization dates, mechanism of injury, clinical signs and symptoms at time of first culture, treatment course and outcomes, microbiologic culture data, and antimicrobials given prior to the development of WTAI. In addition, a composite trauma Injury Severity Score (ISS) was calculated on all patients as detailed by the methods of Baker et al.8
The surgical management of wounds was similar for all patients WIA. Aggressive debridement of all necrotic tissue was performed in the operating room upon arrival to the USNS Comfort. Further follow on wound care included daily wet to dry dressing changes and wound vacuum assisted closure therapy depending on the availability of suction on board Comfort. Additional wound debridements were performed as necessary and dressing changes on large wounds were performed in the operating room to assist in patient comfort.
Microbiologic isolates of wounds were collected in the operating room either as debrided tissue or swabs of the wound bed. Cerebrospinal fluid specimens were collected via sterile lumbar puncture and sterile blood culture technique was used at the time of wound isolate collection or first febrile episode in the hospital. Standard culture techniques were used and identification was performed using API strips (bioMérieux, Inc., Durham, NC). Susceptibility testing was performed by the Kirby Bauer disc method,9 using standard NCCLS criteria.10 In an attempt to correctly classify true infections from normal skin flora and/or colonization status without infection, a case of a WTAI was defined by history of positive culture from a wound or sterile body fluid (ie, blood, cerebrospinal fluid) and at least 2 of the following infection-associated signs/symptoms: fever (temperature >100.4°F), dehiscence, foul smell, peri-wound erythema, hypotension (systolic <90 mm Hg or diastolic <50 mm Hg), and leukocytosis (>10,000 cells/μL). Patients classified as noninfected did not meet the case definition of WTAI.
Characteristic variables were compared between infected and noninfected using χ2 or Fisher exact test tests for categorical data, and Student t tests or Kruskal-Wallis for continuous variables. The univariate results exploring associations between possible predictive variables and the outcome of WTAI were used to develop a multivariate model to adjust for potential confounding. Given the relatively low outcome probability of 26% (<30%), we considered a logistic regression model appropriate for the multivariate analysis. All variables with a P value less than 0.20 were considered eligible for inclusion and a reiterative stepwise approach was used to build a final model. Odds ratios (OR) of potential predictive variables are reported. A second analysis was conducted comparing Acinetobacter spp.-infected cases to non-Acinetobacter spp. WTAI cases. Further description of pathogen, culture site, and antimicrobial susceptibility testing was described. Statistical significance for all associations was set at the P < 0.05 level. All analysis was performed using Stata version 9.0 (College Station, TX). This study was reviewed and approved by the Institutional Review Board of the National Naval Medical Center Bethesda, Maryland.
RESULTS
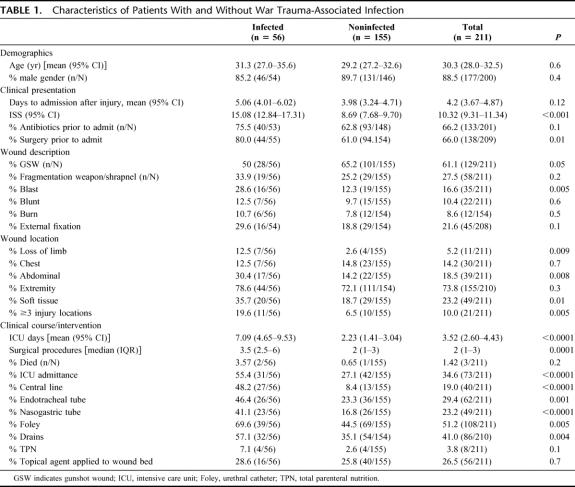
A total of 211 correctly classified WIA patients were admitted to the USNS Comfort between 19 March 2003 and 24 April 2003. A total of 179 (85%) were Iraqi nationals and 32 (15%) were Americans. Eighty-five WIA patients had positive microbiologic cultures at any time during hospitalization and, of these, 56 met the case definition for WTAI. The remaining 155 WIA were designated noninfected. Demographic and clinical characteristics of WTAI and noninfected are displayed in Table 1, the mean age of all WIA was 30 years, and 89% were male. The mean time to admission after injury was 4.2 days. Overall mortality was 1.4%, 2 patients died of sepsis and one from a pulmonary embolism.
TABLE 1. Characteristics of Patients With and Without War Trauma-Associated Infection
There were no differences in age and gender between infected and noninfected. Compared with noninfected, WTAI more often had surgery prior to admission, had a higher ISS, and had injuries qualified as blast, abdominal, soft tissue, ≥3 injury locations, or loss of limb (P < 0.05). No individual factor completely explained case status. Gunshot wounds were less frequently associated with wound infection. (P ≤ 0.05).
The probability of an ICU admission was more than doubled in infected compared with noninfected (55.4% vs. 27.1%, P < 0.0001), and infected had more surgical procedures during admission (median 3.5 vs. 2, P = 0.0001). Consistent with this finding, we also observed procedures and devices typically associated with an ICU admission were also used more frequently in infected. Placement of a central line, nasogastric tube or total parenteral nutrition appeared to be relatively more common among WTAI compared with noninfected (3 or more times higher). There were no differences in the mortality between WTAI case and noninfected, although there were only 3 mortalities, 2 occurring in WTAI and one among noninfected (P = 0.2).
The final model of the multivariate logistic regression for potential predictor variables found that wound type was associated with the outcome of WTAI with soft tissue injuries (OR = 2.4, P = 0.04) or abdominal injury (OR = 2.7, P = 0.04) more frequently being associated with WTAI, and gunshot wounds (OR = 0.4, P = 0.02) being less commonly associated with WTAI. Additionally, increasing ISS seemed to be associated with increasing risk of WTAI (OR = 3.0, P < 0.001) when modeled as an ordinal variable using ISS categories of increasing severity (mild, 0–10; moderate, 11–20; severe, >20). Lastly, other variables that where associated with increased odds in WTAI included delay to ship transport of greater than 3 days (OR = 2.5, P = 0.02) and having an external fixator (OR = 3.0, P = 0.01).
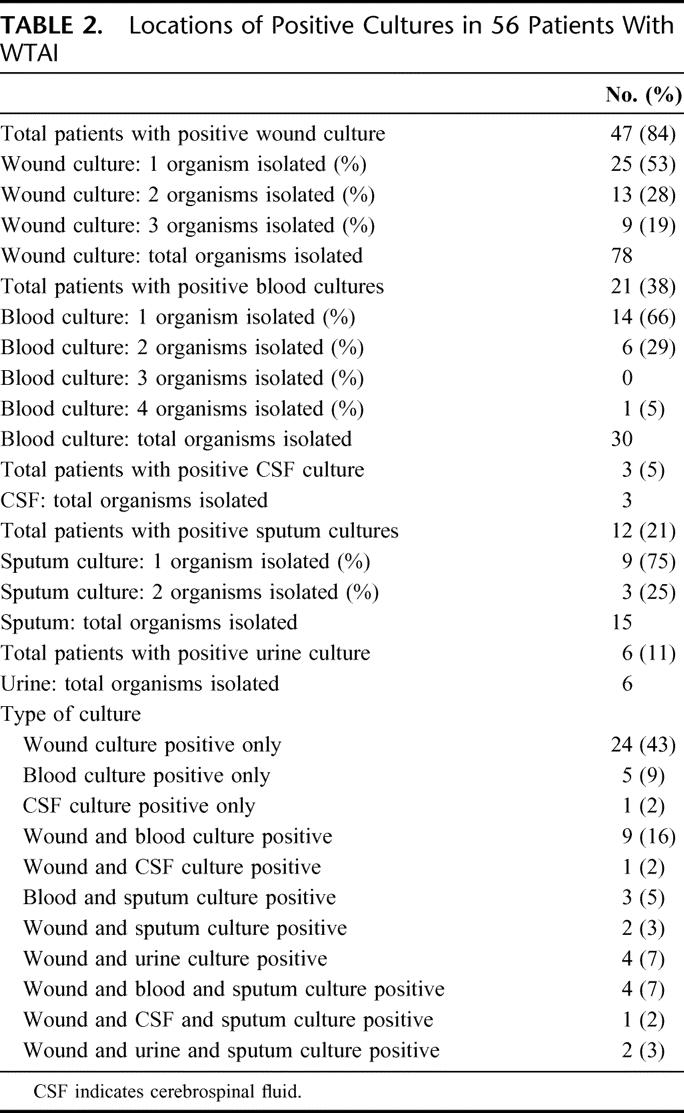
Location and number of organisms per site for infected patients are indicated in Table 2. Overall, 47 (84%) of cases had wound infections followed by 21 (38%) with bloodstream infections 12 (21%) with positive sputum cultures, and 6 (11%) with positive urine cultures. Three (5%) patients had cerebrospinal fluid infection. Of wound cultures, 47% were polymicrobial while bloodstream isolates were 34% polymicrobial.
TABLE 2. Locations of Positive Cultures in 56 Patients With WTAI
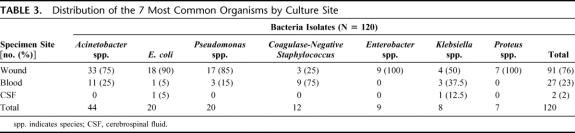
Of a total of 132 unique organisms that were isolated from 56 patients, Acinetobacter spp. were the most common isolate overall (33%, n = 44) and represented 36% of all wound isolates and 41% of all bloodstream isolates. Escherichia coli and Pseudomonas spp. accounted for 14% each, followed by coagulase-negative staphylococcal (CoNS) infection (9%), Klebsiella spp., and Enterobacter spp. (both 6%), and Proteus spp. (5%). All other organisms (35%) represented a mixture of Streptococcus spp. and miscellaneous gram-negatives. Overall, 19% of organisms were gram-positive and 81% were gram-negative. Table 3 displays the distribution of the 7 most common organisms by culture site. Because of incomplete records, we were unable to collect actual rates of antibiotics administered before admission to our ship, but noted most commonly used Echelon II perioperative agents were cefazolin for most injuries, and either ciprofloxacin or gentamicin plus metronidazole; or ampicillin/sulbactam for abdominal injuries.
TABLE 3. Distribution of the 7 Most Common Organisms by Culture Site
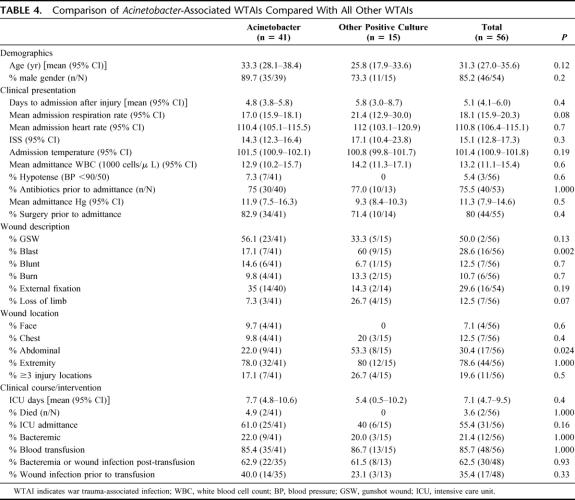
Subset analysis of WTAI associated with A. baumanii is displayed in Table 4. Acinetobacter-WTAI occurred less often in abdominal wounds (P = 0.02) and blast injuries (P = 0.002) and showed a trend toward being more common in limb loss or in patients with dyspnea. Acinetobacter WTAIs had a higher mean number of ICU days (7.7 vs. 5.4) and were associated with both infection-related deaths.
TABLE 4. Comparison of Acinetobacter-Associated WTAIs Compared With All Other WTAIs
Antibiotic susceptibility patterns of 7 most common organisms isolated are displayed in Table 5. Acinetobacter spp. isolates exceeded 80% resistance to all drugs tested except imipenem. E. coli were 85% resistant to ciprofloxacin, and E. coli and Klebsiella spp. were very resistant to third-generation cephalosporins, but all of these isolates were carbapenem susceptible.
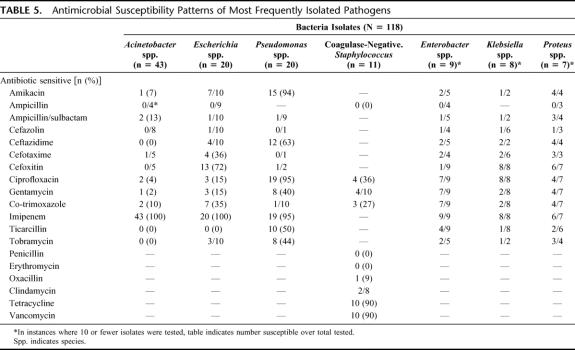
TABLE 5. Antimicrobial Susceptibility Patterns of Most Frequently Isolated Pathogens
DISCUSSION
WTAI has demonstrated a gradual evolution in mortality and bacterial predominance over time with the development of better surgical and antimicrobial treatments. In World War I, the leading microbiologic causes of death and morbidity respectively were Streptococcus and Clostridium perfringens gangrene.11–13 By World War II, although the majority of wound isolates were still anaerobic and aerobic Streptococci14, penicillin and sulfa drugs had significantly decreased mortality by 50%.15 Gram-negatives emerged in the Korean War (12%–69% of all isolates)16 and by the Vietnam War were the predominant organisms isolated.4,5,17 Simchen and Sacks attributed this phenomenon to use of perioperative antibiotics, which are thought to kill streptococci and staphylococci and select for gram-negative flora overgrowth.18
We observed that multiple injuries, extensive soft tissue injury, abdominal injury, higher ISS scores, and severe extremity trauma resulting in amputation were all more common in infected. Wound data collected from experimental gunshot wounds have shown that exit wounds have a high potential for bacterial contamination from the animal's environment.19 Large or multiple soft tissue injuries from blast wounds have an even larger surface area than gunshot wounds that requires debridement; and as surface area of a wound increases, the likelihood of a missed area of necrosis at initial debridement or missed contaminated foreign body increases. Blast injuries can also cause vascular ischemia and DIC, predisposing to infection.20 Abdominal injuries to hollow viscera with leakage of bowel contents are obviously more likely to get infected than extremity injuries. This has been described in the past in non-WTAI traumatic abdominal injuries, hence current guidelines for broader empiric perioperative antibiotic coverage.21 Increasing ISS has been previously shown to have higher risk for infection (sepsis) in civilian trauma patients.22
Surgery and presence of fever at a forward hospital appeared to be more commonly associated with infection. One would have expected early debridement to be protective, so perhaps casualties requiring surgery at Echelon II facilities were more severely injured and at greater risk for infection than those not requiring early surgery. In addition, there might be increased risk for nosocomial infection in the forward installations.
Invasive devices were associated with WTAI in our population. It is unclear whether these devices were portals of infection or surrogate markers of more severely injured patients requiring critical care support and hence more likely to acquire infection. What is interesting about our results is the paucity of pathogens normally associated with device-related infections, such as CoNS and Staphylococcus aureus infections.23 Total parenteral nutrition has been associated with Candidal or CoNS infections24 but not gram-negative infection. Although Acinetobacter has been described in nosocomial outbreaks (particularly pneumonia in ICU settings and wound infection in burn units),25 we found no association with devices or burn injury and increased risk for Acinetobacter infection; however, our subset might have lacked sufficient patients to show significance.
Abdominal injuries were less common in Acinetobacter-associated WTAI, suggesting Acinetobacter is not a colonizer of the lower digestive tract or acquired through visceral perforation; this is consistent with recent epidemiology.26 Blast injuries were also less commonly infected with Acinetobacter: we are not clear why this occurred. Gunshot wounds and external fixation trended toward being associated with increased risk of Acinetobacter. Acinetobacter infection of gunshot wounds of the head27 and femur28 have been reported, but this association with external fixation is a new observation.
The multivariate logistic regression analysis found several possible predictive variables, including type of wound and injury severity that might increase the risk of WTAI development. However, caution must be exercised in interpretation of these results as these data were not collected prospectively; therefore, a causal nature cannot be assured due to lack of knowledge of temporality. These findings suggest further research into a better predictive model of WTAI so that antibiotic therapy can be appropriately and expeditiously employed.
Like the conflicts of the past 3 decades, our results showed an overwhelming preponderance of gram-negative infections. This is similar to Tong's experience in the Vietnam War where Acinetobacter followed by Enterobacter spp., and E. coli were the most common initial wound isolates, and Acinetobacter and Pseudomonas spp were the most common bloodstream isolates.4 As the majority of our infections were in wounds, generally of soft tissue, our findings are quite unique compared with those of standard skin and soft tissue infections in hospitalized patients. In 2000, a SENTRY database of microbiologic causes of over 1400 pyogenic wound infections compiled from 14 North American medical centers demonstrated primarily (46%) staphylococcal infections, of which 30% were MRSA. Gram negatives were much less common and included Pseudomonas, E. coli, Enterobacter, and Klebsiella, accounting for 11%, 7%, 6%, and 5% of all isolates, respectively. Drug resistance among SENTRY gram-negative isolates was generally low, except for Enterobacter resistance to cephalosporins.29 Our observations of extremely low rates of Staphylococcus infection are also similar to Vietnam War data, which was surprising given the increasing prevalence of MRSA in military populations.30,31 Military members in a recruit/training setting and no Southwest Asian deployment history were recently screened for Acinetobacter colonization. The authors found only 14% skin colonization, primarily in the toe webs and no evidence of multidrug resistance, which is also in stark contrast to our findings.32
Our findings are limited in that this was a retrospective study; furthermore, records (especially Echelon II) were not always complete, making data extraction more difficult. No catheter cultures were performed, so it is difficult to say if the bloodstream isolates originated from wounds or from central lines or other devices. Furthermore, we did not abstract data to meet all the CDC criteria for hospital-acquired pneumonia and cannot comment if all positive sputa are true pneumonias versus colonization. We noted 3 patients where bloodstream isolates were matched to wound isolates (2 Acinetobacter and 1 Pseudomonas) and 4 patients where urine isolates matched bloodstream isolates (all Acinetobacter) and 1 concurrent sputum and bloodstream isolate (S. aureus). Without pulse field gel electrophoresis, this is not definitive but suggests wounds and urinary tract may be portals of entry for bloodstream infection in some (38%) WTAIs, particularly in Acinetobacter species, which account for 75% of these findings. Finally, we lacked freezer capabilities to maintain isolates hampering our ability to investigate the possibility of nosocomial infection by further molecular epidemiologic analysis.
Multidrug resistant Acinetobacter and gram-negative infections are emerging as the predominant organisms in civilian mass casualty trauma situations, particularly when there is rapid aeromedical evacuation to distant tertiary care and environmental contamination of wounds with soil and water at time of injury. In Turkey in 1997, following a mass influx of earthquake-related crush injuries, a local hospital experienced an 18% rate of nosocomial infections, predominantly Acinetobacter.33 Following the Bali bombing in 2002, 82% of the most seriously injured trauma victims admitted to an Australian hospital were colonized with multidrug resistant gram-negative pathogens (62% had Acinetobacter infection).34 In both instances, there was a problem with nosocomial transmission to nontrauma patients. Following the 2004 tsunami, 17 patients evacuated to Germany from Thailand with severe orthopedic and soft tissue injuries had predominantly gram-negative infection. Multidrug resistant Acinetobacter was isolated in 18% of cultures despite no previous records of this pathogen in marine infections.35
Like these previous examples, our patients were also wounded far from our hospital and evacuated via ever-escalating levels of care. Like the Turkish earthquake experience, our patients were injured in a similar geographic area and had contaminated wounds with impaired vascular and lymphatic supply. As in Bali, we also had a sizable number of blast and burn victims contaminated with local soil. Finally, tsunami victims had very similar soft tissue injuries as our blast or gunshot wounds. Our patients also received perioperative first generation cephalosporins as in the Vietnam battlefield casualties and the Bali and Thailand victims.
Given these similarities, is something in the Medevac process responsible for multidrug resistant gram-negative infection? One explanation could be nosocomial contamination either in Echelon II or in the evacuation aircraft. Recent data from a forward hospital in Iraq found little Acinetobacter on culture of wounded Americans at admission, instead Staphylococcus and Streptococcus predominated.36 Arguing against a pure nosocomial process is that our patients came from multiple forward installations from multiple countries' medical services, had a multiplicity of organisms isolated from wounds with similarities to the Vietnam and Bosnian conflicts, which predated OIF by years and used different medical equipment. Furthermore, recent analysis of military Acinetobacter strains revealed clonality in only 45 of 170 isolates, with a total of 67 different strains represented among the 170 isolates.37,38 Finally, WTAI with Acinetobacter spp. infection/colonization has not decreased since forward military hospitals have moved geographic location in Iraq, increased infection control measures, or moved from tent to brick and mortar structures.39
A second explanation for the predominance of multidrug resistance gram-negative organisms recovered might be the patients themselves. In the abstract by Yun et al, Iraqi patients admitted to the same military facility had higher rates of Acinetobacter infection.36 The majority of our patients were Iraqi, so there may be higher rates of colonization, behavioral differences, or other host susceptibilities in Southwest Asian natives. Potentially spread of infection could occur from Iraqi patients to coalition forces in Echelon II, which receive both U.S. and foreign national casualties. More likely, there is a selection bias due to the Medevac process. Coalition wounded had priority for evacuation to our ship and often were shipped to the United States via Europe shortly after being stabilized on Comfort, hence shorter ICU and hospital stays and shorter time to admission. Iraqi patients remained at Echelon II longer awaiting transfer and remained on board ship longer for lack of another facility to send them to. A multivariate analysis comparing nationality found longer time to admission after wounding for the Iraqi patients and more severe injuries and higher numbers of procedures and blood transfusions, but was inconclusive due to the bias of the Medevac process and the small number of coalition forces wounded.
Finally, the combat medical care environment with its continued danger and hostile fire might be expected to present great challenges to good infection control practices, especially following massive influxes of allied, civilian, and enemy casualties to a hospital as hospital staffs become distracted and patient areas crowded. During the Balkan conflicts, a Serbian military hospital saw an increase in Acinetobacter wound infections on its surgical wards compared with peacetime operations a year later.40
This emerging phenomenon of highly resistant organisms is most likely due to some combination of all of the above. Regardless of the cause, the ongoing arrival of returning casualties infected or colonized with Acinetobacter, Pseudomonas, and extended-spectrum beta-lactamase enteric organisms, mandates aggressive surgical wound management, infection control measures, as well as judicious antimicrobial usage, at all levels of the Department of Defense, Veterans Administration healthcare systems, as well as civilian hospitals that may care for injured troops.
Footnotes
Conflicts of interest: All authors: No conflicts. Financial Support: All authors: none. Dr. Petersen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Views expressed in this article are those of the authors and do not reflect the official policy or position of the U.S. Navy, Department of Defense, nor the U.S. Government.
Reprints will not be available. Correspondence: Kyle Petersen, DO, NNMC Division of Infectious Diseases, 8901 Wisconsin Ave, Bethesda, MD 20889. E-mail: knpetersen@bethesda.med.navy.mil.
REFERENCES
- 1.Osler W. Medical notes on England at war. JAMA. 1914;63:2303–2305. [Google Scholar]
- 2.Bellamy R, Zajtchuk R. The management of ballistic wounds of soft tissue. In: Bellamy RF, Zajtchuk R, eds. Textbook of Military Medicine: Conventional Warfare–Ballistic, Blast and Burn Injuries, Part 1, vol. 3. Washington, DC: U.S. Government Printing Office, 1991:163–220. [Google Scholar]
- 3.U.S. Department of Defense Military Casualty Information, Directorate for Information Operations and Reports 2005. Available at www.dior.whs.mil/mmid/casualty/castop.htm (Accessed August 12, 2005).
- 4.Tong MJ. Septic complications of war wounds. JAMA. 1972;219:1044–1047. [PubMed] [Google Scholar]
- 5.Matsumoto T, Wyte SR, Moseley RV, et al. Combat surgery in communication zone: I. War wound and bacteriology [preliminary report]. Milit Med. 1969;134:655–665. [PubMed] [Google Scholar]
- 6.Gawande A. Casualties of war: military care for the wounded from Iraq and Afghanistan. N Engl J Med. 2004;351:2471–2475. [DOI] [PubMed] [Google Scholar]
- 7.Petersen K. Acinetobacter, drug resistant-Iraq: request for information. E-mail to Program for Monitoring Emerging Diseases mailing list (ProMEDmail). 2003;17 April: Archive no. 20030417. 0934 Available at: www.promedmail.org (Accessed August 12, 2005).
- 8.Baker SP, O'Neill B, Haddon W Jr, et al. The Injury Severity Score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- 9.Bauer AW, Kirby WM, Sherris JC, et al. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests: Approved Standard, 7th ed. Wayne, PA: National Committee for Clinical Laboratory Standards, 2000. [Google Scholar]
- 11.Haller JS Jr. Treatment of infected wounds during the Great War, 1914 to 1918. South Med J. 1992;85:303–315. [DOI] [PubMed] [Google Scholar]
- 12.Stoddard JL. The occurrence and significance of B welchii in certain wounds. JAMA. 1918;71:1400–1402. [Google Scholar]
- 13.Fleming A. On the bacteriology of septic wounds. Lancet. 1915;638–643. [Google Scholar]
- 14.Rustigan R, Cipriani A. The bacteriology of open wounds. JAMA. 1947;133:224–229. [DOI] [PubMed] [Google Scholar]
- 15.Fisher GH, Florey ME, Grimson TA, et al. Penicillin and clostridial infections. Lancet. 1945;395–399. [Google Scholar]
- 16.Lindberg RB, Wetzler TF, Marshall JD, et al. The bacterial flora of battle wounds at the time of primary debridement. Ann Surg. 1955;141:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovaric JJ, Matsumoto T, Dobek AS, et al. Bacterial flora of one hundred and twelve combat wounds. Mil Med. 1968;132:622–624. [PubMed] [Google Scholar]
- 18.Simchen E, Sacks T. Infection in war wounds: experience during the 1973 October war in Israel. Ann Surg. 1975;182:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tikka S. The contamination of missile wounds with special reference to early antimicrobial therapy. Acta Chir Scand Suppl. 1982;508:281–287. [PubMed] [Google Scholar]
- 20.Sharpnack DD, Johnson AJ, Phillips YY. The pathology of primary blast injury. In: Bellamy RF, Zajtchuk R, eds. Textbook of Military Medicine: Conventional Warfare–Ballistic, Blast and Burn Injuries, Part 1, vol. 3. Washington, DC: U.S. Government Printing Office, 1991:271–294. [Google Scholar]
- 21.Luchette FA, Borzotta AP, Croce MA, et al. Practice Management Guidelines for Prophylactic Antibiotic Use in Penetrating Abdominal Trauma. Allentown, PA: Eastern Association for the Surgery of Trauma, 2000:1–33. [Google Scholar]
- 22.Laupland K, Gregson DB, Kirkpatrick AW, et al. Bloodstream infection complicating trauma. Clin Invest Med. 2004;27:253–258. [PubMed] [Google Scholar]
- 23.Maki D. Nosocomial bacteremia: an epidemiologic overview. Am J Med. 1981;70:719–731. [DOI] [PubMed] [Google Scholar]
- 24.Beekman SE, Henderson DK. Infections due to percutaneous intravascular devices In: Mandell GL, Bennett JE, Dolin R eds. Mandell Douglas and Bennett's Principles and Practice of Infectious Diseases, 6th ed. New York: Churchill Livingstone, 2004:3347–3362. [Google Scholar]
- 25.Villegas MV, Hartstein AI. Acinetobacter outbreaks, 1977–2000. Infect Control Hosp Epidemiol. 2003;24:284–295. [DOI] [PubMed] [Google Scholar]
- 26.Dijkshoorn L, van Aken E, Shunburne L, et al. Prevalence of Acinetobacter baumannii and other Acinetobacter spp. in faecal samples from non-hospitalised individuals. Clin Microbiol Infect. 2005;11:329–332. [DOI] [PubMed] [Google Scholar]
- 27.Aarabi B. Comparative study of bacteriological contamination between primary and secondary exploration of missile head wounds. Neurosurgery. 1987;20:610–616. [DOI] [PubMed] [Google Scholar]
- 28.Volpin G, Krivoy N, Stein H. Acinetobacter sp. osteomyelitis of the femur: a late sequel of unrecognized foreign body implantation. Injury. 1993;24:345–346. [DOI] [PubMed] [Google Scholar]
- 29.Rennie RP, Jones RN, Mutnick AH. Occurrence and antimicrobial susceptibility patterns of pathogens isolated from skin and soft tissue infections: report from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 2000). Diagn Microbiol Infect Dis. 2003;45:287–293. [DOI] [PubMed] [Google Scholar]
- 30.Zinderman CE, Conner B, Malakooti MA, et al. Community-acquired methicillin-resistant Staphylococcus aureus among military recruits. Emerg Infect Dis. 2004;10:941–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell KM, Vaughn AF, Russell KL, et al. Risk factors for community-associated methicillin-resistant Staphylococcus aureus infections in an outbreak of disease among military trainees in San Diego, California, in 2002. J Clin Microbiol. 2004;42:4050–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffith ME, Ceremuga JM, Ellis MW, et al. Acinetobacter skin colonization of US Army soldiers infect control. Hosp Epidemiol. 2006;27:659–661. [DOI] [PubMed] [Google Scholar]
- 33.Oncul O, Keskin O, Acar HV, et al. Hospital-acquired infections following the 1999 Marmara earthquake. J Hosp Infect. 2002;51:47–51. [DOI] [PubMed] [Google Scholar]
- 34.Heath CH, Orrell CT, Lee RC, et al. A review of the Royal Perth Hospital Bali experience: an infection control perspective. Austral Infect Control. 2003;8:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maegele M, Gregor S, Steinhausen E, et al. The long-distance tertiary air transfer and care of tsunami victims: Injury pattern and microbiological and psychological aspects. Crit Care Med. 2005;33:1136–1140. [DOI] [PubMed] [Google Scholar]
- 36.Yun HC, Murray CK, Roop SA, et al. Bacteria recovered from patients admitted to a deployed United States military hospital in Baghdad, Iraq [Abstract 1090]. Presented at the 43rd Meeting of the Infectious Disease Society of America, San Francisco, October 2005.
- 37.Scott PT, Hulten EA, Craft DW, et al. An outbreak of multi-drug resistant Acinetobacter baumannii infections in the Military Health Care System associated with Operation Iraqi Freedom (OIF) [Abstract 684]. Presented at the 43rd Meeting of the Infectious Disease Society of America, San Francisco, October 2005.
- 38.Scott PT, Petersen K, Fishbain J, et al. Acinetobacter baumanii infections among patients at military facilities treating injured U.S. service members, 2002–2004. MMWR Morb Mortal Wkly Rep. 2004;53:1063–1066. [PubMed] [Google Scholar]
- 39.Petruccelli BP. Epidemiological consultation no. 12-HA-01JK-04 Investigating Acinetobacter baumanii infections at U.S. Army military treatment facilities 27 August 2004 to 27 May 2005. Available from: Commander, U.S. Army Center for Health Promotion and Preventive Medicine, ATTN: MCHB-TS-D, Aberdeen Proving Ground, MD 21010–5403.
- 40.Suljagic V, Mirovic V, Tomanovic B. Surveillance of bacterial causes of hospital infections during periods of war and peace. Vojnosanit Pregl. 2003;60:443–447. [DOI] [PubMed] [Google Scholar]