Abstract
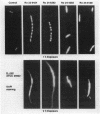
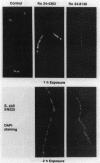
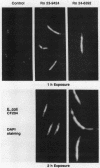
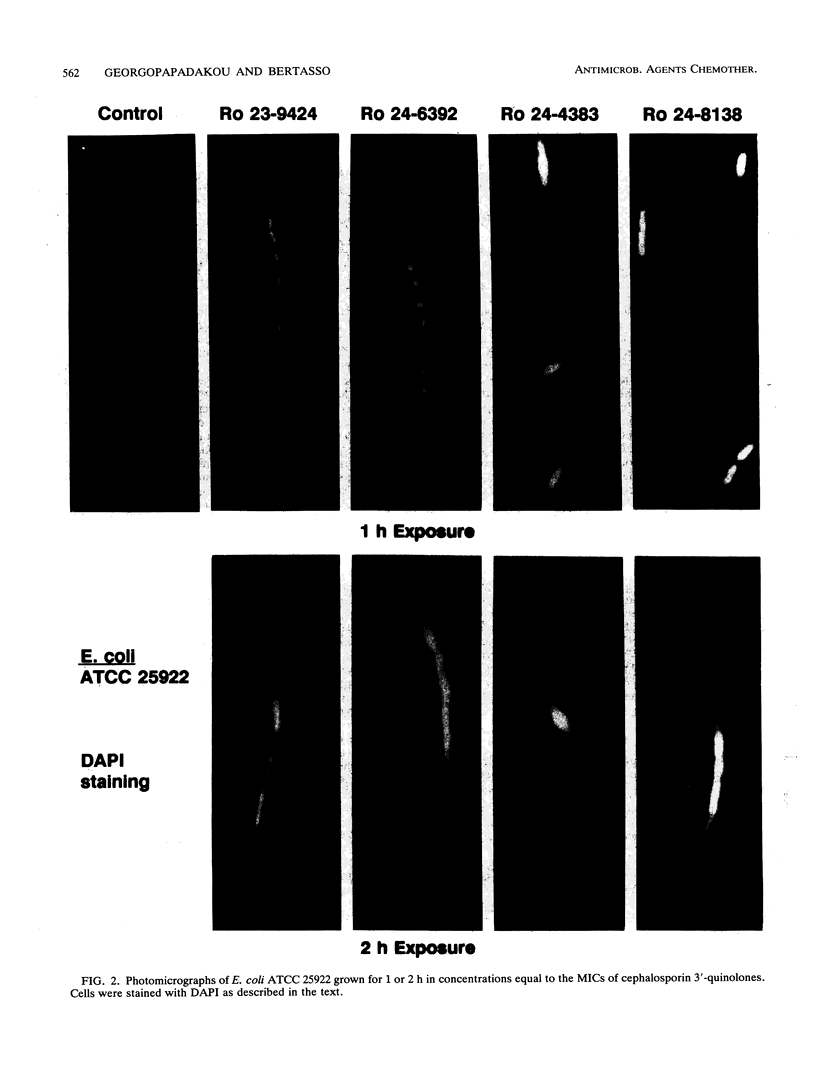
Cephalosporin 3'-quinolone esters, carbamates, and tertiary amines are potent antibiotics whose antibacterial activities reflect the action of both the beta-lactam and the quinolone components. The biological properties of representative compounds from each class were compared in Escherichia coli. All compounds bound to the essential PBP 3, inhibited DNA gyrase, and caused filamentation in growing cells. To distinguish between cephalosporin- and quinolone-induced filaments, nucleoid segregation was also examined, as quinolones disrupt nucleoid segregation while the beta-lactams do not (N. H. Georgopapadakou and A. Bertasso, Antimicrob. Agents Chemother. 35:2645-2648, 1991). The cephalosporin quinolone esters Ro 23-9424 and Ro 24-6392, at concentrations causing filamentation in E. coli ATCC 25922, did not affect nucleoid segregation after 1 h of incubation (cephalosporin response) but did not affect it after 2 h (quinolone response), indicating the release of free quinolone. Accordingly, only the quinolone response was produced in a strain possessing TEM-3, an expanded-spectrum beta-lactamase. The cephalosporin carbamate Ro 24-4383 and the tertiary amine Ro 24-8138 produced a quinolone response in E. coli ATCC 25922, though they produced a cephalosporin response in a quinolone-resistant strain. Carbamate and tertiary amine linkages are chemically more stable than the ester linkage, and both cephalosporin 3'-quinolone carbamates and tertiary amines are more potent inhibitors of DNA gyrase than are the corresponding esters. The results suggest that, while intact cephalosporin 3'-quinolone esters act as cephalosporins, carbamates and amines may possess both cephalosporin and quinolone activity in the intact molecule.
Full text
PDF
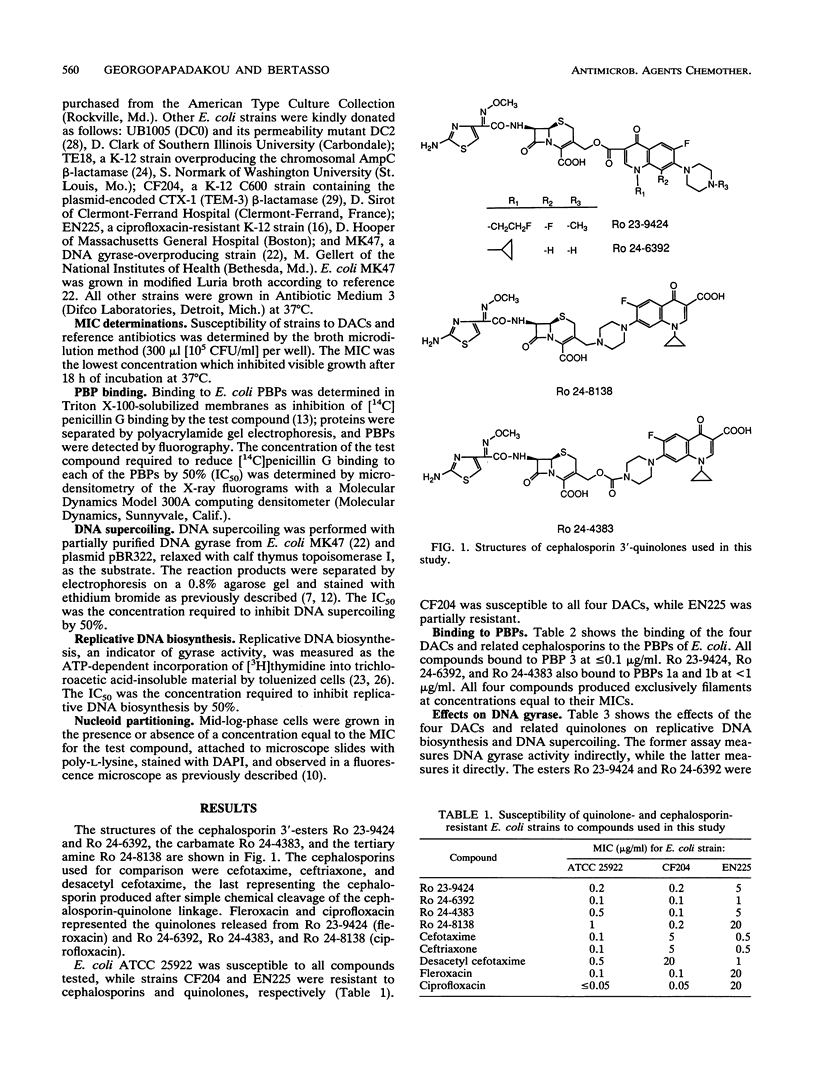


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht H. A., Beskid G., Chan K. K., Christenson J. G., Cleeland R., Deitcher K. H., Georgopapadakou N. H., Keith D. D., Pruess D. L., Sepinwall J. Cephalosporin 3'-quinolone esters with a dual mode of action. J Med Chem. 1990 Jan;33(1):77–86. doi: 10.1021/jm00163a013. [DOI] [PubMed] [Google Scholar]
- Albrecht H. A., Beskid G., Christenson J. G., Durkin J. W., Fallat V., Georgopapadakou N. H., Keith D. D., Konzelmann F. M., Lipschitz E. R., McGarry D. H. Dual-action cephalosporins: cephalosporin 3'-quaternary ammonium quinolones. J Med Chem. 1991 Feb;34(2):669–675. doi: 10.1021/jm00106a031. [DOI] [PubMed] [Google Scholar]
- Albrecht H. A., Beskid G., Christenson J. G., Georgopapadakou N. H., Keith D. D., Konzelmann F. M., Pruess D. L., Rossman P. L., Wei C. C. Dual-action cephalosporins: cephalosporin 3'-quinolone carbamates. J Med Chem. 1991 Sep;34(9):2857–2864. doi: 10.1021/jm00113a026. [DOI] [PubMed] [Google Scholar]
- Bliska J. B., Cozzarelli N. R. Use of site-specific recombination as a probe of DNA structure and metabolism in vivo. J Mol Biol. 1987 Mar 20;194(2):205–218. doi: 10.1016/0022-2836(87)90369-x. [DOI] [PubMed] [Google Scholar]
- Corraz A. J., Dax S. L., Dunlap N. K., Georgopapadakou N. H., Keith D. D., Pruess D. L., Rossman P. L., Then R., Unowsky J., Wei C. C. Dual-action penems and carbapenems. J Med Chem. 1992 May 15;35(10):1828–1839. doi: 10.1021/jm00088a019. [DOI] [PubMed] [Google Scholar]
- Demuth T. P., Jr, White R. E., Tietjen R. A., Storrin R. J., Skuster J. R., Andersen J. A., McOsker C. C., Freedman R., Rourke F. J. Synthesis and antibacterial activity of new C-10 quinolonyl-cephem esters. J Antibiot (Tokyo) 1991 Feb;44(2):200–209. doi: 10.7164/antibiotics.44.200. [DOI] [PubMed] [Google Scholar]
- Domagala J. M., Hanna L. D., Heifetz C. L., Hutt M. P., Mich T. F., Sanchez J. P., Solomon M. New structure-activity relationships of the quinolone antibacterials using the target enzyme. The development and application of a DNA gyrase assay. J Med Chem. 1986 Mar;29(3):394–404. doi: 10.1021/jm00153a015. [DOI] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Bertasso A., Chan K. K., Chapman J. S., Cleeland R., Cummings L. M., Dix B. A., Keith D. D. Mode of action of the dual-action cephalosporin Ro 23-9424. Antimicrob Agents Chemother. 1989 Jul;33(7):1067–1071. doi: 10.1128/aac.33.7.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Bertasso A. Effects of quinolones on nucleoid segregation in Escherichia coli. Antimicrob Agents Chemother. 1991 Dec;35(12):2645–2648. doi: 10.1128/aac.35.12.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Dix B. A., Angehrn P., Wick A., Olson G. L. Monocyclic and tricyclic analogs of quinolones: mechanism of action. Antimicrob Agents Chemother. 1987 Apr;31(4):614–616. doi: 10.1128/aac.31.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Penicillin-binding proteins in bacteria. Antimicrob Agents Chemother. 1980 Jul;18(1):148–157. doi: 10.1128/aac.18.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S., Niki H., Ogura T., Ichinose C., Mori H., Ezaki B., Jaffé A. Chromosome partitioning in Escherichia coli: novel mutants producing anucleate cells. J Bacteriol. 1989 Mar;171(3):1496–1505. doi: 10.1128/jb.171.3.1496-1505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S. Fluoroquinolone antimicrobial agents. N Engl J Med. 1991 Feb 7;324(6):384–394. doi: 10.1056/NEJM199102073240606. [DOI] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Ng E. Y., Swartz M. N. Mechanisms of action of and resistance to ciprofloxacin. Am J Med. 1987 Apr 27;82(4A):12–20. [PubMed] [Google Scholar]
- Ishino F., Matsuhashi M. Peptidoglycan synthetic enzyme activities of highly purified penicillin-binding protein 3 in Escherichia coli: a septum-forming reaction sequence. Biochem Biophys Res Commun. 1981 Aug 14;101(3):905–911. doi: 10.1016/0006-291x(81)91835-0. [DOI] [PubMed] [Google Scholar]
- Kapuściński J., Szer W. Interactions of 4', 6-diamidine-2-phenylindole with synthetic polynucleotides. Nucleic Acids Res. 1979 Aug 10;6(11):3519–3534. doi: 10.1093/nar/6.11.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Manzini G., Barcellona M. L., Avitabile M., Quadrifoglio F. Interaction of diamidino-2-phenylindole (DAPI) with natural and synthetic nucleic acids. Nucleic Acids Res. 1983 Dec 20;11(24):8861–8876. doi: 10.1093/nar/11.24.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi K., Mizuuchi M., O'Dea M. H., Gellert M. Cloning and simplified purification of Escherichia coli DNA gyrase A and B proteins. J Biol Chem. 1984 Jul 25;259(14):9199–9201. [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Normark S. Sensitivity of Escherichia coli to various beta-lactams is determined by the interplay of outer membrane permeability and degradation by periplasmic beta-lactamases: a quantitative predictive treatment. Mol Microbiol. 1987 Jul;1(1):29–36. doi: 10.1111/j.1365-2958.1987.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Pace J., Bertasso A., Georgopapadakou N. H. Escherichia coli resistant to cephalosporins and quinolones is still susceptible to the cephalosporin-quinolone ester Ro 23-9424. Antimicrob Agents Chemother. 1991 May;35(5):910–915. doi: 10.1128/aac.35.5.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrini A. M., Geroldi D., Siccardi A., Falaschi A. Studies on the mode of action of nalidixic acid. Eur J Biochem. 1972 Feb 15;25(2):359–365. doi: 10.1111/j.1432-1033.1972.tb01704.x. [DOI] [PubMed] [Google Scholar]
- Perrone E., Jabés D., Alpegiani M., Andreini B. P., Della Bruna C., Del Nero S., Rossi R., Visentin G., Zarini F., Franceschi G. Dual-action penems. J Antibiot (Tokyo) 1992 Apr;45(4):589–594. doi: 10.7164/antibiotics.45.589. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Clark D. C., Wotton S. Indirect method for assessing the penetration of beta-lactamase-nonsusceptible penicillins and cephalosporins in Escherichia coli strains. Antimicrob Agents Chemother. 1976 Aug;10(2):215–218. doi: 10.1128/aac.10.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot D., Sirot J., Labia R., Morand A., Courvalin P., Darfeuille-Michaud A., Perroux R., Cluzel R. Transferable resistance to third-generation cephalosporins in clinical isolates of Klebsiella pneumoniae: identification of CTX-1, a novel beta-lactamase. J Antimicrob Chemother. 1987 Sep;20(3):323–334. doi: 10.1093/jac/20.3.323. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. R., Drlica K. Bacterial chromosome segregation: evidence for DNA gyrase involvement in decatenation. Cell. 1984 Apr;36(4):1081–1088. doi: 10.1016/0092-8674(84)90058-8. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev. 1989 Oct;2(4):378–424. doi: 10.1128/cmr.2.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]