Abstract
Objective:
To investigate the role of bacterial DNA in development of an excessive inflammatory response and loss of gut barrier loss following systemic hypotension.
Summary Background Data:
Bacterial infection may contribute to development of inflammatory complications following major surgery; however, the pathogenesis is not clear. A common denominator of bacterial infection is bacterial DNA characterized by unmethylated CpG motifs. Recently, it has been shown that bacterial DNA or synthetic oligodeoxynucleotides containing unmethylated CpG motifs (CpG-ODN) are immunostimulatory leading to release of inflammatory mediators.
Methods:
Rats were exposed to CpG-ODN prior to a nonlethal hemorrhagic shock. The role of interferon-gamma (IFN-γ) was investigated by administration of anti IFN-γ antibodies.
Results:
Exposure to CpG-ODN prior to hemorrhagic shock significantly augmented shock-induced release of IFN-γ, tumor necrosis factor-alpha (TNF-α) (P < 0.05), interleukin (IL)-6 (P < 0.05), and nitrite levels (P < 0.05), while there was a defective IL-10 response (P < 0.05). Simultaneously, expression of Toll-like receptor (TLR) 4 in the liver was markedly enhanced. Furthermore, intestinal permeability for HRP significantly increased and bacterial translocation was enhanced in hemorrhagic shock rats pretreated with CpG-ODN. Interestingly, inhibition of IFN-γ in CpG-treated animals reduced TNF-α (P < 0.05), IL-6 (P < 0.05), nitrite (P < 0.05), and intestinal permeability following hemorrhagic shock (P < 0.05) and down-regulated expression of TLR4.
Conclusion:
Exposure to bacterial DNA strongly aggravates the inflammatory response, disrupts the intestinal barrier, and up-regulates TLR4 expression in the liver following hemorrhagic shock. These effects are mediated via an IFN-γ-dependent route. In the clinical setting, bacterial DNA may be important in development of inflammatory complications in surgical patients with bacterial infection.
This study reports that exposure to bacterial DNA prior to hemorrhagic shock, up-regulates expression of TLR4 in the liver, and strongly exacerbates inflammation and loss of gut barrier function via an IFN-γ-dependent mechanism. Bacterial DNA may be important in development of inflammatory complications in surgical patients with bacterial infection.
An excessive systemic inflammatory response following severe blood loss or major surgery may lead to potentially fatal syndromes such as sepsis.1 Although it is not clear what the determinants are for development of an excessive inflammatory response, it is suggested by some that sequential physiologic injuries prime the host for a subsequent trigger.2 In many clinical circumstances, severe blood loss is followed by a “second” hit leading to an excessive inflammatory response to an otherwise low-grade trigger, probably mediated by bacterial ligands such as endotoxin.3
However, bacterial infection preceding an inflammatory “stress” event such as major surgery is also an important prognostic risk factor for development of surgical site infections and inflammatory syndromes such as sepsis.4,5 The underlying mechanisms for this potential priming effect are yet unknown. Recently, it has been shown that bacterial DNA, a common denominator of bacterial infection, is immunostimulatory. Bacterial DNA is structurally different from eukaryotic DNA by the prevalence of unmethylated cytosine-phosphate-guanine dinucleotides, termed CpG motifs.6 Both bacterial DNA and synthetic oligodeoxynucleotides (ODN) containing unmethylated CpG motifs are potent inducers of inflammatory mediators such as TNF-α, and IFN-γ via Toll-like receptor (TLR) 9.7,8
The TH1-like immune response induced by CpG-ODN is increasingly being used as adjuvant in models of vaccination and cancer.9–11 However, this immune-enhancing property of CpG-ODN may also lead to an unwanted and strong inflammatory response as shown in d-galactosamine-sensitized mice leading to a lethal toxic shock.12 An important factor in the pathogenesis of postoperative inflammatory syndromes such as sepsis is the development of an exaggerated (uncontrolled) inflammatory response and loss of gut barrier function. It is thought that low concentrations of IFN-γ cause lipid-raft mediated translocation of bacteria, thereby stimulating the inflammatory response.13 Subsequent release of inflammatory mediators such as TNF-α and IFN-γ disrupts intestinal tight junctions, leading to loss of gut barrier integrity and a viscous circle of inflammation and remote tissue damage.14
In addition, multiple stress sessions or multiple “hits” enhance the inflammatory response and disrupt the colonic epithelial barrier via an IFN-γ-dependent route.15
We hypothesized that release of bacterial DNA contributes to development of an excessive inflammatory response and loss of gut barrier loss following major surgery via IFN-γ. The current study was designed to investigate the effect of exposure to bacterial DNA on the inflammatory response and loss of gut barrier following hemorrhagic shock in rats. The role of IFN-γ was assessed using anti IFN-γ antibodies.
MATERIALS AND METHODS
Reagents
CpG oligodeoxynucleotide (ODN) (5′-TGACTGTGAACGTTCGAGATGA-3′+ phosphorothioate backbone16) and nonimmunostimulatory non-CpG-ODN (5′-GCTTGATGACTCAGCCGGAA-3′) was purchased from Eurogentec (Seraing, Belgium) and dissolved in sterile, pyrogen-free saline to a final concentration of 500 μmol/L. A monoclonal antibody directed against rat IFN-γ was kindly provided by Dr. P. van der Meide (University Medical Center Utrecht, the Netherlands). This is an IgG1 antibody with a high-affinity for rat IFN-γ with proven in vitro and in vivo IFN-γ functional inhibiting capacity.17,18
Animals
Healthy male Sprague-Dawley rats, weighing 303 to 425 g (average, 355 g) were purchased from Charles River (Maastricht, the Netherlands) and housed under controlled conditions of temperature and humidity. Before the start of the experiments, rats were fed ad libitum with standard rodent chow and had free access to water. The experimental protocol was performed according to the guidelines of the Animal Care Committee of the University of Maastricht and approved by the committee.
Isolation and Stimulation of Peritoneal Macrophages
Sprague-Dawley rats (n = 8) were not exposed (controls), injected with non-CpG (180 μg) or CpG (180 μg) with or without anti IFN-γ (5 mg/kg) 18 hours prior to stimulation. Peritoneal macrophages were harvested under sterile conditions, washed 5 times in RPMI (GIBCO Europe, Paisley, UK) with 1% penicillin/ streptomycin and incubated in 96-well culture plates (Costar, Cambridge, MA) at 2 × 105 cells per well for 5 hours with LPS (1 and 10 ng/mL). After incubation, supernatants were collected and TNF-α was determined by sandwich ELISA.19
NF-κB Assay
Peritoneal macrophages were isolated as described above from rats injected with non-CpG, CpG-ODN, or CpG-ODN, and anti IFN-γ and stimulated with LPS (10 ng/mL) for 15 minutes. Next, nuclear extracts were isolated and p65 activation was quantified using an oligonucleotide-based ELISA (Active Motif, Rixensart, Belgium) according to the supplier's instructions. Furthermore, TLR4 was determined using RT-PCR.
Experimental Design and Hemorrhagic Shock Procedure
Rats were allocated to 7 groups (n = 7 per group) before the start of the experiments. All animals were starved overnight and killed at 4 hours after hemorrhagic shock. Group 1: healthy controls not exposed to CpG-ODN; group 2: rats exposed to CpG-ODN (25 nmol in 0.5 mL saline, equal to 180 μg) 18 hours before death; group 3: control rats subjected to hemorrhagic shock; group 4: rats exposed to non-CpG (25 nmol in 0.5 mL saline, equal to 180 μg) for 18 hours before subjection to hemorrhagic shock; group 5: rats exposed to CpG-ODN for 18 hours before subjection to hemorrhagic shock; group 6: control rats injected with rat anti-IFN-γ (5 mg/kg) 18 hours before subjection to hemorrhagic shock; and group 7: rats exposed to CpG-ODN and injected with rat anti-IFN-γ (5 mg/kg) 18 hours before subjection to hemorrhagic. A nonlethal hemorrhagic shock model was used as previously described.20,21 In short, rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p); the femoral artery was dissected and cannulated with polyethylene tubing (PE-10). Mean arterial pressure (MAP) and heart rate (HR) were continuously recorded during a 50-minute observation period. At the time of shock (t = 0), 2.1 mL blood per 100 g of body weight was taken at a rate of 1 mL/min (representing 30%–40% of the total blood volume). The severity of the hemorrhagic shock insult as reflected by changes in MAP, HR, and hematocrit was similar for all hemorrhagic shock groups and comparable with our earlier data, using the same hemorrhagic shock model.20,21 At the time of death (t = 4 hours), blood was taken and segments of small bowel were harvested for determination of gut permeability. Plasma was separated by centrifugation, frozen immediately and stored (−20°C) until analysis.
Cytokine Analysis
TNF-α concentrations in supernatants of stimulated cells were determined using a sandwich-ELISA.19 TNF-α, IFN-γ, IL-6, and IL-10 concentrations in arterial blood were determined using standard ELISA's for rat TNF-α and rat IFN-γ (both kindly provided by Hbt, Uden, the Netherlands), rat IL-6 (BD Biosciences, San Diego, CA), and rat IL-10 (Biosource, Camarillo, CA).
Measurement of NO· Production
Nitrite (NO2−) in rat plasma was measured using Griess reagent. This primary oxidation product of NO· after reaction with oxygen was used as an indicator of NO synthesis.
Intestinal Permeability and Microbiologic Methods
Intestinal permeability for macromolecules was assessed by measuring translocation of the 44-kDa enzyme horseradish peroxidase (HRP, Sigma) by the everted gut sac method as described.21
Bacterial translocation to distant organs was assessed as described.20,21 In short, mesenteric lymph nodes (MLN), the midsection of the spleen and liver-segment (IV) were collected aseptically in preweighed thioglycolate broth tubes (Becton Dickinson, BBL, Microbiology Europe, Maylan, France) in all rats. Tissue fragments were homogenized and the entire suspension was transferred to agar plates (Columbia III blood agar base supplemented with 5% vol/vol sheep blood, BBL, duplicate plates, and Chocolate PolyviteX agar, BioMérieux, Marcy L'Etoile, France). After 48 hours of incubation, colonies were counted, determined using conventional techniques, adjusted to tissue weight, and expressed as number of colony forming units (CFU) per gram tissue.
Statistical Analyses
Bacterial translocation data are represented as median and range; all other data are represented as mean ± SEM. A Mann–Whitney U test was used for between-group comparisons. Differences were considered statistically significant at P < 0.05.
RESULTS
Preexposure to CpG-ODN Enhanced Plasma IFN-γ Levels After Hemorrhagic Shock
Hemorrhagic shock was followed by a rise in circulating IFN-γ levels already after 90 minutes (data not shown) and became more pronounced at 4 hours after shock (1.3 ± 0.1 ng/mL) in control rats and rats pretreated with non-CpG (0.9 ± 0.1 ng/mL) (Fig. 1). Interestingly, administration of CpG-ODN 18 hours prior to hemorrhagic shock doubled circulating plasma levels of IFN-γ at 4 hours after shock (2.6± 0.5 ng/mL, #P < 0.05). In contrast, exposure to CpG-ODN alone resulted in detectable, however, low plasma IFN-γ levels (34 pg/mL, P = 0.003) after 18 hours.
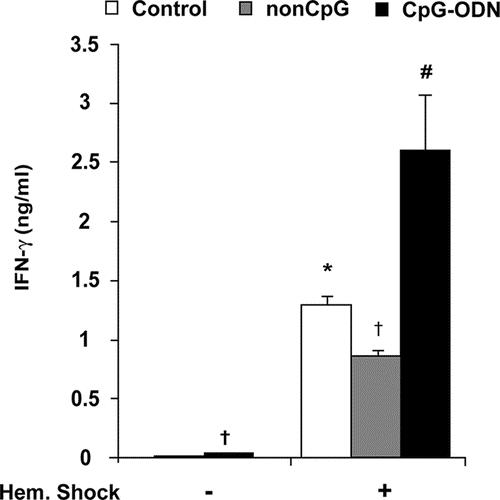
FIGURE 1. Preexposure to CpG-ODN results in significantly higher plasma IFN-γ levels after hemorrhagic shock. Plasma IFN-γ levels were measured in (nonexposed) controls and rats preexposed to non-CpG or CpG-ODN at 18 hours after exposure and at 4 hours after hemorrhagic shock (n = 7 per group). Administration of CpG-ODN alone resulted in detectable, significantly increased plasma IFN-γ (34 ± 2 pg/mL, †P = 0.003) compared with nonexposed control rats after 18 hours. Hemorrhagic shock caused a rise of circulating IFN-γ levels at 4 hours after shock in control (1.3 ± 0.1 ng/mL, *P < 0.01) and non-CpG treated rats (0.9 ± 0.1 ng/mL, †P < 0.01) compared with nonexposed control rats not subjected to shock. Preexposure to CpG-ODN followed by hemorrhagic shock doubled circulating plasma levels of IFN-γ at 4 hours after shock (2.6 ± 0.5 ng/mL, #P < 0.05) compared with control rats and non-CpG treated rats (#P < 0.05) subjected to hemorrhagic shock.
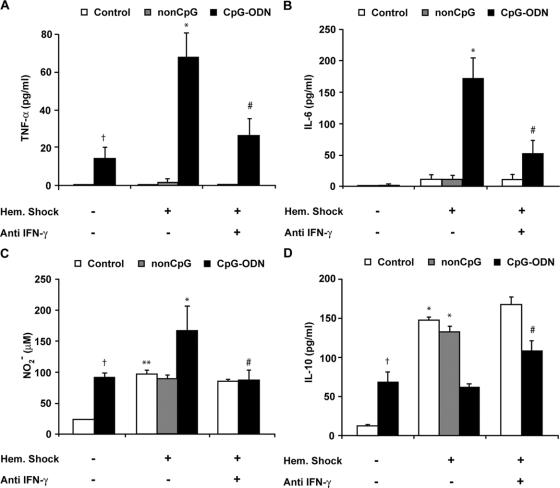
Preexposure to CpG-ODN Significantly Elevated TNF-α, IL-6, and NO Release in Plasma After Hemorrhagic Shock, Whereas IL-10 Was Down-Regulated
Hemorrhagic shock typically results in a TNF-α response that peaks at 90 minutes and rapidly fades.21,22 As expected, circulating TNF-α was no longer detectable in control rats and non–CpG-pretreated rats at 4 hours after hemorrhagic shock (Fig. 2A), while circulating IL-6 levels were still detectable at low levels (control, 12 ± 6 pg/mL; and non-CpG, 12 ± 5 pg/mL) (Fig. 2B). Exposure to CpG-ODN alone led to a mild increase of plasma TNF-α levels (14 ± 6 pg/mL) even after 18 hours after exposure, whereas IL-6 was not demonstrable (Fig. 2A, B). In contrast, administration of CpG-ODN prior to hemorrhagic shock resulted in elevated TNF-α (68 ± 13 pg/mL, P < 0.001) and plasma IL-6 levels (171 ± 33 pg/mL, P < 0.005) compared with control shock rats at 4 hours after hemorrhagic shock. Next, nitrite (NO2−) was measured as marker for nitric oxide (NO) release. Administration of CpG-ODN alone led to elevated NO2− levels after 18 hours compared with background levels in nonexposed controls. Hemorrhagic shock by itself enhanced NO2− levels (97 ± 7 μmol/L). However, hemorrhagic shock in CpG-ODN rats caused a significant rise in NO2− levels (169 ± 38 μmol/L, *P < 0.05) (Fig. 2C). Preexposure to CpG-ODN resulted in a significant rise in circulating IL-10 levels (68 ± 12 pg/mL) compared with background levels in controls (13 ± 11 pg/mL, P < 0.01), in line with previous in vitro studies.23 Hemorrhagic shock resulted in a marked increase of plasma IL-10 in nontreated rats (148 ± 13 pg/mL, *P < 0.01) and non–CpG-treated rats (133 ± 7 pg/mL, *P < 0.01) compared with rats not subjected to shock (Fig. 2D). However, this increase of circulating IL-10 after hemorrhagic shock was not observed in rats exposed to CpG-ODN (62 ± 4 pg/mL). These data show that, although exposure to CpG-ODN alone has relatively modest effects, inflammation is strongly aggravated in combination with hemorrhagic shock.
FIGURE 2. Preexposure to CpG-ODN significantly elevated TNF-α, IL-6, and nitrite (NO2−) and down-regulated IL-10 via an IFN-γ-dependent route. TNF-α, IL-6, nitrite, and IL-10 levels were determined in plasma of controls and CpG-ODN-exposed rats at 18 hours after exposure and at 4 hours after hemorrhagic shock (n = 7 per group). A, Administration of CpG-ODN alone enhanced plasma TNF-α (14 ± 6 pg/mL, †P < 0.05). CpG-ODN preexposure together with hemorrhagic shock elevated TNF-α (68 ± 13 pg/mL, *P < 0.001). Anti-IFN-γ reduced TNF-α after hemorrhagic shock in CpG-ODN-treated rats (27 ± 8 pg/mL, #P < 0.05). B, Preexposure to CpG-ODN strongly enhanced hemorrhagic shock-induced IL-6 (171 ± 33 pg/mL, *P < 0.01), which was reduced by administration of anti IFN-γ (52 ± 20 pg/mL, #P < 0.05). C, Administration of CpG-ODN alone increased nitrite (NO2−) (91± 7 μmol/L, †P < 0.01). Hemorrhagic shock caused a marked increase in NO2− compared with controls (97 ± 6 μmol/L, **P < 0.01). CpG-ODN preexposure elevated hemorrhagic shock-induced NO2− (169 ± 38 μmol/L, *P< 0.05). Treatment with anti IFN-γ reduced nitrite levels following hemorrhagic shock in CpG-ODN rats (83 ± 12 μmol/L, #P < 0.05). D, Administration of CpG-ODN alone enhanced plasma IL-10 levels (68 ± 12 pg/mL, †P < 0.006). Hemorrhagic shock caused a marked increase of plasma IL-10 (148 ± 13 pg/mL, *P < 0.01) in control and non-CPG-treated rats (133 ± 7 pg/mL, *P < 0.01). CpG-ODN preexposure together with hemorrhagic shock caused a defective IL-10 response that was restored by administration of anti IFN-γ (109 ± 12 pg/mL, #P < 0.01): **,†compared with control; *compared with control hemorrhagic shock; #compared with CpG-ODN shock.
Preexposure to CpG-ODN Impairs Intestinal Barrier Function and Further Enhances Hemorrhagic Shock-Induced Intestinal Permeability
Intestinal permeability for the 44-kDa macromolecule HRP was significantly elevated following CpG-ODN exposure (5.5 ± 0.5 μg/mL) compared with controls (1 ± 0.1, P = 0.006) (Fig. 3). As expected, hemorrhagic shock resulted in an increased leakage of HRP (36 ± 3 μg/mL). Preexposure to CpG-ODN followed by hemorrhagic shock almost doubled leakage of HRP (60 ± 11 μg/mL, P < 0.01) compared with non–CpG-treated shock rats (33 ± 1 μg/mL).
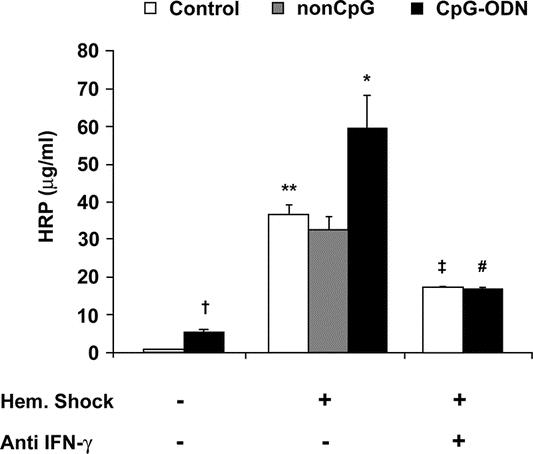
FIGURE 3. Preexposure to CpG-ODN followed by hemorrhagic shock enhanced intestinal permeability for horseradish peroxidase (HRP) via an IFN-γ-dependent route. Exposure to CpG-ODN increased permeability for HRP (5.5 ± 0.5 μg/mL, †P < 0.01) compared with control rats (1.0 ± 0.1 μg/mL). Hemorrhagic shock caused a substantial leakage of HRP (36 ± 3 μg/mL, **P < 0.01). Preexposure to CpG-ODN followed by hemorrhagic shock strongly aggravated intestinal permeability for HRP (60 ± 11 μg/mL, *P < 0.05). Administration of anti IFN-γ markedly reduced permeability for HRP in both control (‡P < 0.05) and CpG-ODN-treated rats (#P < 0.01) subjected to hemorrhagic shock: **,†compared with control; *compared with control hemorrhagic shock; #compared with CpG-ODN shock; ‡compared with control hemorrhagic shock.
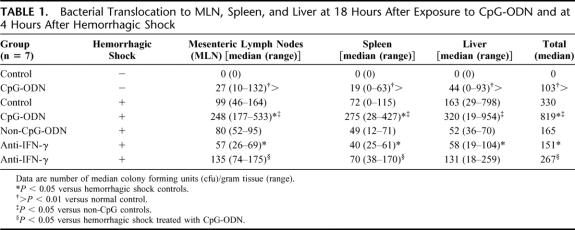
In line, exposure to CpG-ODN caused a mild bacterial translocation in all rats with a median total number of 103 CFU/g tissue, whereas bacterial cultures of control rats were sterile (Table 1). As reported, hemorrhagic shock caused bacterial translocation to MLN, spleen, and liver.20,21 Exposure to CpG-ODN prior to hemorrhagic shock more than doubled bacterial translocation (CpG-treated shock rats, total 819 CFU/g vs. nontreated, total 330 CFU/g, P < 0.05; and non–CpG-treated shock rats, total 165 CFU/g, P < 0.05). In conclusion, these data indicate that exposure to CpG-ODN impaired gut barrier function and significantly worsened hemorrhagic shock-induced intestinal barrier failure.
TABLE 1. Bacterial Translocation to MLN, Spleen, and Liver at 18 Hours After Exposure to CpG-ODN and at 4 Hours After Hemorrhagic Shock
Administration of anti-IFN-γ reduces TNF-α, IL-6, and NO release in plasma, restored the defective IL-10 response, and preserved gut barrier function in CpG-exposed rats after hemorrhagic shock.
The increased IFN-γ levels observed after CpG-ODN administration and the synergistic effect of CpG-ODN preexposure and hemorrhagic shock stimulated us to investigate the role of IFN-γ in the aggravated host response to shock. Anti IFN-γ pretreatment did not significantly affect plasma TNF-α, IL-6, nitrite (NO2−), and IL-10 levels following hemorrhagic shock in nonexposed rats (Fig. 2A–D). However, anti-IFN-γ markedly reduced circulating TNF-α, plasma IL-6, NO2−, and the enhanced permeability for HRP (17 ± 0.5 μg/mL, P = 0.002) in rats exposed to CpG-ODN 18 hours before hemorrhagic shock (Figs. 2A–C, 3). Furthermore, the defective IL-10 response upon hemorrhagic shock in rats preexposed to CpG-ODN was partly restored (109 ± 12 pg/mL, P < 0.01) (Fig. 2D). A similar effect was observed with bacterial translocation (Table 1). Bacterial translocation to distant organs was markedly reduced in anti IFN-γ-treated rats (**P < 0.01). In conclusion, it is shown that the enhanced inflammatory response and gut barrier failure caused by CpG-ODN exposure prior to hemorrhagic shock can be effectively reduced by treatment with anti-IFN-γ.
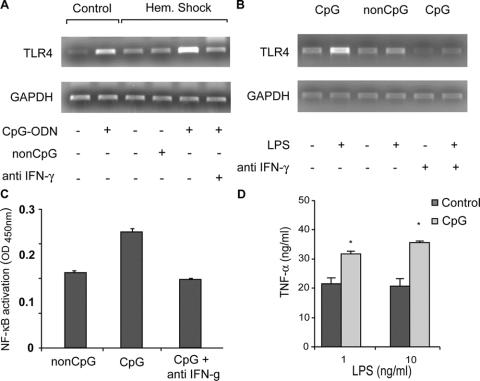
Preexposure to CpG-ODN Enhances Expression of TLR4 In Vitro and In Vivo and Increases Intracellular Signaling via an IFN-γ-Dependent Route
Endotoxin is a major player in the inflammatory response following hemorrhagic shock22,24 and triggers inflammatory cells via TLR4. Our group previously showed that IFN-γ is involved in regulation of the expression of TLR4 in the kidney following ischemia and reperfusion.25 To further delineate the IFN-γ-dependent exacerbating effect of CpG-ODN, we investigated a possible role for TLR4. TLR4 expression was enhanced following 18 hours exposure to CpG-ODN and strongly increased after hemorrhagic shock in the liver, importantly involved in the inflammatory response (Fig. 4A). This enhancement of TLR4 was not observed in non–CpG-treated shock rats and abolished by pretreatment with anti-IFN-γ. To measure the impact of this enhanced TLR4 expression translocation of NF-κB to the nucleus was determined in peritoneal macrophages. Peritoneal macrophages isolated from CpG-treated rats did not show an enhanced expression before stimulation with LPS compared with non–CpG-treated controls (Fig. 4B). However, stimulation with LPS strongly augmented TLR4 expression and resulted in increased translocation of NF-κB to the nucleus (Fig. 4C) via an IFN-γ-dependent route. Stimulation of peritoneal macrophages from rats exposed to CpG-ODN to endotoxin also caused a significantly enhanced release of TNF-α, compared with controls (*P < 0.01, Fig. 4D).
FIGURE 4. Exposure to CpG-ODN up-regulates expression of TLR4 in macrophages and in the liver leading to increased intracellular signaling via an IFN-γ-dependent route. Cell lysates and nuclear extracts were isolated from peritoneal macrophages of rats exposed to non-CpG, CpG-ODN or CpG-ODN and anti-IFN-γ before and after stimulation with LPS. Exposure to CpG-ODN alone caused enhanced expression of TLR4 in vivo in the liver, which was strongly enhanced 90 minutes following hemorrhagic shock in an IFN-γ-dependent manner (A). Expression of TLR4 and translocation of NF-κB to the nucleus in peritoneal macrophages following stimulation were markedly enhanced following CpG-ODN treatment (B, C). This enhanced expression of TLR4 and translocation of NF-κB to the nucleus were reduced after administration with anti IFN-γ. Stimulation of peritoneal macrophages from rats exposed to CpG-ODN with LPS (1 and 10 ng/mL) caused a significantly increased release of TNF-α (D), (*P < 0.01 compared with control).
DISCUSSION
Bacteria can trigger the immune system via several specific pathways. In the past decade Toll-like receptors have been discovered, which are able to bind specific bacterial components like bacterial DNA leading to an inflammatory response via an intracellular signaling pathway.26 Bacterial DNA is the common denominator of bacterial infection and causes release of inflammatory cytokines such as IFN-γ via Toll-like receptor 9.27,28
The current data show that IFN-γ is released in the circulation shortly following hemorrhagic shock. The meaning of this elevation of IFN-γ is not clear. Previous studies have shown that exogenous administration of IFN-γ increases the ability of the host defense system to combat bacterial infections after hemorrhage; therefore, IFN-γ may be essential in the host response to bacterial infection.29,30 However, IFN-γ is also involved in modulation of intestinal barrier integrity. It has recently been described that low levels of IFN-γ may cause bacterial translocation via an active lipid raft-mediated process.13 Furthermore, both IFN-γ and TNF-α lead to degradation of intestinal integrity by breakdown of intestinal tight junction proteins, such as zonula occludens proteins.31,32 This is in line with our data in which is shown that exposure to CpG-ODN strongly enhanced IFN-γ levels following hemorrhagic shock, which was associated with an increased loss of gut barrier integrity. The finding that anti-IFN-γ prevented hemorrhagic shock-induced gut barrier failure in nonexposed control rats is new and indicates an important role of this lymphokine in the pathogenesis of shock-induced intestinal permeability. Our data further demonstrate that exposure to CpG-ODN appears to induce a so-called “two-hit” phenomenon,33 which could be controlled by inhibition of IFN-γ. CpG-ODN seems to be a stress factor that on itself is rather harmless but in combination with another event such as severe blood loss primes the host and drives a stronger and potentially harmful response resulting in exacerbation of the inflammatory response.
These data must be seen in the light of a time-dependent effect of bacterial DNA in which prolonged exposure to CpG-ODN (for several days) may provide protection to a second challenge, whereas short-term exposure might exacerbate the inflammatory response to a subsequent stimulus.34,35 The potential effects of prolonged exposure to CpG-ODN before hemorrhagic shock remain subject of investigation.
Release of endotoxin following hemorrhagic shock is thought to be pivotal in development of the observed inflammatory response.24 Therefore, enhanced release of inflammatory mediators in response to combined exposure to CpG-ODN and hemorrhagic shock confirms earlier in vitro findings from our group in which we showed that CpG-ODN augmented the inflammatory response to endotoxin in RAW 264.7 cells.36 Nevertheless, others demonstrated that CpG-ODN interacts with bacterial toxins such as endotoxin in an ambiguous manner.37,38 CpG-ODN can synergize with bacterial toxins but may also induce cross-tolerance leading to a hyporesponse. Cytokines such as IFN-γ, IL-10, and potentially suppressors of cytokine signaling proteins are thought to play a role in this interrelation.23,34,39 Interestingly, DNA isolated from probiotic bacteria has a potent anti-inflammatory effect,36,40 indicating that different kinds of bacterial DNA have different effects.
The discrepancy between the rather mild effects of administration of CpG-ODN without an extra stimulus and the strong IFN-γ-dependent synergistic effect of CpG-ODN followed by hemorrhagic shock indicate a priming effect of CpG-ODN. IFN-γ is known to prime macrophages via the Toll-like receptor that binds with endotoxin (TLR4) and induce LPS responsiveness through up-regulation of TLR4 expression in intestinal epithelial cells.41,42 We show an association at the molecular level between inhibition of IFN-γ, expression of TLR4, and increased translocation of NF-κB to the nucleus in CpG-ODN-treated animals and cells. These findings suggest that TLR4 up-regulation via IFN-γ may be importantly involved in the underlying mechanism of the found effects. Although up-regulation of IFN-γ via bacterial DNA is detrimental in the current setting, release of IFN-γ is also essential in the host response to bacterial infection.30 Therefore, further research is needed to clarify the exact role of IFN-γ in modulation of these processes.
Besides a sensitizing effect of IFN-γ, the defective IL-10 response following hemorrhagic shock in CpG-ODN-treated animals may yet provide an additional explanation for the observed events.43 IL-10 has an important role as inhibitor of TH1-type cytokine production and functions primarily to limit the TH1-type inflammatory response.44 In this way, the balance necessary for controlling inflammation is dysregulated, favoring an uncontrolled pro-inflammatory response.
CONCLUSION
Exposure to bacterial DNA strongly aggravates the inflammatory response and disrupts the intestinal barrier following hemorrhagic shock. Release of IFN-γ seems pivotal in this exacerbating effect of CpG-ODN prior to hemorrhagic shock and is associated with up-regulation of TLR4 in the liver. In the clinical setting, bacterial DNA may play a role in the increased postoperative inflammatory complications in surgical patients with preexisting bacterial infection. However, in most clinical circumstances, hypovolemic shock, severe hemorrhage, or major surgery often precedes an infectious event, leading to priming of the host and an excessive response to an otherwise low-grade inflammatory trigger. Whether exposure to bacterial DNA after a stress event such as hemorrhagic shock or major surgery has a role in development of an increased inflammatory response is unknown and remains yet to be investigated.
ACKNOWLEDGMENTS
The authors thank Dr. M. G. A. Oude Egbrink (University Maastricht) for facilitating the hemorrhagic shock experiments.
Footnotes
Supported by AGIKO-stipendium 920-03-271 (to M.D.L.) and a clinical fellowship grant (NWO 907-00-033, to C.H.D.) from the Netherlands Organisation for Health Research and Development.
Reprints: Misha D. Luyer, MD, PhD, Department of Surgery, University of Maastricht, P.O. Box 616, 6200 MD Maastricht, The Netherlands. E-mail: M.Luyer@ah.unimaas.nl.
REFERENCES
- 1.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. [DOI] [PubMed] [Google Scholar]
- 2.Fan J, Kapus A, Marsden PA, et al. Regulation of Toll-like receptor 4 expression in the lung following hemorrhagic shock and lipopolysaccharide. J Immunol. 2002;168:5252–5259. [DOI] [PubMed] [Google Scholar]
- 3.Turnbull RG, Talbot JA, Hamilton SM. Hemodynamic changes and gut barrier function in sequential hemorrhagic and endotoxic shock. J Trauma. 1995;38:705–712. [DOI] [PubMed] [Google Scholar]
- 4.Brun-Buisson C, Doyon F, Carlet J, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults: a multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA. 1995;274:968–974. [PubMed] [Google Scholar]
- 5.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97–132; quiz 133–134; discussion 96. [PubMed]
- 6.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. [DOI] [PubMed] [Google Scholar]
- 7.Hacker G, Redecke V, Hacker H. Activation of the immune system by bacterial CpG-DNA. Immunology. 2002;105:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. [DOI] [PubMed] [Google Scholar]
- 9.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. [DOI] [PubMed] [Google Scholar]
- 10.Rhee EG, Mendez S, Shah JA, et al. Vaccination with heat-killed leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against leishmania major infection. J Exp Med. 2002;195:1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang XS, Sheng Z, Ruan YB, et al. CpG oligodeoxynucleotides inhibit tumor growth and reverse the immunosuppression caused by the therapy with 5-fluorouracil in murine hepatoma. World J Gastroenterol. 2005;11:1220–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sparwasser T, Miethke T, Lipford G, et al. Bacterial DNA causes septic shock. Nature. 1997;386:336–337. [DOI] [PubMed] [Google Scholar]
- 13.Clark E, Hoare C, Tanianis-Hughes J, et al. Interferon gamma induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology. 2005;128:1258–1267. [DOI] [PubMed] [Google Scholar]
- 14.Bruewer M, Luegering A, Kucharzik T, et al. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172. [DOI] [PubMed] [Google Scholar]
- 15.Ferrier L, Mazelin L, Cenac N, et al. Stress-induced disruption of colonic epithelial barrier: role of interferon-gamma and myosin light chain kinase in mice. Gastroenterology. 2003;125:795–804. [DOI] [PubMed] [Google Scholar]
- 16.Carpentier AF, Xie J, Mokhtari K, et al. Successful treatment of intracranial gliomas in rat by oligodeoxynucleotides containing CpG motifs. Clin Cancer Res. 2000;6:2469–2473. [PubMed] [Google Scholar]
- 17.van der Meide PH, Dubbeld M, Vijverberg K, et al. The purification and characterization of rat gamma interferon by use of two monoclonal antibodies. J Gen Virol. 1986;67:1059–1071. [DOI] [PubMed] [Google Scholar]
- 18.van der Meide PH, Borman AH, Beljaars HG, et al. Isolation and characterization of monoclonal antibodies directed to rat interferon-gamma. Lymphokine Res. 1989;8:439–449. [PubMed] [Google Scholar]
- 19.Engelberts I, Moller A, Schoen GJ, et al. Evaluation of measurement of human TNF in plasma by ELISA. Lymphokine Cytokine Res. 1991;10:69–76. [PubMed] [Google Scholar]
- 20.Luyer MD, Jacobs J, Vreugdenhil AC, et al. Enteral administration of high-fat nutrition before and directly after hemorrhagic shock reduces endotoxemia and bacterial translocation. Ann Surg. 2004;239:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luyer MD, Buurman WA, Hadfoune M, et al. Pretreatment with high-fat enteral nutrition reduces endotoxin and TNF-α and preserves gut barrier function early after hemorrhagic shock. Shock. 2004;21:65–71. [DOI] [PubMed] [Google Scholar]
- 22.Jiang J, Bahrami S, Leichtfried G, et al. Kinetics of endotoxin and tumor necrosis factor appearance in portal and systemic circulation after hemorrhagic shock in rats. Ann Surg. 1995;221:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crabtree TD, Jin L, Raymond DP, et al. Preexposure of murine macrophages to CpG oligonucleotide results in a biphasic tumor necrosis factor alpha response to subsequent lipopolysaccharide challenge. Infect Immun. 2001;69:2123–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bahrami S, Yao YM, Leichtfried G, et al. Monoclonal antibody to endotoxin attenuates hemorrhage-induced lung injury and mortality in rats. Crit Care Med. 1997;25:1030–1036. [DOI] [PubMed] [Google Scholar]
- 25.Wolfs TG, Buurman WA, van Schadewijk A, et al. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol. 2002;168:1286–1293. [DOI] [PubMed] [Google Scholar]
- 26.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. [DOI] [PubMed] [Google Scholar]
- 27.Latz E, Schoenemeyer A, Visintin A, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–198. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Ishii K, Hisaeda H, et al. IL-18 gene therapy develops Th1-type immune responses in Leishmania major-infected BALB/c mice: is the effect mediated by the CpG signaling TLR9? Gene Ther. 2004. [DOI] [PubMed]
- 29.Meldrum DR, Ayala A, Perrin MM, et al. Diltiazem restores IL-2, IL-3, IL-6, and IFN-gamma synthesis and decreases host susceptibility to sepsis following hemorrhage. J Surg Res. 1991;51:158–164. [DOI] [PubMed] [Google Scholar]
- 30.Ertel W, Morrison MH, Ayala A, et al. Interferon-gamma attenuates hemorrhage-induced suppression of macrophage and splenocyte functions and decreases susceptibility to sepsis. Surgery. 1992;111:177–187. [PubMed] [Google Scholar]
- 31.Han X, Fink MP, Delude RL. Proinflammatory cytokines cause NO*-dependent and -independent changes in expression and localization of tight junction proteins in intestinal epithelial cells. Shock. 2003;19:229–237. [DOI] [PubMed] [Google Scholar]
- 32.Chakravortty D, Kumar KS. Modulation of barrier function of small intestinal epithelial cells by lamina propria fibroblasts in response to lipopolysaccharide: possible role in TNFalpha in inducing barrier dysfunction. Microbiol Immunol. 1999;43:527–533. [DOI] [PubMed] [Google Scholar]
- 33.Fan J, Marshall JC, Jimenez M, et al. Hemorrhagic shock primes for increased expression of cytokine-induced neutrophil chemoattractant in the lung: role in pulmonary inflammation following lipopolysaccharide. J Immunol. 1998;161:440–447. [PubMed] [Google Scholar]
- 34.Krieg AM. CpG DNA: trigger of sepsis, mediator of protection, or both? Scand J Infect Dis. 2003;35:653–659. [DOI] [PubMed] [Google Scholar]
- 35.Slotta JE, Scheuer C, Menger MD, et al. Immunostimulatory CpG-oligodeoxynucleotides (CpG-ODN) induce early hepatic injury, but provide a late window for protection against endotoxin-mediated liver damage. J Hepatol. 2006;44:576–855. [DOI] [PubMed] [Google Scholar]
- 36.Luyer MD, Buurman WA, Hadfoune M, et al. Strain-specific effects of probiotics on gut barrier integrity following hemorrhagic shock. Infect Immun. 2005;73:3686–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao JJ, Xue Q, Papasian CJ, et al. Bacterial DNA and lipopolysaccharide induce synergistic production of TNF-alpha through a post-transcriptional mechanism. J Immunol. 2001;166:6855–6860. [DOI] [PubMed] [Google Scholar]
- 38.Jin L, Raymond DP, Crabtree TD, et al. Preexposure of murine macrophages to CpG-containing oligonucleotides results in nuclear factor kappaB p50 homodimer-associated hyporesponsiveness. Surgery. 2002;132:245–251. [DOI] [PubMed] [Google Scholar]
- 39.Dalpke AH, Opper S, Zimmermann S, et al. Suppressors of cytokine signaling (SOCS)-1 and SOCS-3 are induced by CpG-DNA and modulate cytokine responses in APCs. J Immunol. 2001;166:7082–7089. [DOI] [PubMed] [Google Scholar]
- 40.Rachmilewitz D, Katakura K, Karmeli F, et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520–528. [DOI] [PubMed] [Google Scholar]
- 41.Bosisio D, Polentarutti N, Sironi M, et al. Stimulation of toll-like receptor 4 expression in human mononuclear phagocytes by interferon-gamma: a molecular basis for priming and synergism with bacterial lipopolysaccharide. Blood. 2002;99:3427–3431. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki M, Hisamatsu T, Podolsky DK. Gamma interferon augments the intracellular pathway for lipopolysaccharide (LPS) recognition in human intestinal epithelial cells through coordinated up-regulation of LPS uptake and expression of the intracellular Toll-like receptor 4-MD-2 complex. Infect Immun. 2003;71:3503–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donnelly RP, Freeman SL, Hayes MP. Inhibition of IL-10 expression by IFN-gamma up-regulates transcription of TNF-alpha in human monocytes. J Immunol. 1995;155:1420–1427. [PubMed] [Google Scholar]
- 44.Moore KW, de Waal Malefyt R, Coffman RL, et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. [DOI] [PubMed] [Google Scholar]