Abstract
Objective:
We present and analyze long-term outcomes following multimodal therapy for esophageal cancer, in particular the relative impact of histomorphologic tumor regression and nodal status.
Patients and Methods:
A total of 243 patients [(adenocarcinoma (n = 170) and squamous cell carcinoma (n = 73)] treated with neoadjuvant chemoradiotherapy in the period 1990 to 2004 were followed prospectively with a median follow-up of 60 months. Pathologic stage and tumor regression grade (TRG) were documented, the site of first failure was recorded, and Kaplan-Meier survival curves were plotted.
Results:
Thirty patients (12%) did not undergo surgery due to disease progression or deteriorated performance status. Forty-one patients (19%) had a complete pathologic response (pCR), and there were 31(15%) stage I, 69 (32%) stage II, and 72 (34%) stage III cases. The overall median survival was 18 months, and the 5-year survival was 27%. The 5-year survival of patients achieving a pCR was 50% compared with 37% in non-pCR patients who were node-negative (P = 0.86). Histomorphologic tumor regression was not associated with pre-CRT cTN stage but was significantly (P < 0.05) associated with ypN stage. By multivariate analysis, ypN status (P = 0.002) was more predictive of overall survival than TRG (P = 0.06) or ypT stage (P = 0.39).
Conclusion:
Achieving a node-negative status is the major determinant of outcome following neoadjuvant chemoradiotherapy. Histomorphologic tumor regression is less predictive of outcome than pathologic nodal status (ypN), and the need to include a primary site regression score in a new staging classification is unclear.
A prospective analysis of 243 patients with localized esophageal cancer treated with neoadjuvant chemoradiation followed by surgery was undertaken, and long-term outcomes reported. Pathologic node-negative status (ypN0) was the most powerful (P = 0.002) determinant of survival. Neither histomorphologic tumor regression (TRG; P = 0.06) nor depth of invasion (ypT; P = 0.39) was significantly associated with survival.
Carcinoma of the esophagus and the esophagogastric junction is increasing in incidence in the western world, and the diagnosis confers a poor prognosis even in patients undergoing curative resection.1–4 The most radical surgery with 2 or 3 field lymphadenectomy results is at best a 3-year survival of approximately 50%.5–7 Neoadjuvant approaches have been developed to improve these poor outcomes, and a number of phase II and III studies of either chemotherapy alone or combined with radiation therapy have been published. The interpretation of randomized clinical trials (RCTs), however, is confused and conflicting. For chemotherapy alone, two adequately powered phase III studies of neoadjuvant 5-fluoruracil and cisplatin, the Intergroup8 and Medical Research Council (MRC) trials,9 show a survival benefit solely in the MRC study. Analysis of RCTs of combination chemotherapy and radiation therapy prior to surgery,10–16 and meta-analysis,17,18 is controversial. Notwithstanding the fact that the largest and most adequately powered studies are negative,13,15,16 the use of multimodal therapy has increased; the Patterns of Care studies showed that preoperative chemoradiation therapy increased from 10.4% during 1992 to 1994 to 26.6% in 1996 to 1999.19
In making sense of the existing literature, it is unassailable that a subset of patients, those achieving a complete pathologic response (pCR) from neoadjuvant therapy, have improved outcomes.20–24 This occurs in 15% to 30% of cases receiving preoperative chemotherapy and radiation therapy, with 3-year survival rates of approximately 50% irrespective of the applied protocol, type of histology, and tumor stage. Patients achieving a pCR also appear to have a different pattern of relapse compared with those who do not attain a pCR, with recurrence predominantly systemic rather than locoregional.21
A further subdivision of pathologic response to neoadjuvant regimens, the tumor regression grade, initially proposed by Mandard et al in 1994,25 may also identify patterns of incomplete response that may impact on treatment outcome and response. The inclusion of a measure of histomorphologic response to neoadjuvant therapy at the primary site into a new pTMN staging system has been recently advocated.26,27
We report herein a consecutive series of 243 patients treated by neoadjuvant chemoradiation followed by esophagectomy with curative intent, with a median follow-up of 60 months. The primary goal is to detail the outcomes achieved in a series with long-term follow-up, with a particular focus on the relative value of histomorphologic responses compared with standard pTNM assessment of prognosis. We report the relative importance of both post-treatment pathologic nodal status and the histomorphologic response at the primary site, and highlight the unique significance of node-negative status.
PATIENTS AND METHODS
Eligibility Criteria
Patients at this Institution were considered for an IRB-approved multimodal regimen if they fulfilled the following criteria: age <77 years; satisfactory performance status and medical fitness for surgery; a biopsy proven invasive tumor of the esophagus or esophagogastric junction; and a staged tumor deemed resectable by the primary surgeon. All patients had in addition a leukocyte count greater than 3500 per m3, a platelet count above 100,000 per m3, serum creatinine less than 1.4 mg/dL (124 μmol/L), no previous chemotherapy or radiation therapy, and no previous cancer other than skin. Patients with any one of the following were excluded from consideration: age >77 years; high-grade dysplasia or carcinoma in situ; emergency esophagectomy following esophageal rupture; surgery determined preoperatively to be palliative based on tumor extent or patient performance; bronchial evidence of invasion based on CT imaging and bronchoscopy, and tumor classified as T4, Nany by the multidisciplinary esophageal panel.
From 1990 to 1995, evaluation was by esophagoscopy, barium study, liver ultrasound, and occasional computed tomography (CT). Since 1995, all patients have had CT of the neck, thorax, and abdomen. Now considered routine, 18-F-deoxyglucose PET scans did not become available in this unit until mid-2003. Similarly endoscopic ultrasound (EUS) was not freely available during this study period. Using CT criteria, the mediastinal, left gastric, and celiac lymph nodes were classified as N1 (invaded) if the maximal transverse diameter of these nodes were larger than 1 cm. Resectable disease was defined as T1-3, N0-1. All tumors at the esophagogastric junction were assigned as type I, II, or III, as per Siewert and Stein28: type I was adenocarcinoma of the distal esophagus, usually arising in specialized intestinal metaplasia; type II is a true adenocarcinoma of the cardia arising immediately at the esophagogastric junction; and type III is a subcardial gastric carcinoma infiltrating the esophagogastric junction and distal esophagus from below.
Treatment Protocol
The majority of patients (n = 185) in the neoadjuvant treatment arm were given a standard protocol of chemoradiotherapy consisting of 40 Gy/15 fractions on days 1 to 5, 8 to 12, and 15 to 19, and concurrent chemotherapy of 5-fluorouracil (15 mg/kg) on days 1 to 5 and cisplatin (75 mg/m2) on day 7.12 Chemotherapy was repeated on week 6. Since 2002, an increasing number of patients (n = 41) have been given 44 Gy in 22 fractions. Three patients in this analysis were referred from another institution where they had received 50 Gy in 25 fractions. In the remaining patients, 8 received 40 Gy in 20 fractions, and one patient 45 Gy in 25 fractions. Patients were restaged by CT and OGD at week 8 and scheduled for surgery on week 9. Surgery took place if the neutrophil count was >2 × 106/mL−1, if performance status had not significantly deteriorated, and if there was no evidence of local or systemic progression of disease on imaging.
All patients had a thoracotomy as a component of their surgical management, either combined with an abdominal and neck exploration (3-stage) for mid- and upper-esophageal cancers, or cancer arising in long-segment Barrett esophagus, or with an abdominal exploration (2-stage) for most lower third and junctional tumors, or combined with a total gastrectomy for junctional tumors with significant gastric extension. A 2-field lymphadenectomy (abdominal and thoracic) was performed in all cases.29,30 In the abdomen, nodal dissection routinely involved resection of N1 nodes as well as nodes along the left gastric artery, common hepatic artery, and splenic artery. In the thorax, clearance was obtained of nodes up to and including subcarinal nodes in all cases, and in selected cases paratracheal nodes were resected. Dissection of cervical lymph nodes was not performed.
Pathology
All hematoxylin and eosin-stained sections from resected esophagectomy specimens, including lymph nodes, were assessed without knowledge of patient treatment or follow-up status. Each specimen was evaluated for extent of residual cancer, depth of invasion, and lymph node metastasis. The mean number of blocks examined per case was 10 (range, 4–17). Extent of residual carcinoma in the esophagectomy specimen was assigned to one of five categories as per Mandard et al25: TRG1 represents fibrosis within the esophageal wall with no identifiable residual cancer cells (pCR); TRG2 represents rare residual cancer cells scattered throughout the fibrosis; TRG3 represents an increase in the number of residual cancer cells, but fibrosis still predominated. TRG4 represents residual cancer cells outgrowing fibrosis; and TRG5 represents a complete absence of regression change.
The tumor stage was defined according to the TMN staging system and the American Joint Committee on Cancer classification.31 The prefix y is included as recommended to denote pathologic staging following neoadjuvant therapy.31 The absence of residual tumor was defined as a complete pathologic response (stage 0). The tumor was classified as stage 1 if there was residual tumor in the mucosa or submucosa and the lymph nodes were free of tumor. Stage 2 defines tumor in the muscularis or adventitia but negative lymph nodes. If the tumor was present in the esophageal wall and lymph nodes, this was defined as stage 3.
All patients were followed up with 6 monthly endoscopy and annual CT scans or where clinically indicated. Locoregional recurrence was defined as that occurring at the site of anastomosis, in the mediastinum or cervical region. Distant recurrence was defined as that occurring within a solid organ. Cytologic, histologic, or radiologic proof was required to confirm a diagnosis of recurrent disease.
Statistical Analysis
Data were analyzed with Stata, version 8. Two endpoints were analyzed: time to death, and time to death or relapse. Apart from intention-to-treat analysis, nonoperated patients, in-hospital mortality, and lack of availability of specimen blocks were excluded from survival analyses that related to histomorphologic tumor regression and cancer outcomes. Disease-free and overall survival rates were calculated according to the Kaplan-Meier method and group comparisons were based on the log-rank test. The Pearson χ2 test or Fisher exact test was used to compare comparisons between combinations of the 2 groups. Analysis of variance was used to compare the distribution of parameters among all 3 groups.
Univariate and multivariate Cox regression analyses were used to examine the effect of clinical and pathologic factors on overall and disease-free survival. Cox proportional hazards models were fitted for multivariate analysis. After interactions between variables, a backward stepwise procedure was used to derive the best fitting model.
RESULTS
Patient and Tumor Characteristics
Between May 1990 and December 2004, 243 patients were enrolled for multimodal therapy (Table 1). The majority of patients had adenocarcinoma (n = 170) and 73 had squamous cell cancer. Thirty patients (12%) did not proceed to surgery, 8 manifesting systemic metastases, and 7 had local progression to inoperability, 14 showed significant deterioration of performance status and became medically unfit, and 1 patient refused surgery.
TABLE 1. Patient Characteristics

Pathologic Assessment and Histomorphologic Grading of Tumor Regression
Of resected patients (n = 213), 41 (19%) patients had a complete pathologic response, 31 (15%) had stage 1 disease, 69 (32%) had stage 2 disease, and 72 (34%) had stage 3 disease. Sex, age, and histologic cell type distribution were similar for all pathologic stages.
The tumor regression grade is shown in Table 2. A total of 178 patients had TRG scores assigned and were suitable for analysis. Thirty-seven had complete tumor regression (TRG1) in the primary tumor, 38 had rare residual cancer cells (TRG2), 48 had an increase in the number of residual cancer cells (TRG3), 42 demonstrated residual cancer outgrowing fibrosis (TRG4), and 13 showed an absence of regressive changes (TRG5). In 20 patients no blocks were available to score, and the remainder represent in-hospital deaths excluded from analysis.
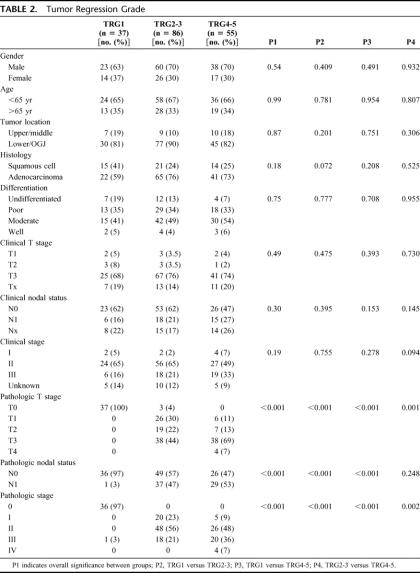
TABLE 2. Tumor Regression Grade
TRG1 was compared with TRG2-3, and TRG4-5 (Table 2). There was no association between TRG and tumor site, type, or differentiation. Clinical N and T stage did not predict response; the percentage of cT3 tumors was similar in all TRG groups. The percentage of cN0 patients was nonsignificantly greater in TRG1 (P = 0.2) and TRG2-3 (P = 0.09) compared with TRG4-5. There was a significant association between TRG and ypT stages (P < 0.01). Forty-four percent of TRG2-3 patients had pT3 pathology, compared with 69% of the TRG4-5 group (P = 0.001).
For ypN status, TRG1 was significantly (P < 0.05) associated with ypN0 compared with both other groups. In the TRG1 cohort, 36 patients (97%) were ypN0 compared with 49 (57%) in TRG2-3, and 26 (47%) in TRG4-5 (P = 0.248 TRG2-3 vs. TRG4-5). When each TRG group was looked at separately, percent ypN0 was 97, 68, 48, 41, and 49 from TRG1 to TRG5, respectively.
Survival
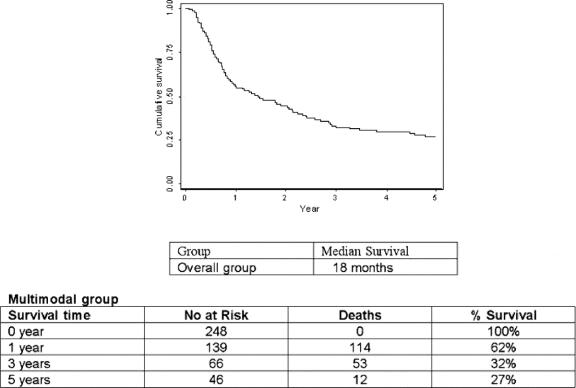
After a median follow-up of 60 months, by intention-to-treat analysis, the median survival was 18 months, and the 1-, 3-, and 5-year survival was 62%, 32%, and 27%, respectively (Fig. 1).
FIGURE 1. Overall survival by intention to treat.
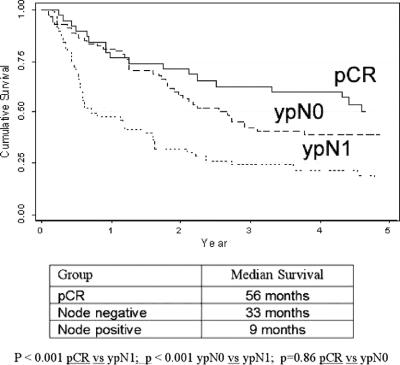
The postoperative survival curves for patients achieving a pCR was compared with node-negative patients (excluding complete responders) and node-positive patients (Fig. 2). The median survival was 56 months in the pCR group, compared with 33 months in the node-negative group (P = 0.86) and 9 months in the node-positive group (P < 0.001). Comparing pCR with node-negative subgroups, the 1 (76% vs. 81%), 3 (63% vs. 42%), and 5-year overall survival (50% vs. 37%) were not significantly different (P = 0.62). When pCR was combined with the ypT1N0 group (n = 65), and compared with ypT2-3N0 patients (n = 52), there was a significant (P = 0.03) survival benefit, with a 3-year survival, respectively, of 61% versus 35%, and a 5-year survival of 53% versus 30%, and a median survival respectively of 67 months compared with 27 months.
FIGURE 2. Survival of compete pathologic responders (pCR) compared with ypN0 and ypN1 groups.
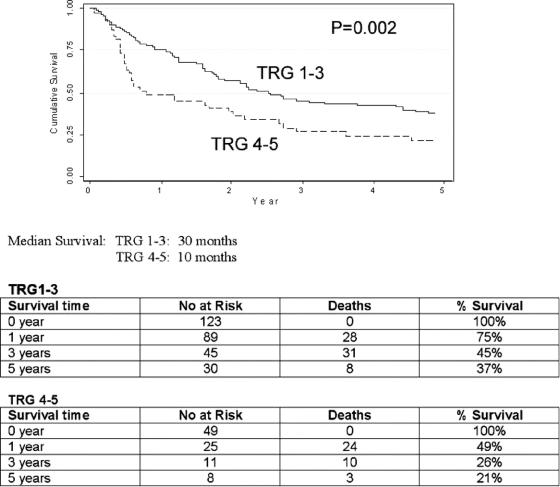
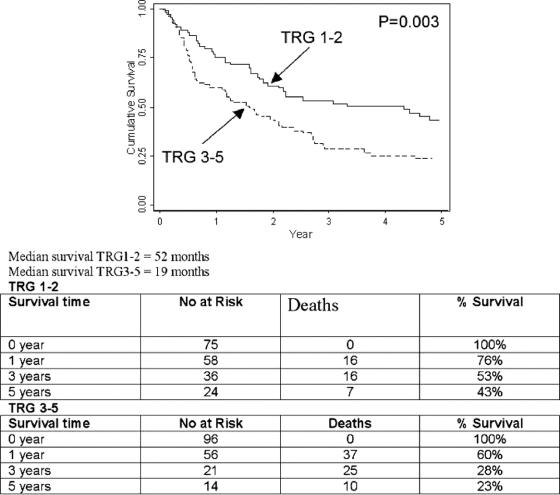
The median and overall survival at 1, 3, and 5 years for all pathologic groups is shown in Table 3, and the impact of tumor regression grade on survival is shown in Table 4. The median survival in TRG1 was 53 months, compared with 27 months (TRG2), 22 months (TRG3), 7 months (TRG4) and 10 months (TRG5). The 5-year survival in TRG 5 was 8% compared with 45%, 41%, 26%, and 27% for TRG1-4 respectively. A highly significant (P = 0.002) survival benefit was observed when TRG1-3 was compared with TRG4-5 (Fig. 3), with a median survival of 30 months versus 10 months, and a 5-year survival of 37% compared with 23%. When TRG1-2 was compared with TRG3-5 (Fig. 4), the median survival was 52 months versus 19 months, and the 5-year survival 43% versus 23%, respectively (P = 0.003).
TABLE 3. Median and Overall Survival for All Pathologic Groups
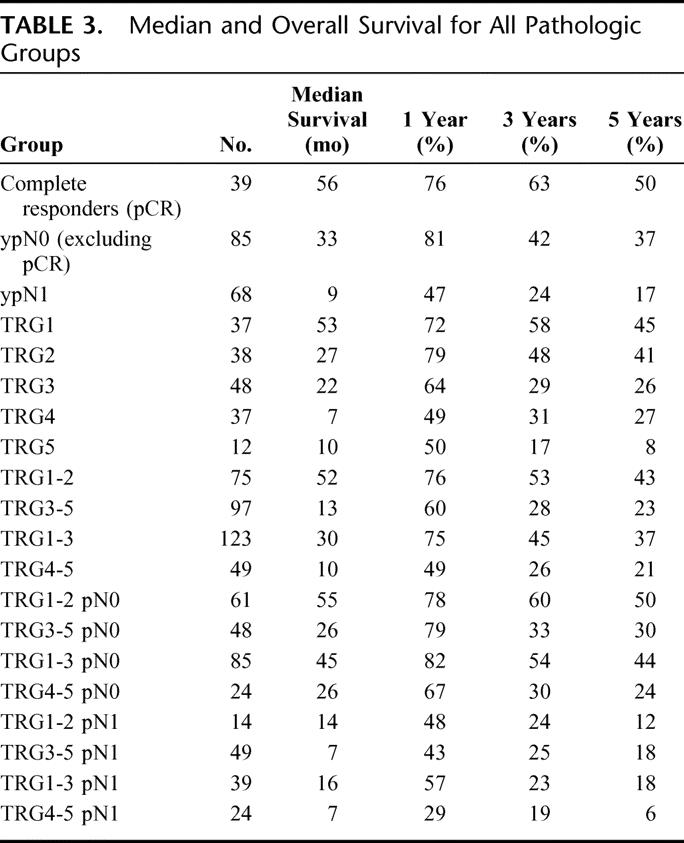
TABLE 4. Survival and Tumor Regression Grade (TRG) 1–5
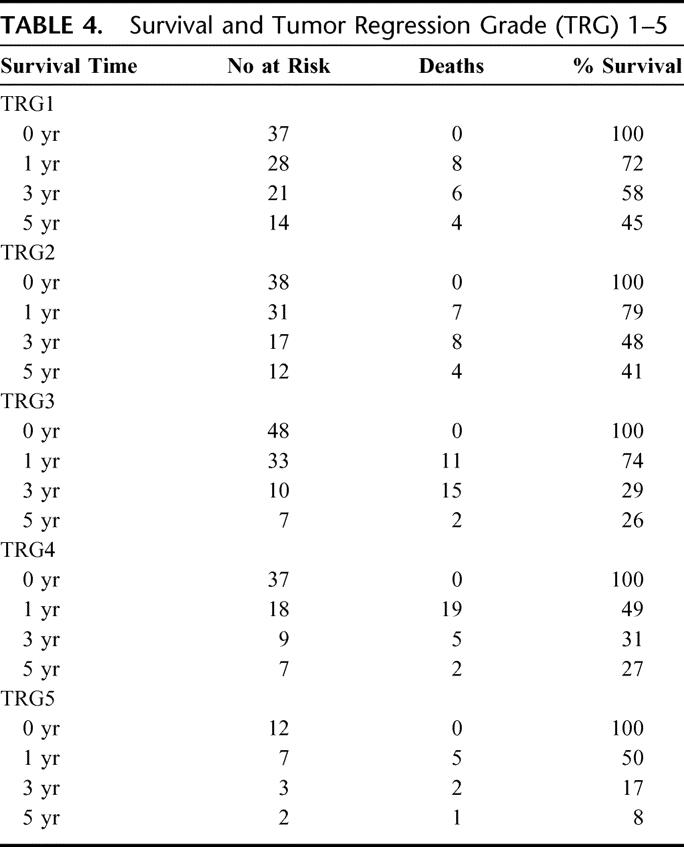
FIGURE 3. TRG 1-3 versus TRG 4-5.
FIGURE 4. TRG 1-2 versus TRG 3-5.
When ypN status is factored into the TRG subgroups, and TRG1-3 is compared with TRG4-5, no significant difference was evident between TRG1-3 ypN0 patients and TRG 4–5 ypN0 patients (P = 0.06), nor between TRG1-3 ypN1 patients and TRG4-5 ypN1 patients (P = 0.7).
Disease Recurrence and Patterns of First Failure
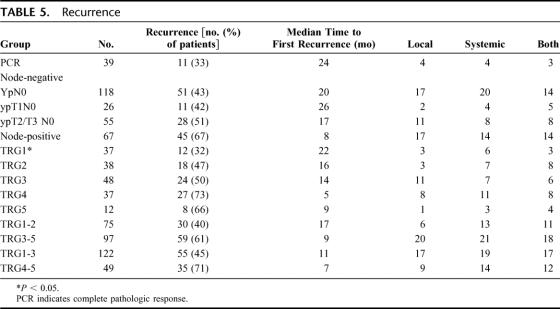
The pattern of failure and median time to relapse is shown in Table 5. A total of 118 patients (48%) have relapsed. The median time to relapse was significantly (P < 0.05) decreased in ypN1 patients compared with pCR and ypN0 patients, but there was no difference between pCR and ypN0. For TRG groups, the median time to recurrence was significantly (P < 0.05) prolonged only in the TRG1 group compared with all other groups. The pattern of failure, local, systemic, or a combination was not significantly different between groups.
TABLE 5. Recurrence
Prognostic Factors: Overall and Disease-Free Survival
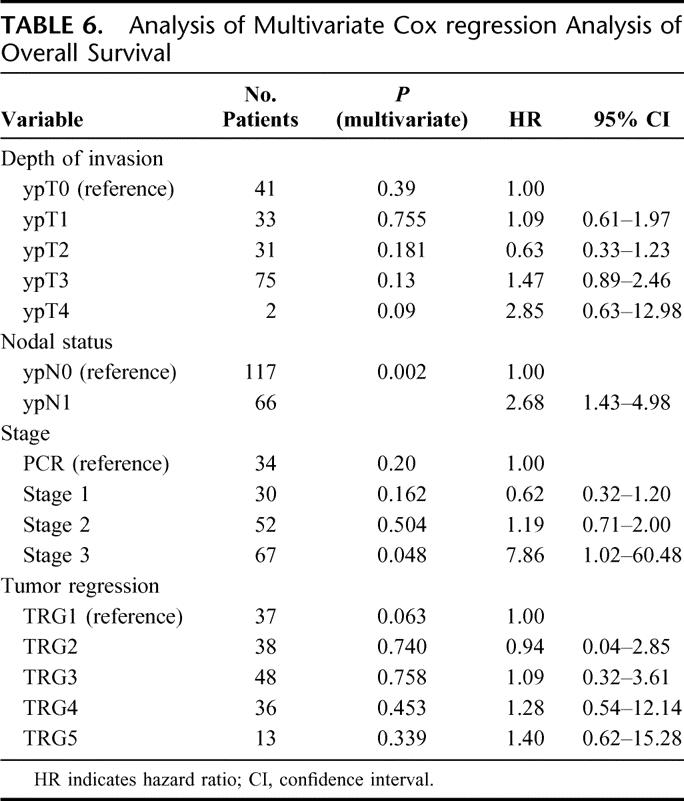
In a univariate analysis ypN status and TRG were significantly (P < 0.05) associated with overall and disease-free survival. By multivariate analysis (Table 6), the only variable associated with overall survival was ypN status (P = 0.002). pT was not significant (P = 0.39) and TRG approached significance (P = 0.06). There were no observed differences between squamous cell tumors and adenocarcinoma.
TABLE 6. Analysis of Multivariate Cox regression Analysis of Overall Survival
DISCUSSION
The achievement of a complete pathologic response following neoadjuvant chemotherapy alone or in combination with radiotherapy for esophageal tumors is a surrogate marker of survival advantage.32–34 In this regard, the combination of chemotherapy and radiation therapy achieves higher response rates than chemotherapy alone. In a review of induction chemoradiotherapy, Geh et al21 reported on 2704 patients from published nonrandomized trials, 69% with squamous cell cancer and 31% with adenocarcinoma, and recorded 643 pCRs, representing 24% of the total number of patients treated or 32% of those undergoing resection. In randomized trials published to date,1 the pCR rate has ranged from 10% to 28%, and the 3-year survival from 17% to 39%. In this study, at a median follow-up of 60 months, 19% of patients resected obtained a pCR, and the 3-year survival by intention to treat was 32%, consistent with published data.
A 5-year survival of 50% and median survival of 56 months in patients achieving a pCR compares favorably with 27% and 18 months, respectively, in the entire group of 243 patients based on intention to treat. Several elements of the overall experience need emphasis. First, the failure of patients, all with good performance indices at commencement of treatment, to complete the full regimen and progress to surgery was observed in 12% in this series, due equally to disease progression or deteriorating performance indices. Second, the study shows that it is nodal status rather than ypT status or the attainment of pCR that is the most significant determinant of prognosis. Although the median survival was 56 months in the pCR group (n = 39) compared with 33 months in the ypN0 groups (n = 118), there was no significant difference in 1-, 3-, and 5-year survival. Within the ypT node-negative groups, the combination of pCR with ypT1N0 (n = 65) was associated with a median survival of 67 months and 5-year survival of 53%, compared (P = 0.03) with 25 months and 30%, respectively, for ypT2-3N0 (n = 52). Therefore, although progressive benefit was observed with pCR and ypT1, the data using standard histopathologic TNM reporting identified ypN status as the most important determinant of outcome.
The primacy of nodal status is important as the focus of recent pathologic studies of response to neoadjuvant therapies has been on the primary rather than nodal sites. In this study, the Mandard tumor regression grade was used; the original report in 1994 showing a significant association of histomorphologic regression with survival in patients with predominantly esophageal squamous cell cancer who were treated with neoadjuvant chemoradiotherapy.25 In the Mandard series, 58 of 93 (62%) had a good histomorphologic response, defined as TRG1-3, similar to 123 of 172 (72%) patients in this study. Others have used similar parameters of histomorphologic response. The M. D. Anderson26,33 group have defined 3 pP groups: P0, no residual cancer; P1, 1% to 50% residual; and P2, >50% residual. Schneider et al27 adapted regression grades of prognostic significance in non-small cell lung cancer35 and estimated percentage of vital residual tumor cells (VTRCs), with grade 1 meaning >50% VRTCs, grade II denoting 10% to 50% VRTCs, grade III, nearly complete response with <10% VRTCs, and grade IV, complete response (pCR, ypT0), and a major histomorphologic regression as grades III/IV. TRG1 approximates to P0 or grade IV response, and the combination of TRG1-2 to grades III/IV, TRG 2 is grade II, TRG2-3 is P1, and TRG4-5 approximately associates with P3 and grade I.
In this series, approximately 1 of 5 patients had a complete response, and the majority of patients, 72% in this study, had a response where fibrosis outweighs residual cancer cells. Pretreatment clinical stage had no predictive value on histomorphologic response, consistent with a recent report.33 Downstaging, defined as post-therapy pathologic stage lower than pretherapy clinical stage, was evident in 97% of patients achieving TRG1, 60% in the TRG1-3 group, 44% in the TRG2-3 group, and 24% in the TRG4-5 group. The association of TRG with ypN was evident, with 97% of TRG1 patients and 68% of TRG2 patients node-negative, consistent with the response classification grade III and IV of Schneider et al.27 Finally, with respect to overall survival, the association of TRG1-3 was associated with significantly improved survival compared with TRG4-5, confirming the original observation of Mandard et al.25 This also confirms the reports from the M. D. Anderson on the equivalent P0 and P1 groups,26 and the survival analysis comparing TRG1-2 with TRG3-5 confirms the fact that there is little difference in outcomes between complete regression and microscopic residual disease, results consistent with equivalent analyses.27 Although node-negative status was the only significant prognostic determinant by multivariate analysis, the histomorphologic response at the primary site measured by TRG approached significance (P = 0.06), and the study suggests that assessment of histomorphologic response is important complimentary information in the overall management of patients within multimodal protocols.
What are the practical implications of these findings? First, this study shows that ypN status is the strongest determinant of outcome, more important than the achievement of a complete pathologic response or of histomorphologic regression at the primary site. These data do not therefore support an imperative to incorporate an assessment of histomorphologic response in a revised pTNM system, and more research is required in this area. The value of histomorphologic response may be to mirror tumor biology, in particular inherent chemoradioresistance, and may be useful as a surrogate of response in novel designs of clinical trials.36 Second, the histomorphologic response may be a proxy for nodal status and residual tumor volume, and where patients have apparent TRG1 based on clinical evaluation and numerous biopsies, the likelihood of nodal disease is small. One could speculate that in this scenario a nonoperative approach could be cautiously considered within trials, an approach already adopted in some studies in rectal cancer.37 Finally, the study invites the question: if patients based on CT, EUS, and FDG-PET appear to have truly node-negative disease, does neoadjuvant chemoradiotherapy have any rationale? This fact has yet to be established. Conversely, we suggest that studies identifying true histomorphologic regression of nodes with multimodal therapy, rather than at the primary site, need to be developed. Further prospective studies are required, and this, in combination with FDG-PET as an early response marker, and the better use of molecular markers, may better classify patients before, during, and after neoadjuvant regimens.38,39 At this time, these data demonstrate the primacy of ypN status in predicting outcomes after multimodal therapy for esophageal cancer, and the relative and linked value of histomorphologic response.
Footnotes
Reprints: John Vincent Reynolds, MD, Department of Surgery, Trinity Centre, St. James’s Hospital and Trinity College Dublin, Dublin 8, Ireland. E-mail: reynoljv@tcd.ie.
REFERENCES
- 1.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. [DOI] [PubMed] [Google Scholar]
- 2.Daly JM. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg. 2000;190:562–572. [DOI] [PubMed] [Google Scholar]
- 3.Blot WJ, De Vesa SS, Kneller RW, et al. Rising incidence of adenocarcinoma of the oesophagus and gastric cardia. JAMA. 1991;265:1287–1289. [PubMed] [Google Scholar]
- 4.Pera M, Cameron A, Trastek V, et al. Increasing incidence of adenocarcinoma of the esophagus and eosphagogastric junction. Gastroenterology. 1993;104:510–513. [DOI] [PubMed] [Google Scholar]
- 5.Altorki N, Kent M, Ferrara RN, et al. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg. 2002;236:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lerut T, Coosemans W, De Leyn P, et al. Reflections on 3-field lymphadenectomy in carcinoma of the esophagus and gastroesophageal junction. Hepatogastroenterology. 1999;46:717–725. [PubMed] [Google Scholar]
- 7.Siewert JR, Stein HJ, Feith M, et al. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1000 consecutive resections at a single centre in the western world. Ann Surg. 2001;232:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1974–1984. [DOI] [PubMed] [Google Scholar]
- 9.Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727–1733. [DOI] [PubMed] [Google Scholar]
- 10.Nygard K, Hagen S, Hansen HS, et al. Preoperative radiotherapy prolongs survival in operable esophageal cancer: a randomised, multicenter study of pre-operative radiotherapy and chemotherapy: the second Scandinavian trial in esophageal carcinoma. World J Surg. 1992;16:1104–1110. [DOI] [PubMed] [Google Scholar]
- 11.Le Prise E, Etienne PL, Meunier P, et al. A randomised study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer. 1994;73:1179–1184. [DOI] [PubMed] [Google Scholar]
- 12.Walsh T, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–467. [DOI] [PubMed] [Google Scholar]
- 13.Bosset J-F, Gignoux M, Tiboulet J-P, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous cell cancer of the esophagus. N Engl J Med. 1997;337:161–167. [DOI] [PubMed] [Google Scholar]
- 14.Urba SG, Orriner MB, Turrisi A, et al. Randomised trial of preoperative chemoradiotherapy versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–313. [DOI] [PubMed] [Google Scholar]
- 15.Law S, Kwong D, Tung H, et al. Preoperative chemoradiation for squamous cell esophageal cancer: a prospective randomised trial [Abstract]. Can J Gastroenterol. 1998;12(suppl B):56. [Google Scholar]
- 16.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for respectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659–668. [DOI] [PubMed] [Google Scholar]
- 17.Urschel JD, Vasan H. A meta-analysis of randomised controlled that compared neo-adjuvant chemoradiation and surgery to surgery alone for respectable esophageal cancer. Am J Surg. 2003;185:538–543. [DOI] [PubMed] [Google Scholar]
- 18.Fiorica F, DiBona D, Schepis F, et al. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut. 2004;53:925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suntharalingam M, Mougham J, Coia LR, et al. The national practice for patients receiving radiation therapy for carcinoma of the esophagus: results of the 1996–1999 Patterns of Care Study. Cancer. 1999;85:2499–2505. [DOI] [PubMed] [Google Scholar]
- 20.Ancona E, Ruol A, Santi S, et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients respectable esophageal squamous cell carcinoma: final report of a randomised trial of preoperative chemotherapy versus surgery alone. Cancer. 2001;91:2165–2174. [PubMed] [Google Scholar]
- 21.Geh JI, Crellin AM, Glynne-Jones R. Preoperative (neoadjuvant) chemoradiotherapy in oesophageal cancer. Br J Surg. 2001;88:338–356. [DOI] [PubMed] [Google Scholar]
- 22.Forasitere AA, Orringer MB, Perez-Tamayo C, et al. Preoperative chemoradiotherapy followed by transhiatal esophagectomy for carcinoma of the esophagus: final report. J Clin Oncol. 1993;11:1118–1123. [DOI] [PubMed] [Google Scholar]
- 23.Donington JS, Miller DL, Allen MS, et al. Tumor response to induction chemoradiation: influence on survival after esophagectomy. Eur J Cardiothorac Surg. 2003;24:631–637. [DOI] [PubMed] [Google Scholar]
- 24.Swisher SG, Ajani JA, Komaki R, et al. Long-term outcomes of phase II trial evaluating chemotherapy, chemoradiotherapy, and surgery for locoregionally advanced esophageal cancer. Int J Radiat Oncol Biol Phys. 2003;57:120–127. [DOI] [PubMed] [Google Scholar]
- 25.Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Cancer. 1994;73:2680–2686. [DOI] [PubMed] [Google Scholar]
- 26.Swisher SG, Hofsetter W, Wu TT, et al. Proposed revision of the esophageal cancer staging system to accommodate pathologic response (pP) following preoperative chemoradiation (CRT). Ann Surg. 2005;241:810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider PM, Baldus SE, Metzger R, et al. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer. Ann Surg. 2005;242:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophago-gastric junction. Br J Surg. 1998;85:1457–1459. [DOI] [PubMed] [Google Scholar]
- 29.Kitajima M, Kitagawa Y. Surgical treatment of esophageal cancer: the advent of the era of individualization. N Engl J Med. 2002;347:1705–1709. [DOI] [PubMed] [Google Scholar]
- 30.Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus N Engl J Med. 2002;347:1662–1669. [DOI] [PubMed] [Google Scholar]
- 31.Greene FL, Page DL, Fleming ID, et al., eds. American Joint Committee on Cancer: AJCC Cancer Staging Manual, 6th ed. New York: Springer-Verlag, 2002. [Google Scholar]
- 32.Vogel SB, Mendenhall WM, Sombeck MD, et al. Downstaging of esophageal cancer after preoperative radiation and chemotherapy. Ann Surg. 1995;221:685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chireac LR, Swisher SG, Ajani JA, et al. Post-therapy pathologic predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103:11347–11355. [DOI] [PubMed] [Google Scholar]
- 34.Kaklamanos IG, Walker GR, Ferry K, et al. Neoadjuvant treatment for resectable cancer of the esophagus and the gastroesophageal junction: a meta-analysis of randomized clinical trials. Ann Surg Oncol. 2003;10:754–761. [DOI] [PubMed] [Google Scholar]
- 35.Junker K, Thomas M, Schulmann K, et al. Tumour regression in non-small-cell lung cancer following neoadjuvant therapy: histological assessment. J Cancer Res Clin Oncol. 1997;123:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rohatgi PR, Swisher SG, Correa AM, et al. Failure patterns correlate with the proportion of residual carcinoma after preoperative chemoradiotherapy for carcinoma of the esophagus. Cancer. 2005;104:1349–1355. [DOI] [PubMed] [Google Scholar]
- 37.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for Stage 0 distal rectal cancer following chemoradiation therapy: long term results. Ann Surg. 2004;240:711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber WA, Ott K, Becker K, et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol. 2001;19:3058–3065. [DOI] [PubMed] [Google Scholar]
- 39.Abdel-Laetif MM, O’Riordan J, Windle HJ, et al. NF-κB activation in esophageal adenocarcinoma: relationship to Barrett’ metaplasia, survival and response to neoadjuvant chemoradiotherapy. Ann Surg. 2004;239:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]