Abstract
Background:
Pancreatic infections and sepsis are major complications in severe acute pancreatitis (AP) with significant impact on management and outcome. We investigated the value of Procalcitonin (PCT) for identifying patients at risk to develop pancreatic infections in severe AP.
Methods:
A total of 104 patients with predicted severe AP were enrolled in five European academic surgical centers within 96 hours of symptom onset. PCT was measured prospectively by a semi-automated immunoassay in each center, C-reactive protein (CRP) was routinely assessed. Both parameters were monitored over a maximum of 21 consecutive days and in weekly intervals thereafter.
Results:
In contrast to CRP, PCT concentrations were significantly elevated in patients with pancreatic infections and associated multiorgan dysfunction syndrome (MODS) who all required surgery (n = 10) and in nonsurvivors (n = 8) early after onset of symptoms. PCT levels revealed only a moderate increase in patients with pancreatic infections in the absence of MODS (n = 7), all of whom were managed nonoperatively without mortality. A PCT value of ≥3.5 ng/mL on 2 consecutive days was superior to CRP ≥430 mg/L for the assessment of infected necrosis with MODS or nonsurvival as determined by ROC analysis with a sensitivity and specificity of 93% and 88% for PCT and 40% and 100% for CRP, respectively (P < 0.01). The single or combined prediction of the two major complications was already possible on the third and fourth day after onset of symptoms with a sensitivity and specificity of 79% and 93% for PCT ≥3.8 ng/mL compared with 36% and 97% for CRP ≥430 mg/L, respectively (P = 0.002).
Conclusion:
Monitoring of PCT allows early and reliable assessment of clinically relevant pancreatic infections and overall prognosis in AP. This single test parameter significantly contributes to an improved stratification of patients at risk to develop major complications.
Early and reliable assessment of pancreatic infections is of major importance for timely goal-directed treatment in acute pancreatitis. The results of our prospective international multicenter study in patients with predicted severe disease has shown that a single laboratory test, Procalcitonin, significantly contributes to an early stratification of patients at risk to develop clinically relevant pancreatic infections or death.
Acute pancreatitis usually takes a mild, self-limiting course with complete restitutio ad integrum. However, about 20% to 30% of all patients experience a severe attack, which is almost uniformly associated with the morphologic correlate of intrapancreatic and extrapancreatic necrosis.1 Depending on the presence and extent of necrosis, pancreatic infections are observed in 30% to 70% of patients and are associated with a substantial increase of morbidity and mortality.2–4 Timely and accurate diagnosis of pancreatic infections is of major importance because it strongly influences further therapeutic decision-making. Whereas most patients with sterile necrosis can be successfully managed by conservative means,3–5 proven pancreatic infections with systemic signs of sepsis are an established indication for interventional or surgical therapy.6,7
Facing this clinical dilemma, there is major interest in a valid tool for the diagnosis of pancreatic infections and sepsis. Beyond several multifactorial scoring systems,8 a multitude of biochemical variables9 have been studied in acute pancreatitis and proven to be good predictors of disease severity. However, it is well documented that they are of little value in discriminating pancreatic infections and associated sepsis from systemic inflammatory response syndrome (SIRS) in the absence of infections.10,11 Currently, guided fine-needle aspiration (FNA) is still the procedure of choice to establish the diagnosis of pancreatic infections.11,12 Unfortunately, the demand for high standard technical equipment and personal experience as well as the potential risk of complications do not render guided FNA an easily available and cost-effective approach. An accurate and readily available biochemical parameter for identifying patients at risk to develop pancreatic infections would definitely contribute to an easier and safer diagnosis.
Procalcitonin (PCT) is the inactive 116 amino acid pro-peptide of the biologically active hormone calcitonin. In 1993, Assicot et al first described significantly increased concentrations of PCT in patients with bacterial and fungal infections and sepsis.13 Hence, it has been largely confirmed that PCT is the only one among a large array of biochemical parameters, which closely correlates with the inflammatory host response to microbial infections.14 In acute pancreatitis, PCT has been shown to predict the development of infected necrosis accurately.15–18 In other series, PCT was found to be an excellent predictor of severity19 and organ failure20,21 within the first 24 hours after hospital admission or onset of symptoms. However, a number of subsequent studies have shown opposite results,22–25 and the clinical usefulness of this parameter in acute pancreatitis still remains controversial. In the absence of representative studies, we addressed this issue by conducting the first prospective international multicenter trial in patients with severe acute pancreatitis.
MATERIALS AND METHODS
Patients
Patients were recruited from December 1999 to March 2004 at the Department of General Surgery, University of Ulm, Ulm, Germany, at the Department of General-, Visceral-,and Vascular Surgery, University of the Saarland, Homburg/Saar, at the Department of Surgery, Helsinki University Central Hospital, Helsinki, Finland, at the Department of Surgery and Gastroenterology, Pancreatic Unit, University of Verona, Italy, and the Department of Visceral- and Transplantation Surgery, University of Bern, Bern, Switzerland. General exclusion criteria were 1) a time interval between onset of abdominal symptoms and study inclusion >96 hours, 2) absence of SIRS,26 3) age of less than 18 years, 4) hepatitis B, C, or HIV infection, and 5) psychoses except delirium tremens. In addition, previous pancreatic interventions or surgery due to the current attack of acute pancreatitis was also an exclusion criterion.
General inclusion criteria for acute pancreatitis were defined as 1) a time interval between onset of typical abdominal symptoms and study inclusion of 96 hours and less, 2) the presence of SIRS, and 3) informed consent according to local rules. Specific inclusion criteria for severe acute pancreatitis were 1) at least 3-fold elevated serum amylase or lipase levels, 2) the presence of intrapancreatic/extrapancreatic necrosis documented by contrast-enhanced CT or a C-reactive protein (CRP) of ≥250 mg/L27 or alternatively at least one failing organ system (pulmonary failure: arterial pO2 <60 mm Hg at room air or mechanical ventilation, renal failure: creatinine >180 μmol/L or hemofiltration/dialysis, shock: systolic blood pressure <80 mm Hg over >15 minutes or pressure support) according to the Atlanta classification system.28
Infection of pancreatic necrosis was diagnosed by guided FNA and/or by intraoperative findings. FNA was performed whenever infection of intrapancreatic/extrapancreatic necrosis was suspected by persisting or new onset clinical and/or laboratory signs of sepsis after other sources of infections had been ruled out. Beyond the intraabdominal bacteriology, further microbiologic cultures from central venous/arterial lines, blood, bronchoalveolar fluid, or urine were taken and documented whenever suspicion of new onset or persistent infection was raised. Multiorgan dysfunction syndrome (MODS) was defined as the presence of 2 or more failing organ systems requiring specific ICU treatment, such as mechanical ventilation, hemofiltration/dialysis, or pressure support. Septic MODS was defined as MODS in the presence of an infectious focus documented by positive bacteriology.
In all study centers, initial treatment of acute pancreatitis was conservative, including intensive care support and administration of adequate prophylactic antibiotics according to local treatment protocols. If biliary pancreatitis was suspected, early endoscopic retrograde cholangiography with papillotomy was performed. Indications for surgery were either documented infection of pancreatic necrosis with systemic signs of sepsis or persistent organ failure/abdominal symptoms despite maximum intensive care support in the absence of positive FNA results.
Study Design
PCT (upper reference range 0.5 ng/mL in healthy subjects) was prospectively analyzed in a real time fashion in each study center by a semi-automated chemoluminescent immunoassay (LUMITEST-PCT, BRAHMS Diagnostica AG, Hennigsdorf, Germany). CRP (upper reference range 5 mg/L in healthy subjects) was determined as a routine parameter on automated analyzers in each center. Both parameters were measured over a maximum of 21 consecutive days and thereafter in weekly intervals until hospital discharge or death. APACHE II (acute and chronic health evaluation)29 and SOFA (sequential organ failure assessment)30 scores were calculated in 24-hour intervals after study inclusion during the total observation period. All clinically relevant data such as results of diagnostic imaging procedures, surgical procedures, type and duration of specific ICU and antibiotic treatment, vital parameters, and routine laboratory variables were documented in standardized case report forms (CRFs). In 6-month intervals, each patient's completed CRF was reviewed at an investigator's monitoring visit to ascertain eligibility for the study and to check for appropriate documentation. Each center obtained approval from the local research ethics committees.
Statistics
The primary endpoint analyzed was bacteriologically proven pancreatic infection, secondary endpoints were pancreatic infections with and without organ failure/MODS, type of organ failure, MODS in general, and nonsurvival. Descriptive data are presented as absolute numbers (percentages) or as medians with interquartile ranges or 95% confidence intervals. For comparison of independent samples, we used exact Wilcoxon rank sum tests and for comparison of proportions Fisher's exact tests. P values <0.05 at an α < 0.05 were considered significant.
To study the differences in PCT as well as in CRP courses of patients with or without MODS, infected necrosis or both, Gaussian repeated measurement mixed models were fitted to the observed log PCT or log CRP values. The model class was chosen to account for autocorrelation between repeated measurements and to correct for differences in observation time. Logarithmic transformations were used to better meet the normal assumptions of the Gaussian model. To test whether patients with infected necrosis, MODS, or the combination of both had elevated PCT or CRP levels as compared with patients without these complications revealed different curve shapes, each factor was included twice in the model, as main factor as well as interaction term with time. Further, to test whether the PCT or CRP levels of patients with infected necrosis and MODS were just the sum of the 2 effects or not, an interaction term between infected necrosis and MODS was added to the model. The resulting group-specific PCT or CRP curves are presented graphically on a log scale as modeled.
Similarly, in a second step, center was added to the model. If significant center differences effects were present, it was tested whether these differences could be explained by age or sex differences, by the etiology or by differences in time from symptom onset to inclusion. Finally, the presence of necrosis, pulmonary or renal insufficiency, cardiovascular shock, and nonsurvival were tentatively added to the model to clarify whether PCT levels reflect these parameters. They were kept in the final model if their regression coefficients were significant.
Receiver operating characteristic (ROC) curves and the respective areas under the curve were calculated for the maximum value of each parameter reached on at least 2 days during the whole observation period to determine overall cutoff levels. To assess the early predictive value, the highest PCT and CRP concentrations on day 3 and day 4 after onset of symptoms were subjected to ROC analysis. The best cutoff was chosen as the value, which maximized the Phi-statistic that is based on Pearson's χ2 test. The predictive power of indicators was additionally demonstrated by calculating sensitivity, specificity, PPV (positive predictive value), and NPV (negative predictive value) of the sample in the usual way, using the optimal cutoff.
RESULTS
Disease Severity, Treatment, and Outcome
A total of 113 patients with severe acute pancreatitis were enrolled in the study, of whom 9 were excluded because they did not meet the inclusion criteria; 104 patients with predicted severe acute pancreatitis were eligible and underwent further analysis. The patient numbers recruited by each center were as follows: Bern, Switzerland, n =12; Helsinki, Finland, n= 55; Homburg, Germany, n = 4; Ulm, Germany, n = 20; Verona, Italy, n = 13. There were 73 male (70%) and 31 female (30%) patients, the median time between symptom onset and study inclusion was 48 hours (range, 1–96 hours), the median age of the study population was 50 years (range, 19–91 years). The median time interval between symptom onset and study inclusion revealed no difference between the centers. The median patients' age considerably differed between the centers (P = 0.00001).
The etiology of pancreatitis was alcoholic in 60 patients (58%), biliary in 28 patients (27%), and related to other factors in 16 patients (15%). The incidence of local and infectious complications, as well as specific treatment is summarized in Table 1. Among the 17 patients with documented pancreatic infections, 12 had primary infected necrosis and 5 patients developed secondary pancreatic infections after surgery for sterile necrosis. Pancreatic infections were diagnosed 21 days (median, range 2–36 days) after onset of symptoms. Ten patients with infected necrosis were treated operatively by open necrosectomy, all of them presented with early and persistent MODS, 3 of them died (30%). Seven patients with FNA-proven infected necrosis were managed nonoperatively (six completely conservatively, one by interventional, CT-guided percutaneuos drainage), none of them presented with persistent organ failure or MODS at any time during the hospital stay and all of them survived. Five deaths occurred in patients with sterile necrosis (9.1%) who all suffered from persistent MODS. One death occurred within 72 hours and another 2 within the first week after onset of symptoms, the remaining 5 deaths were observed beyond the the first week, 2 of them after surgical treatment. Median age and etiology did not differ between survivors and nonsurvivors. The overall disease severity in terms of APACHE II and SOFA scores within the first 24 hours after study inclusion and the incidence and onset of organ failure related to the day of acute pancreatitis is summarized in Table 2. The overall mortality rate was 7.7% (Table 2).
TABLE 1. Overall Disease Severity, Local/Infectious Complications, and Treatment in Patients With Severe Acute Pancreatitis

TABLE 2. Overall Disease Severity, Incidence, and Onset of Organ Failure/Mortality in Patients With Severe Acute Pancreatitis

Overall Course of PCT and CRP
Patients who developed infected necrosis revealed an early and sustained PCT increase with higher concentrations than in sterile necrosis or edematous pancreatitis. PCT elevation was most expressed in patients with infected necrosis and associated MODS with higher levels compared to patients with infected necrosis in whom persistent organ failure and MODS were absent or in patients with a sterile course (Fig. 1A). In all nonsurviving patients with severe acute pancreatitis, a similar course of PCT was observed. PCT concentrations remained significantly elevated throughout the course of the disease in nonsurvivors, whereas levels quickly returned to normal ranges in surviving patients (Fig. 2A). In contrast, CRP values did not show differences between patients with infected necrosis and MODS, infected necrosis without organ failure, and sterile necrosis/edematous pancreatitis (Fig. 1B), surviving and nonsurviving patients (Fig. 2B), within the first week after onset of symptoms.

FIGURE 1. Course of PCT (A) and CRP (B) (medians, upper and lower quartiles) in severe acute pancreatitis in the patient groups with infected necrosis and MODS, infected necrosis without organ failure, and sterile necrosis/edematous pancreatitis irrespective of organ failure. Values are related to the onset of symptoms. Significant differences between patients with infected necrosis and MODS versus the other 2 groups were observed from day 3 to 21 (P < 0.005–0.0001) for PCT and from day 5 to 21 (P < 0.006–0.0001) for CRP.

FIGURE 2. Course of PCT (A) and CRP (B) (medians, upper and lower quartiles) in nonsurvivors and survivors with severe acute pancreatitis. Values are related to the onset of symptoms. Significant differences between nonsurvivors and survivors were observed from day 2 to 21 (P < 0.03–0.001) for PCT and from day 8 to 18 (P < 0.05–0.004) for CRP.
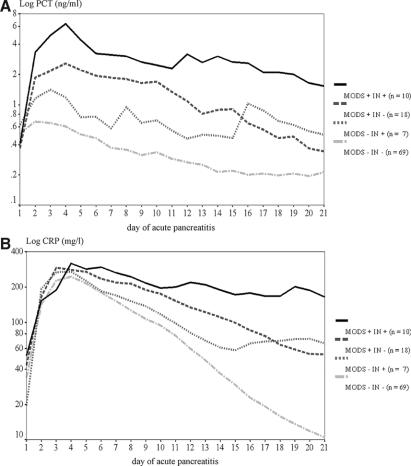
Gaussian repeated measurement mixed-model analysis including all values up to day 21 after disease onset revealed that PCT levels were higher in patients with infected necrosis (by factor 2.2; 95% CI, 1.1–4.4, P = 0.001) and in patients with MODS (by factor 2.4; 95% CI, 1.4–4.0, P < 0.001). If patients developed MODS and infected necrosis, the effects were additive (interaction test, not significant). However, the curve shape was significantly different, if MODS is present (P < 0.001), because PCT elevations persisted longer (Fig. 3A). PCT levels differed significantly between centers (P < 0.001). The differences could not be explained by age, sex, etiology, or differences in inclusion times. Further elevations of PCT levels were observed in patients with renal insufficiency or dialysis/hemofiltration (P < 0.001), with shock or pressure support (P = 0.017) and in patients who subsequently died (by factor 3.1; 95% CI, 1.5–6.4, P = 0.003, after adjustment for all other significant regressors).
FIGURE 3. Gaussian repeated measurement mixed models of PCT (A) and CRP (B) in severe acute pancreatitis in patients with infected necrosis and associated MODS (MODS+ IN+), infected necrosis without MODS (MODS− IN+), MODS without pancreatic infections (MODS+ IN−), and patients without pancreatic infections or MODS (MODS− IN−). Values are related to the onset of symptoms.
Gaussian repeated measurement mixed-model analysis comprising all values up to day 21 after disease onset showed that CRP levels were higher in patients with infected necrosis (by factor 5.4; 95% CI, 3.1–9.3, P = 0.009) and in patients with MODS (by factor 2.0; 95% CI, 1.5–2.8, P < 0.001). If patients developed MODS and infected necrosis, the effects were simply additive (interaction test, not significant). However, other as with PCT, the curve shape was significantly different if infection was present (P < 0.001) because CRP elevations persisted longer (Fig. 3B). CRP levels did not differ between centers. Further elevations of CRP levels were observed in patients with pulmonary insufficiency or mechanical ventilation (P = 0.005) and in patients with shock or pressure support (P = 0.013), but not in patients who subsequently died.
Overall Predictive Value of PCT and CRP
In patients with severe acute pancreatitis who developed infected necrosis, infected necrosis with MODS, or subsequently died, significantly higher maximum PCT concentrations were found as compared to patients in whom these complications were absent. No comparable differences were observed for CRP (Table 3). The overall pancreatitis-specific cutoff levels assessed by ROC analysis were based on the maximum PCT and CRP concentration, which was reached on at least 2 consecutive days within the total observation period. PCT was found to have the closest correlation with the development of infected necrosis associated with MODS (AUC = 0.86; 95% CI, 0.80–0.90) and nonsurvival (AUC = 0.91; 95% CI, 0.87–0.95) or the combination of both (AUC = 0.89; 95% CI, 0.84–0.93). The presence of infected necrosis alone revealed a lower correlation (AUC = 0.78; 95% CI, 0.72–0.84). In contrast, CRP revealed a lower or no correlation with infected necrosis and associated MODS (AUC = 0.79; 95% CI, 0.73–0.82; P = not significant, CRP vs. PCT), nonsurvival (AUC = 0.58; 95% CI, 0.51–0.64; P < 0.002 CRP vs. PCT) or the combination of both (AUC = 0.67; 95% CI, 0.60–0.74, P < 0.01 CRP vs. PCT), or infected necrosis alone (AUC = 0.68; 95% CI, 0.61–0.74; P = not significant, CRP vs. PCT). Table 4 shows the optimum cutoff levels with the respective sensitivity, specificity, and positive and negative predictive values at the calculated cutoff levels for the assessment of each complication.
TABLE 3. Maximum PCT and CRP Concentrations in Patients With Infected Necrosis, Infected Necrosis, and MODS, and Nonsurvivors (median, 95% CI)
TABLE 4. Sensitivity, Specificity, PPV, NPV, and Optimum Cutoff Levels for the Overall Assessment of Major Complications in Severe Acute Pancreatitis

Early Predictive Value of PCT and CRP
To assess the clinical usefulness of both parameters for the early assessment of septic complications and overall outcome, PCT and CRP values of the third and fourth day of the disease were analyzed. As shown by Figures 1 to 3, PCT concentrations revealed striking differences early in the course of the disease depending on the presence or absence of infected necrosis associated with MODS and nonsurvival, whereas CRP values did not differ. In patients with severe acute pancreatitis, the optimum cutoff levels and the results for the early assessment of septic complications and nonsurvival were similar to those obtained for the overall analysis. For ROC analysis, the peak PCT and CRP concentrations of day 3 and day 4 of severe acute pancreatitis were used and revealed corresponding results with the same cutoff levels for day 3 and for day 4 as well as for the peak concentrations of day 3 or 4, which are shown in Table 5. Comparing the calculated AUCs of PCT and CRP for predicting infected necrosis (PCT: AUC = 0.76; 95% CI, 0.67–0.84; CRP: AUC = 0.60; 95% CI, 0.50–0.69; P < 0.08), infected necrosis associated with MODS (PCT: AUC = 0.83; 95% CI, 0.75–0.90; CRP: AUC = 0.64; 95% CI, 0.54–0.74; P = 0.06), nonsurvival (PCT: AUC = 0.91; 95% CI, 0.84–0.96; CRP: AUC = 0.60; 95% CI, 0.50–0.69; P = 0.006), and the combination of infected necrosis with MODS and nonsurvival (PCT: AUC = 0.87; 95% CI, 0.78–0.92; CRP: AUC = 0.60; 95% CI, 0.50–0.69; P = 0.002) identified PCT superior to CRP.
TABLE 5. Sensitivity, Specificity, PPV, NPV, and Optimum Cutoff Levels for the Early Assessment (day 3 and 4) of Major Complications in Severe Acute Pancreatitis

DISCUSSION
The results of our prospective multicenter trial could show that PCT does not allow the prediction of pancreatic infections in general. However, PCT was found to be a reliable means to predict clinically relevant infected necrosis, which was always associated with MODS and ultimately required operative intervention. In addition, PCT proved to be an excellent variable to assess overall prognosis throughout the course of severe acute pancreatitis. Unlike other proposed laboratory parameters for severity stratification such as trypsinogen activation peptide,31 the diagnostic accuracy of PCT was not limited to a specific time interval after onset of symptoms. PCT was able to identify patients at risk to develop the 2 major complications, infected necrosis and death, before they ultimately occurred with high sensitivity and specificity. In both instances, PCT was superior to the widely used biochemical “gold standard” CRP. We could also confirm that PCT is no parameter for depicting “severe” cases as defined by the Atlanta system. The current study thus contributes to shed further light on the still existing controversies about the usefulness of PCT determinations in acute pancreatitis. In this context, confusion arose from hardly comparable monocentric studies, which comprised limited patient numbers and suffered from nonuniform definitions of endpoints or complications.15–25
At present, increasing demands for optimum medical and ICU care have to be covered despite limited healthcare resources. Abdominal infections require a multitude of specific diagnostic and therapeutic measures and considerably add to overall healthcare expenses.32 The high specificity and negative predictive values for clinically relevant infected necrosis and nonsurvival could therefore serve as a helpful means to select those patients in whom further cost-intensive diagnostic and therapeutic procedures such as repeated CT scans, guided FNA, prolonged antibiotic treatment, and ICU therapy are not necessary. In the daily clinical practice, PCT determinations have previously been shown as helpful guide for goal-directed antibiotic therapy in lower respiratory tract infections33 and in elective colonic surgery.34 In scientific respect, PCT could contribute to improved severity stratification and a better selection of patients for diagnostic or therapeutic trials, which is still a compelling problem in acute pancreatitis.35,36 Since a fully automated test system for PCT analysis has recently been introduced, single determinations with a laboratory turnaround time of about 30 minutes are possible.37 In the present study, PCT determinations were still performed by a semi-automated technique requiring manual pipetting of the samples. This may in part explain the center-specific differences of this parameter in contrast to CRP, which, however, had no influence on the overall results of the study.
It is important to emphasize that PCT is no substitute for careful history and clinical examination of the individual patient. The cutoff levels of PCT for predicting septic complications or overall prognosis are disease dependent and vary considerably among different inflammatory conditions.14 Moreover, PCT is a nonspecific marker of bacterial/fungal infection and sepsis and does not provide any information about the underlying source of infection. In severe abdominal inflammation, sources other than the abdomen such as pulmonary, urinary tract, or catheter infections are frequently observed in critically ill patients and need to be carefully taken into account when interpreting PCT measurements. However, an interesting observation in the current series was the fact that, in general, only abdominal infections lead to the most expressed systemic PCT release. In the absence of an abdominal septic focus, other sites of infections had by far a lesser influence on PCT levels.
Despite the fact that PCT allowed identification of major complications already on the third and fourth day after symptom onset, this may not be early enough to depict patients at risk at hospital admission, especially those with early severe disease. Because patient recruitment was restricted to cases with predicted severe disease, true admission PCT values were available in few patients only, which precludes any meaningful analysis; there is no doubt that further work is needed in searching for the optimum prognostic tool in this specific context. On the other hand, a delay of 24 to 48 hours from onset of symptoms to hospital admission or referral is common in the majority of patients with acute pancreatitis20,21,35,36; therefore, PCT still enables risk stratification on the first or second day of admission.20,21 The high incidence of early mortality in acute pancreatitis remains a continuing challenge in this respect.38 However, unlike previous reports, early mortality within 72 hours after disease onset occurred in only 1 of 8 deaths in our study, and this patient was correctly identified by dramatically elevated PCT concentrations upon admission.
Although the cellular source and pathophysiologic role of PCT are still incompletely understood, increasing clinical evidence suggests that the term “sepsis parameter” does not embrace the real properties of this parameter.39 In accordance with previous findings,15 excessively high PCT concentrations were already present early after the onset of symptoms, which was days or even weeks before the infectious abdominal focus was ultimately diagnosed. Moreover, a similar course of PCT was observed in patients with severe acute pancreatitis who died early and had no evidence of infection but uniformly suffered from MODS. The Gaussian repeated measurement mixed-model analysis also revealed a MODS-dependent rise of PCT concentrations even in the absence of any infections in acute pancreatitis.20 Our clinical observations are well in accordance with recent experimental studies suggesting a role for PCT in the pathophysiology of severe sepsis.40,41 However, beyond the proposed role in sepsis, it could be hypothesized that the degree of the systemic PCT release reflects an impaired immunologic response, rendering the host susceptible to severe infections or unable to overcome the initial (infectious or noninfectious) local insult, thus ultimately resulting in death.
Our study population of patients with acute pancreatitis raises further questions as far as the currently used “gold standards” for defining “severe acute pancreatitis” and “infected necrosis/pancreatic infections,” including its therapeutic consequences, are concerned. All patients enrolled had predicted severe disease according to the widely used Atlanta classification system. The incidence of organ failure was 65%, and all patients had either CT-proven necrosis or a CRP value of at least 250 mg/L at study inclusion. Surprisingly, mortality was only 7.7% and only 16% of the patients developed documented infection. This is in contrast to the expected incidence of pancreatic infections of at least 30% to 40% upon planning the study.1–4 On the other hand, these observations are in line with previous multicenter trials using comparable definitions of severity.35,36 In the present study, 35 patients with predicted severe acute pancreatitis experienced a completely uneventful course without any organ failure or systemic complications, although 18 of them had intrapancreatic necrosis. In other terms, one third of the patients in our study did not suffer from clinically relevant severe disease and probably received unnecessary over-treatment. The current recommendation of surgical or interventional treatment in the presence of documented infection of necrosis is another issue, which needs future discussion with redefinition and reevaluation. Seven of 17 patients with documented infection of necrosis and associated SIRS experienced an uneventful course, none of them developed relevant organ failure or required surgery, and in 6 patients treatment was completely conservative. This is clearly in opposition to the current treatment algorithm of early surgical debridement for infected necrosis and has been observed by a previous series as well.42 In this specific setting, PCT determinations could help to select those patients in whom conservative treatment can be continued and surgery may be further delayed. In an overall sense, these data underscore the need for a revision of the current definitions for disease severity and infected necrosis in acute pancreatitis. Any future revision to the current severity classification system will require to withdraw the longstanding emphasis on local pathology such as necrosis and infection and to stress the systemic aspects of acute pancreatitis.
ACKNOWLEDGMENTS
The authors thank all study participants who contributed to patient recruitment, collecting clinical data, and analyzing PCT samples in the laboratories:
University of Ulm: Dr. Gerald Steinbach MD, Department of Clinical Chemistry/Pathobiochemistry; Ms. Astrid Bothe, medical student, Department of General Surgery
University of the Saarland, Homburg/Saar: Ms. Berit Kopp,study nurse, Dr. Colin M. Krüger, MD, Martin K. Schilling, MD, Department of General, Visceral, and Vascular Surgery; Mr. and Mrs. Pape, Department of Clinical Chemistry
University of Verona: Dr. Isabella Frigerio, MD, Department of Surgery and Gastroenterology, Pancreatic Unit
University of Helsinki: Ms. Maarit Leinimaa, study nurse, Research Laboratory, Department of Surgery
University of Bern: Prof. Dr. Waldemar Uhl, MD, Dr. Oliver Strobel, MD, and Dr. Christoph Müller, MD, Department of Visceral- and Transplantation Surgery
The authors also thank Dr. Wilfried Beier, PhD (from BRAHMS) for organizing the investigators' monitoring visits.
Footnotes
Dr. Rau has served as consultant and received payments from BRAHMS to attend meetings related to this trial, for travel expenses, and speaking engagements.
Reprints: Bettina M. Rau, MD, Department of General, Visceral, and Vascular Surgery, University of the Saarland, Kirrberger Strasse 66421 Homburg/Saar, Germany. E-mail: bettina.rau@uniklinikum-saarland.de.
REFERENCES
- 1.Beger HG, Rau B, Isenmann R. Natural history of necrotizing pancreatitis. Pancreatology. 2003;3:93–101. [DOI] [PubMed] [Google Scholar]
- 2.Beger HG, Bittner R, Block S, et al. Bacterial contamination of pancreatic necrosis. Gastroenterology. 1986;49:433–438. [DOI] [PubMed] [Google Scholar]
- 3.Bradley EL III, Allen K. A prospective longitudinal study of observation versus surgical intervention in the management of necrotizing pancreatitis. Am J Surg. 1991;161:19–25. [DOI] [PubMed] [Google Scholar]
- 4.Büchler MW, Gloor B, Müller CA, et al. Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann Surg. 2000;232:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rau B, Pralle U, Uhl W, et al. Management of sterile necrosis in instances of severe acute pancreatitis. J Am Coll Surg. 1995;181:279–288. [PubMed] [Google Scholar]
- 6.Dervenis C, Johnson CD, Bassi C, et al. Diagnosis, objective assessment of severity, and management of acute pancreatitis: Santorini Consensus Conference. Int J Pancreatol. 1999;25:195–210. [DOI] [PubMed] [Google Scholar]
- 7.King NK, Siriwardena AK. European survey of surgical strategies for the management of severe acute pancreatitis. Am J Gastroenterol. 2004;99:719–728. [DOI] [PubMed] [Google Scholar]
- 8.Sandberg AA, Borgstrom A. Early prediction of severity in acute pancreatitis: is this possible? JOP. 2002;3:116–125. [PubMed] [Google Scholar]
- 9.Rau B, Schilling MK, Beger HG. Laboratory markers of severe acute pancreatitis. Dig Dis Sci. 2004;22:247–257. [DOI] [PubMed] [Google Scholar]
- 10.Marshall JC, Vincent JL, Fink MP, et al. Measures, markers, and mediators: toward a staging system for clinical sepsis. A report of the fifth Toronto Sepsis Roundtable, Toronto, Ontario, Canada, October 25–26, 2000. Crit Care Med. 2003;31:1560–1567. [DOI] [PubMed] [Google Scholar]
- 11.Nathens AB, Curtis JR, Beale RJ, et al. Management of the critically ill patients with severe acute pancreatitis. Crit Care Med. 2004;32:2524–2536. [DOI] [PubMed] [Google Scholar]
- 12.Gerzof SG, Banks PA, Robbins AH, et al. Early diagnosis of pancreatic infection by computed tomography-guided aspiration. Gastroenterology. 1987;93:1315–1320. [DOI] [PubMed] [Google Scholar]
- 13.Assicot M, Gendrel D, Carsin H, et al. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon L, Gauvin F, Amre DK, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39:206–217. [DOI] [PubMed] [Google Scholar]
- 15.Rau B, Steinbach G, Gansauge F, et al. The potential role of procalcitonin and interleukin-8 in the prediction of infected necrosis in acute pancreatitis. Gut. 1997;41:832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandi Y, Farkas G, Takacs T, et al. Diagnostic relevance of procalcitonin, IL-6, and sICAM-1 in the prediction of infected necrosis in acute pancreatitis. Int J Pancreatol. 2000;28:41–49. [DOI] [PubMed] [Google Scholar]
- 17.Riche FC, Cholley BP, Laisne MJ, et al. Inflammatory cytokines, C reactive protein, and procalcitonin as early predictors of necrosis infection in acute necrotizing pancreatitis. Surgery. 2003;133:257–262. [DOI] [PubMed] [Google Scholar]
- 18.Olah A, Belagyi T, Issekutz A, et al. Value of procalcitonin quick test in the differentiation between sterile and infected forms of acute pancreatitis. Hepatogastroenterology. 2005;52:243–245. [PubMed] [Google Scholar]
- 19.Ammori BJ, Becker KL, Kite P, et al. Calcitonin precursors: early markers of gut barrier dysfunction in patients with acute pancreatitis. Pancreas. 2003;27:239–243. [DOI] [PubMed] [Google Scholar]
- 20.Kylänpää-Bäck ML, Takala A, Kemppainen E, et al. Procalcitonin strip test in the early detection of severe acute pancreatitis. Br J Surg. 2001;88:222–227. [DOI] [PubMed] [Google Scholar]
- 21.Kylänpää-Bäck ML, Takala A, Kemppainen EA, et al. Procalcitonin, soluble interleukin-2 receptor, and soluble E-selectin in predicting the severity of acute pancreatitis. Crit Care Med. 2001;29:63–69. [DOI] [PubMed] [Google Scholar]
- 22.Müller CA, Uhl W, Printzen G, et al. Role of procalcitonin and granulocyte colony stimulating factor in the early prediction of infected necrosis in severe acute pancreatitis. Gut. 2000;46:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pezzilli R, D'Eril GVM, Morselli-Labate AM, et al. Serum amyloid A, procalcitonin, and C-reactive protein in early assessment of severity of acute pancreatitis. Dig Dis Sci. 2000;45:1072–1078. [DOI] [PubMed] [Google Scholar]
- 24.Frasquet J, Saez J, Trigo C, et al. Early measurement of procalcitonin does not predict severity in patients with acute pancreatitis. Br J Surg. 2003;90:1129–1130. [DOI] [PubMed] [Google Scholar]
- 25.Modrau IS, Floyd AK, Thorlacius-Ussing O. The clinical value of procalcitonin in early assessment of acute pancreatitis. Am J Gastroenterol. 2005;100:1593–1597. [DOI] [PubMed] [Google Scholar]
- 26.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International sepsis definitions conference. Intensive Care Med. 2003;29:530–538. [DOI] [PubMed] [Google Scholar]
- 27.Rau B, Steinbach G, Baumgart K, et al. Serum amyloid A versus C-reactive protein in acute pancreatitis: clinical value of an alternative acute-phase reactant. Crit Care Med. 2000;28:736–742. [DOI] [PubMed] [Google Scholar]
- 28.Bradley EL 3rd. A clinically based classification system for acute pancreatitis: summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11–13, 1992. Arch Surg. 1993;128:586–590. [DOI] [PubMed]
- 29.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 30.Moreno R, Vincent JL, Matos R, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care: results of a prospective, multicentre study. Working Group on Sepsis Related Problems of the ESICM. Intensive Care Med. 1999;25:686–696. [DOI] [PubMed] [Google Scholar]
- 31.Neoptolemos JP, Kemppainen EA, Mayer JM, et al. Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: a multicentre study. Lancet. 2000;355:1955–1960. [DOI] [PubMed] [Google Scholar]
- 32.Burchardi H, Schneider H. Economic aspects of severe sepsis: a review of intensive care unit costs, cost of illness and cost effectiveness of therapy. Pharmacoeconomics. 2004;22:793–813. [DOI] [PubMed] [Google Scholar]
- 33.Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363:600–607. [DOI] [PubMed] [Google Scholar]
- 34.Chromik AM, Endter F, Uhl W, et al. Preemptive antibiotic treatment vs ‘standard' treatment in patients with elevated serum procalcitonin levels after elective colorectal surgery: a prospective randomised pilot study. Langenbeck's Arch Surg. 2006;299:189–194. [DOI] [PubMed] [Google Scholar]
- 35.Johnson CD, Kingsnorth AN, Imrie CW, et al. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut. 2001;48:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isenmann R, Runzi M, Kron M, et al. German antibiotics in Severe Acute Pancreatitis Study Group. Prophylactic antibiotic treatment in patients with predicted severe acute pancreatitis: a placebo-controlled, double-blind trial. Gastroenterology. 2004;126:997–1004. [DOI] [PubMed] [Google Scholar]
- 37.Steinbach G, Rau B, Debard AL, et al. Multicenter evaluation of a new immunoassay for PCT measurement on the Kryptor System. Clin Chem Lab Med. 2004;42:440–449. [DOI] [PubMed] [Google Scholar]
- 38.McKay CJ, Imrie CW. The continuing challenge of early mortality in acute pancreatitis. Br J Surg. 2004;91:1243–1244. [DOI] [PubMed] [Google Scholar]
- 39.Rau B, Krüger CM, Schilling MK. Procalcitonin: improved biochemical severity stratification and postoperative monitoring in severe abdominal inflammation and sepsis. Langenbeck's Arch Surg. 2004;389:134–144. [DOI] [PubMed] [Google Scholar]
- 40.Nylen ES, Whang KT, Snider RH Jr, et al. Mortality is increased by procalcitonin and decreased by an antiserum reactive to procalcitonin in experimental sepsis. Crit Care Med. 1998;26:1001–1006. [DOI] [PubMed] [Google Scholar]
- 41.Martinez JM, Wagner KE, Snider RH, et al. Improved physiologic and metabolic parameters and increased survival with late procalcitonin immunoneutralization in septic pigs. Surg Infect. 2001;2:193–204. [DOI] [PubMed] [Google Scholar]
- 42.Rünzi M, Niebel W, Goebell H, et al. Non-surgical treatment of infected necrosis in severe acute pancreatitis. Pancreas. 2005;30:195–199. [DOI] [PubMed] [Google Scholar]