Abstract
Context:
Although hospital procedure volume is clearly related to operative mortality with many cancer procedures, its effect on late survival is not well characterized.
Objective:
To examine relationships between hospital volume and late survival after different types of cancer resections.
Design:
Using the national Surveillance Epidemiology and End Results (SEER)-Medicare linked database (1992–2002), we identified all patients undergoing major resections for lung, esophageal, gastric, pancreatic, colon, and bladder cancer (n = 64,047). Relationships between hospital volume and survival were assessed using Cox proportional hazards models, adjusting for patient characteristics and use of adjuvant radiation and chemotherapy. Study Participants: U.S. Medicare patients residing in SEER regions. Main Outcome Measures: 5-year survival.
Results:
Although there were statistically significant relationships between hospital volume and 5-year survival with all 6 cancer types, the relative importance of volume varied markedly. Absolute differences in 5-year survival probabilities rates between low-volume hospitals (LVHs) and high-volume hospitals (HVHs) ranged from 17% for esophageal cancer resection (17% vs. 34%, respectively) to only 3% for colon cancer resection (45% vs. 48%). Absolute differences in 5-year survival between LVHs and HVHs fell between these ranges for lung (6%), gastric (6%), pancreatic (5%), and bladder cancer (4%). Volume-related differences in late survival could not be attributed to differences in rates of adjuvant therapy.
Conclusions:
Along with lower operative mortality, HVHs have better late survival rates with selected cancer resections than their lower-volume counterparts. Mechanisms underlying their better outcomes and thus opportunities for improvement remain to be identified.
Using the SEER-Medicare database, we identified all patients undergoing major resections for lung, esophageal, gastric, pancreatic, colon, and bladder cancer between 1992 and 1999. Although the magnitude of the volume-outcome effect varied markedly, there were statistically significant relationships between hospital volume and 5-year survival with all 6 cancer types. Volume-related differences in late survival could not be attributed to differences in rates of adjuvant therapy.
For many cancer procedures, patients undergoing surgery at high-volume hospitals have lower rates of perioperative morbidity and mortality than those at lower-volume centers. Several large population-based studies and structured literature reviews have confirmed relationships between hospital volume and operative mortality with many types of cancer resection.1–5 Hospital volume also seems to be related to risks of nonfatal complications, including perioperative pneumonia, septicemia, and renal failure.6
However, it is less clear to what extent procedure volume is associated with patient outcomes after the perioperative period. Numerous studies, most examining a single procedure type, have described relationships between hospital volume and late survival after cancer surgery, with conflicting results.7 One explanation for inconsistent findings across studies may simply be that hospital volume is more important for late survival with some procedures than with others, as it is for operative mortality. Among other potential reasons, studies to date have varied widely in their study populations, their sample sizes and statistical power, and their ability to account for tumor stage, use of adjuvant therapy, and other potentially confounding variables.
To provide a more comprehensive view of volume-survival relationships after cancer surgery, we used data from the national Surveillance Epidemiology and End Results (SEER)-Medicare linked database to examine late survival among patients undergoing several different types of cancer resection. In addition to describing the relative importance of hospital volume with different procedures, we sought to explore to what extent observed volume-outcome relationships might be attributable to differences in the use of adjuvant chemotherapy and radiation therapy.
METHODS
Subjects and Databases
For this study, we used the 1992 to 2002 national SEER-Medicare linked database. As detailed elsewhere,8 these files provide a rich source of information on Medicare patients included in SEER, a nationally representative collection of population-based registries of all incident cancers from diverse geographic areas in the United States. During this study period, there were 11 SEER areas, representing approximately 14% of the U.S. population. For each Medicare patient in SEER, the SEER-Medicare linked files contain 100% of Medicare claims from the inpatient, outpatient, physician, home health, and hospice files.
From these files, we identified all patients aged 65 to 99 undergoing major resection for one of 6 different cancers (lung, esophagus, stomach, pancreas, colon, and bladder) between 1992 and 1999. All Medicare patients with incident cases of these cancers were identified by the appropriate cancer codes from the SEER files. Those patients undergoing major surgical resections were identified from the Medicare Inpatient file using the appropriate procedure codes from the International Classification of Diseases, version 9. Inpatient, outpatient, and physician claims files were used to identify patients receiving neoadjuvant or adjuvant radiation and chemotherapy,9,10 defined as therapy occurring within 6 months before or after surgery.
Categorization of Hospital Volume
Relying on SEER-Medicare data alone to assess procedure volumes may misclassify the volume status of some hospitals.11 SEER patients often travel to hospitals outside SEER region boundaries for surgical care. Hospitals within SEER boundaries treat a variable mix of SEER and non-SEER patients. For these reasons, we estimated total hospital volumes for each of the 6 procedures using methods previously described.1 In brief, we used the 100% Medicare Inpatient file to determine the average number of procedures per year performed on Medicare patients by each hospital. Using the 1997 Nationwide Inpatient Sample, we then estimated total (all-payer) volumes by determining the proportion of patients undergoing each procedure covered by Medicare (approximately 50% overall) and dividing each provider's observed Medicare volume by these procedure-specific proportions. After ranking hospitals in order of increasing total volume, volume thresholds were selected that most closely sorted patients into 3 evenly sized groups (low, medium, and high hospital volume terciles).
Analysis
Vital status was determined at 5 years from the date of resection or through December 31, 2002, the end of our follow-up period. We used Cox proportional hazards models to examine relationships between hospital volume and mortality, adjusting for patient characteristics, censoring at the end of the follow-up period. We used the patient as the unit of analysis, with the exposure (volume) measured at the hospital level. We adjusted for age group (65–69, 70–74, 75–79, 80–84, 85+ years), sex, race (black, nonblack), and their interactions, year of procedure, and acuity of the index admission (elective, urgent, emergent), and patient comorbidities. Comorbidities were identified using information from the index admission and inpatient encounters from the preceding 6 months, based on methods described by Elixhauser et al.12 Our models also included tumor grade and stage (from the SEER clinical registries) and median household income and college education, assessed at the zip code level using data from the U.S. Census file. Although we adjusted only for patient characteristics in our baseline analysis, we subsequently included adjuvant radiation and chemotherapy as separate variables in the models, to explore their potential roles as mechanisms underlying observed volume-outcome relationships.
Since patients admitted to the same hospital may have correlated outcomes, we used marginal survival models that incorporated clustering by hospital to adjust the standard errors.13 All P values are two-tailed. The institutional review board of the University of Michigan approved the study protocol.
RESULTS
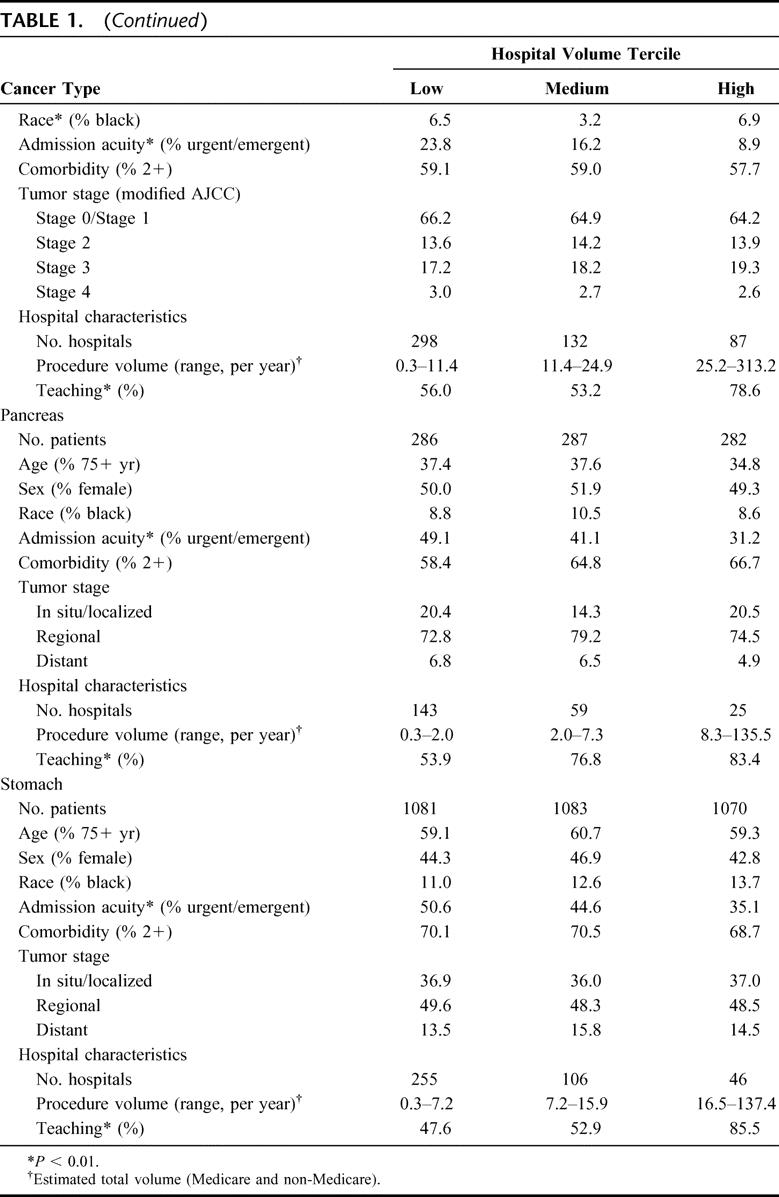
Approximately 64,047 Medicare patients residing in the 11 SEER regions underwent 1 of the 6 cancer resections. As seen in Table 1, patients at lower volume hospitals tended to be older, less affluent, and have more comorbidities and higher-stage tumors, although volume-related differences in these variables were relatively small in magnitude. Low-volume and high-volume hospitals treated similar proportions of black patients for most procedures. However, patients at low-volume hospitals were substantially more likely to be admitted nonelectively.
TABLE 1. Characteristics of Patients Undergoing 1 of 6 Cancer Resections at Low-, Medium-, and High-Volume Hospitals, Based on Data From the 1992–2002 SEER-Medicare Database
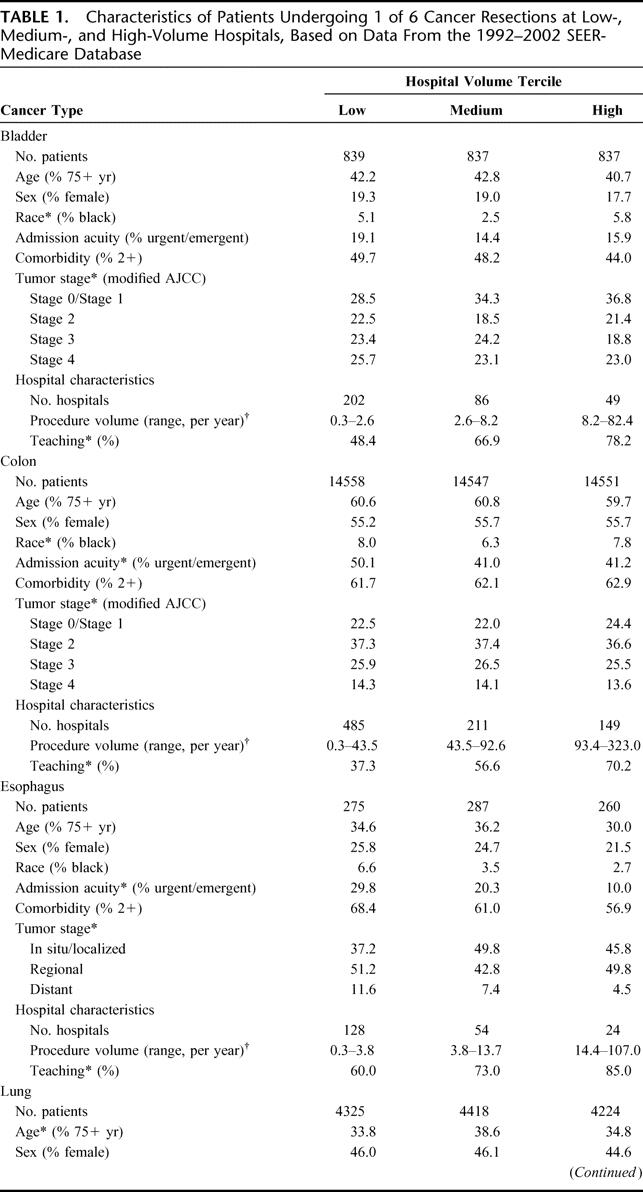
TABLE 1. (Continued)
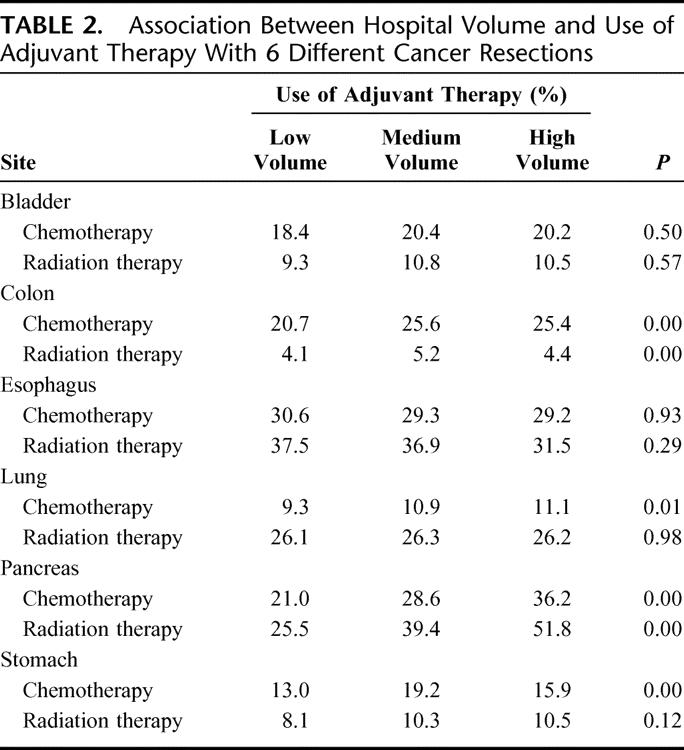
Hospital volume was not related to the use of adjuvant therapy for most procedures. (Table 2) Patients at low-volume hospitals were substantially less likely to receive either adjuvant radiation therapy or chemotherapy for pancreatic cancer. They were also less likely to receive adjuvant chemotherapy for colon cancer. The use of adjuvant radiation therapy and chemotherapy did not vary systematically by hospital volume with the other 4 cancer resections.
TABLE 2. Association Between Hospital Volume and Use of Adjuvant Therapy With 6 Different Cancer Resections
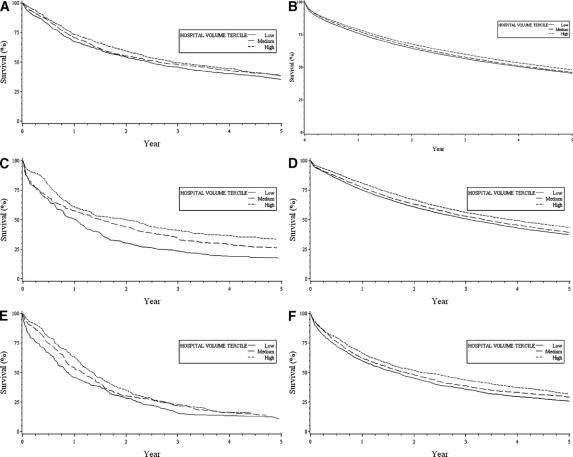
Although hospital volume was related to late survival after each of the 6 procedures (P < 0.001), the magnitude of the volume effect varied widely by cancer type (Fig. 1). Five-year Kaplan-Meier survival probabilities after esophageal resection were 17% lower, in absolute terms, at low-volume hospitals than at high-volume hospitals (17.4% vs. 33.7%, respectively). Relatively large risk differences in 5-year survival were also observed for lung cancer (37.5% vs. 43.5%) and gastric cancer (25.6% vs. 32.0%). Small differences in 5-year survival between low-volume and high-volume hospitals were noted for pancreatic (10.8% vs. 15.9%), bladder (35.4% vs. 39.0%), and colon (45.4% vs. 48.1%) cancer.
FIGURE 1. Kaplan-Meier plots describing 5-year survival among patients undergoing cancer resection at low-, medium-, and high-volume hospitals, based on data from the SEER-Medicare linked database, 1992–2002. Cancer types include those related to bladder (A), colon (B), esophagus (C), lung (D), pancreas (E), and stomach (F).
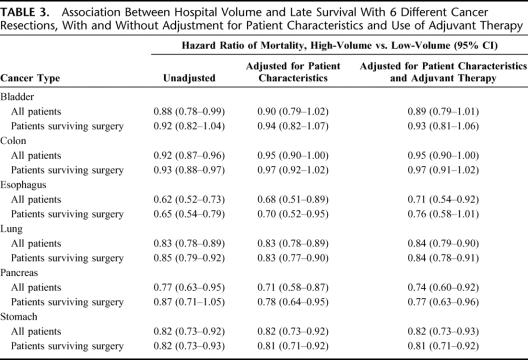
In terms of relative mortality rates, adjusting for patient characteristics tended to attenuate volume effects with surgery for colon and esophageal cancer, but not with the other 4 procedures. (Table 3) Nonetheless, volume-outcome relationships remained statistically significant for 4 of the 6 procedures. Adjusting for differences in the use of adjuvant radiation and chemotherapy had a negligible effect in attenuating differences in survival between low-volume and high-volume hospitals. To isolate the effects of hospital volume on operative and subsequent mortality, we repeated these analyses in patients surviving the perioperative period. Adjusted hazard ratios of late mortality associated with hospital volume were remained essentially unchanged after this restriction (Table 3).
TABLE 3. Association Between Hospital Volume and Late Survival With 6 Different Cancer Resections, With and Without Adjustment for Patient Characteristics and Use of Adjuvant Therapy
DISCUSSION
Although associations between hospital volume and perioperative outcomes are well described, our study suggests that patients with some types of cancer can improve their odds of long-term survival by having surgery at higher-volume centers. The importance of volume varied widely by cancer type. Volume-related differences in 5-year survival were largest with surgery for esophageal, gastric, pancreatic, and lung cancer, but smallest with colon and bladder cancer. Relationships between hospital volume and late survival after cancer surgery could not be explained by volume-related differences in patient characteristics or in the use of adjuvant therapy. They were also not fully explained by differences in operative mortality: Hospital volume remained a strong predictor of later survival after some procedures among patients surviving the perioperative period.
Although our analysis is the largest to date, previous studies have examined relationships between hospital volume and late survival after cancer surgery. To date, all such studies of surgery for breast, lung, pancreatic, liver, and rectal cancer have found significant volume-outcome effects.14–19 For example, in one earlier study of SEER-Medicare data, 5-year survival after resection for lung cancer was only 33% at low-volume hospitals, compared with 44% at high-volume hospitals (P < 0.001).17 Consistent with our findings, the association between hospital volume and late mortality after resection for colon cancer appears to be weaker than with other cancer types. As summarized in one recent review,7 only 1 of 4 previous studies, all based on different study populations, was able to document a statistically significant effect of hospital volume on late mortality.
Our findings should be interpreted in light of several limitations. First, we studied only Medicare patients 65 years and older. Based our recent (unpublished) analysis of data from the Nationwide Inpatient Sample, such patients constitute approximately half of all U.S. patients undergoing these procedures. Although there is little evidence that volume-relationships are influenced by patient age, we cannot confirm the generalizability of our findings to younger patients empirically. Second, our measure of hospital volume may be imprecise. To minimize misclassification occurring as a result of patients crossing SEER boundaries, we assessed volume from national Medicare data rather than SEER-Medicare data alone. Our previous analyses suggest that hospital volume estimates based on Medicare data and all-payer data are highly correlated (>0.90 for most procedures). To the extent that Medicare data remains an imperfect proxy for total hospital volume, we would expect this misclassification to be largely random and bias our findings toward the null hypothesis (no volume-outcome effect). Some would also criticize our approach to categorizing hospital volume. As with our previous work,1 volume cutpoints were selected prospectively in the manner that divided the study population into evenly sized groups (terciles in this study). We used this approach to avoid inflating the apparent effect of volume, which occurs routinely when cutpoints are selected post hoc based on the shape of volume-outcome “curve.” Nonetheless, we acknowledge that our volume cutpoints are arbitrary and do not necessarily reflect thresholds for optimal performance.
The third and most important limitation of our analysis relates to risk adjustment. Based on data available in the SEER-Medicare linked database, we did not find large differences in patient characteristics at low-volume and high-volume hospitals. Among important predictors of survival after cancer surgery, age, race, and tumor stage distributions were relatively similar across hospital volume strata. Patients at lower-volume hospitals were substantially more likely to be admittedly nonelectively than those at high-volume hospitals. However, since the cancer procedures we studied are rarely “urgent,” we think that this variable reflects differences in referral pathways by which patients get to surgery rather than true differences in clinical acuity. Cancer stage, a strong predictor of prognosis after surgery, did not vary substantially according to hospital volume. Although some would criticize SEER staging conventions as too imprecise for some cancers (eg, esophageal and pancreatic cancer), we have no reason to believe that cancer stage varies systematically by hospital type and thus is likely to be major confounder of observed volume-survival associations. Although we also found few differences in comorbidity rates by hospital volume, the limitations of Medicare data in capturing coexisting illnesses are well recognized. One recent study confirms that differences in comorbidity prevalence and noncancer mortality may be important factors underlying disparities in survival after a cancer diagnosis.20 Finally, our study cannot rule out volume-related differences in socioeconomic status, a strong predictor of survival after a cancer diagnosis.21 Although we adjusted for zip-code level measures of income and education, these measures are only proxies of the socioeconomic status of individual patients.
Our findings provide additional empirical support for ongoing volume-based referral initiatives. As one example, the purchaser-coalition Leapfrog Group is using hospital procedure volume and other criteria as the basis for its evidence-based hospital referral initiative for esophagectomy, pancreatectomy, and 3 other noncancer procedures.22 Although the primary motivation for these efforts has been the strong link between hospital volume and operative mortality, our data suggest that associations between volume and late mortality may be an equally strong rationale for selective referral policies.
Nonetheless, volume-based referral strategies are ultimately limited in their potential to improve patient outcomes after cancer surgery. Although procedure volume can reliably identify groups of providers with better results on average, they do not reliably predict performance for individual hospitals. On a practical level, volume-based referral initiatives in the United States will never get all high-risk procedures into high-volume centers. Even well-organized blocs of private payers lack the negotiating strength to dictate surgical referral patterns in most markets23; public sector payers, Medicare in particular, may not be willing to take on hospitals over this contentious issue. Thus, given patient preferences, geography, and provider incentives to hold onto surgical cases, many patients will undoubtedly remain at low-volume hospitals.
For this reason, strategies for improving care in all hospitals, even low volume ones, are paramount. Achieving this goal will require a better understanding of mechanisms underlying volume-outcome relationships in cancer surgery. Volume-related differences in late mortality may be attributable to differences in the quality of the initial surgical care not fully reflected by operative mortality rates. One recent study demonstrated that patients experiencing nonfatal surgical complications had markedly increased mortality in the year following discharge, after accounting for other risk factors.24 These data suggest that perioperative events may affect late survival in ways not well understood at the present time.
Alternatively, volume-outcome relationships may reflect differences in the quality of care patients receive after surgery. For example, even if overall rates of adjuvant therapy do not vary by volume, higher-volume hospitals may target such therapy more effectively to patients more likely to benefit, or deliver it more expertly. Patients at high-volume hospitals may receive more intensive surveillance and treatment of recurrent cancer. Finally, patients treated at these centers may be better treated for their coexisting illnesses.
Potential mechanisms underlying relationships between volume and late cancer mortality have not been characterized by this study or previous research and thus remain speculative. Ultimately, however, such information will be essential for targeting future quality improvement efforts and ultimately reducing disparities in patient outcomes after cancer surgery.
Footnotes
Supported by the National Cancer Institute (No. 1 R01 CA098481-01A1).
The views expressed herein do not necessarily represent the views of Center for Medicare and Medicaid Services or the United States Government.
Reprints: John D. Birkmeyer, MD, 2920 Taubman Center, 1500 East Medical Center Drive, Ann Arbor, MI 48109. E-mail: jbirkmey@umich.edu.
REFERENCES
- 1.Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 2.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–1751. [DOI] [PubMed] [Google Scholar]
- 3.Dudley RA, Johansen KL, Brand R, et al. Selective referral to high volume hospitals: estimating potentially avoidable deaths. JAMA. 2000;283:1159–1166. [DOI] [PubMed] [Google Scholar]
- 4.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511–520. [DOI] [PubMed] [Google Scholar]
- 5.Killeen SD, O'Sullivan MJ, Coffey JC, et al. Provider volume and outcomes for oncological procedures. Br J Surg. 2005;92:389–402. [DOI] [PubMed] [Google Scholar]
- 6.Dimick J, Pronovost P, Cowan JAJ, et al. Variation in postoperative complication rates after high-risk surgery in the United States. Surgery. 2003;134:534–540. [DOI] [PubMed] [Google Scholar]
- 7.Birkmeyer JD. Impact of hospital and surgeon volume on outcomes following surgery for cancer. Principles Practice Oncol Updates. 2004;18:1–12. [Google Scholar]
- 8.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(suppl 8):3–18. [DOI] [PubMed] [Google Scholar]
- 9.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(suppl 8):55–61. [DOI] [PubMed] [Google Scholar]
- 10.Virnig BA, Warren JL, Cooper GS, et al. Studying radiation therapy using SEER-Medicare-linked data. Med Care. 2002;40(suppl 8):49–54. [DOI] [PubMed] [Google Scholar]
- 11.Schrag D, Bach PB, Dahlman C, et al. Identifying and measuring hospital characteristics using the SEER-Medicare data and other claims-based sources. Med Care. 2002;40(suppl 8):96–103. [DOI] [PubMed] [Google Scholar]
- 12.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 13.Lin DY. Cox regression analysis of multivariate failure time data: the marginal approach. Stat Med. 1994;13:2233–2247. [DOI] [PubMed] [Google Scholar]
- 14.Fong Y, Gonen M, Rubin D, et al. Long-term survival is superior after resection for cancer in high-volume centers. Ann Surg. 2005;242:540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roohan PJ, Bickell NA, Baptiste MS, et al. Hospital volume differences and five-year survival from breast cancer. Am J Public Health. 1998;88:454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birkmeyer JD, Warshaw AL, Finlayson SR, et al. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery. 1999;126:178–183. [PubMed] [Google Scholar]
- 17.Bach PB, Cramer LD, Schrag D, et al. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345:181–188. [DOI] [PubMed] [Google Scholar]
- 18.Simunovic M, Baxter N, Balshem A, et al. Hospital procedure volume and teaching status do not influence treatment and outcome measures of rectal cancer surgery in a large general population. J Gastrointest Surg. 2000;4:324–330. [DOI] [PubMed] [Google Scholar]
- 19.Schrag D, Cramer LD, Bach PB, et al. Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA. 2000;284:3028–3035. [DOI] [PubMed] [Google Scholar]
- 20.Tammemagi CM, Nerenz D, Neslund-Dudas C, et al. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294:1765–1772. [DOI] [PubMed] [Google Scholar]
- 21.Ward E, Jemal A, Cokkinedes V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. [DOI] [PubMed] [Google Scholar]
- 22.www.leapfroggroup.org. Last accessed January 15, 2006.
- 23.Galvin RS, Delbanco S, Milstein A, et al. Has the leapfrog group had an impact on the health care market? Health Aff. 2005;24:228–233. [DOI] [PubMed] [Google Scholar]
- 24.Khuri SF, Henderson WG, DePalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–341. [DOI] [PMC free article] [PubMed] [Google Scholar]