Abstract
Objective:
This study compared the postoperative pancreatic anastomosis leakage rate of a new binding technique with the conventional technique of pancreaticojejunostomy after pancreaticoduodenectomy.
Summary Background Data:
Leakage from pancreatic anastomoses remains the single most important morbidity after pancreaticoduodenectomy and contributes to prolonged hospitalization and mortality. The reported incidence after conventional pancreaticojejunostomy ranged from 10% to 29%. We previously reported a new binding pancreaticojejunostomy technique with a leakage of 0%.
Methods:
We conducted a prospective randomized study on 217 patients who underwent pancreaticoduodenectomy for benign and malignant diseases of the pancreatic head and the periampullary region comparing the 2 techniques of pancreaticojejunostomy.
Results:
Of the 111 patients randomized to the conventional group, pancreaticojejunostomy leakage occurred in 8 patients, while no patient in the 106 patients randomized to the binding group developed leakage (χ2 test, P = 0.014). The overall postoperative complications developed in 41 patients (36.9%) in the conventional group compared with 26 patients (24.5%) in the binding group (χ2 test, P = 0.048). Seven patients (6.3%) died in the perioperative period in the conventional group compared with 3 patients (2.8%) in the binding group (χ2 test, P = 0.37). The postoperative hospital stay (mean ± SD) for the conventional group was 22.4 ± 10.9 days, which was significantly longer than the binding group (18.4 ± 4.7 days) (Mann-Whitney U test, P < 0.001).
Conclusions:
Binding pancreaticojejunostomy after panceaticoduodenectomy significantly decreased postoperative complication and pancreaticojejunostomy leakage rates and shortened hospital stay when compared with conventional pancreaticojejunostomy.
Pancreatic anastomotic leakage remains the most significant cause of postoperative morbidity after pancreaticoduodenectomy. This prospective randomized study showed that binding pancreaticojejunostomy decreased pancreaticojejunostomy leakage rate and shortened hospital stay significantly when compared with conventional pancreaticojejunostomy.
Dramatic improvement in operative mortality has expanded the indications for pancreaticoduodenectomy from periampullary tumors, to pancreatic head cancer, benign neoplasms and other non-neoplastic conditions such as chronic pancreatitis.1 However, postoperative morbidity remains high even in large series.2–4 Leakage from pancreatic anastomosis remains the single most important cause of morbidity, and it also contributes significantly to prolonged hospitalization and mortality.1 The incidence of pancreatic leakage varies greatly in different reports because of the various definitions used. In a review by Bassi et al,5 the incidence of pancreatic leakage ranged between 9.9% and 28.5%, and different definitions of pancreatic leakage were applied with high statistical differences between them. Thus, it is important to have the same definition of pancreatic leakage before any series of patients can be compared.
Many factors have been identified that are associated with a significantly higher incidence of pancreatic leakage after pancreaticoduodenectomy.1 The surgeon has been shown to be one of the most, if not the most, important factor in the prevention of pancreatic anastomosis leakage,6,7 and it is logical to relate operative morbidity and mortality to the surgical volume handled.8
The best technique in pancreatic anastomosis is still debated. The technique most commonly employed after pancreaticoduodenectomy is pancreaticojejunostomy. We established a new pancreaticojejunostomy anastomosis technique, binding pancreaticojejunostomy with a leakage rate of 0%.9,10 This prospective randomized study aimed to compare the new binding with the conventional pancreaticojejunostomy using a predetermined definition, and controlling the known compounding factors, of pancreatic leakage after pancreaticoduodenectomy.
PATIENTS AND METHODS
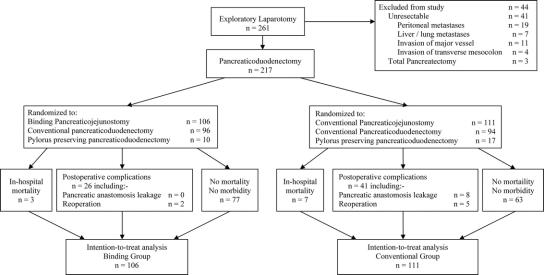
Between June 2001 and September 2004, 261 consecutive patients underwent elective exploratory laparotomy for various benign and malignant diseases of the pancreatic head and periampullary region, and 217 patients were enrolled in this study. Forty-four patients were excluded from this study before randomization for various reasons (Fig. 1). All patients were adequately investigated with hematologic, biochemical, and radiologic investigations, and the lesions were assessed to be resectable preoperatively. Written inform consent was obtained from every patient to enter into this study before exploratory laparotomy. Pancreaticoduodenectomy was performed by just 3 experienced surgeons who had done more than 40 pancreaticoduodenectomy with either the conventional or the binding pancreaticojejunostomy. This study was approved by the Ethical Committee of the School of Medicine, Zhejiang University, and it followed the ethical guidelines of the 1975 Declaration of Helsinki. The study was designed according to the CONSORT guidelines.
FIGURE 1. Flow chart.
Randomization
After resection of the lesion in the pancreatic head or the periampullary region, the patients were randomized to receive either the binding pancreaticojejunostomy (binding group) or the conventional pancreaticojejunostomy (conventional group). Randomization was done by using random numbers generated from a computer in a central registry for this study by a research nurse. The treatment allocation was concealed to the operating surgeons until after the resection of the lesions but before the anastomoses to reconstruct the gastrointestinal tract. Patients were kept blind to the treatment allocation. The outcome measurements were determined by 2 independent assessors who were blinded to the types of pancreaticojejunostomy that the patients received. If there was a discrepancy in the outcome measurement between the 2 assessors, a discussion was held between them to establish a mutually acceptable measurement.
Binding Pancreaticojejunostomy
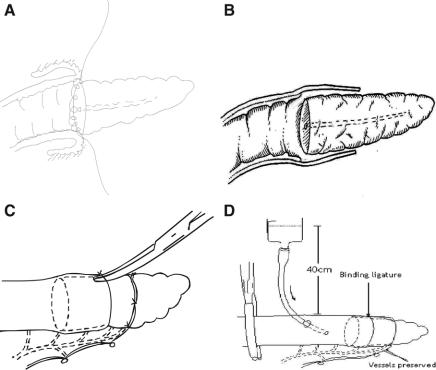
Our technique of binding pancreaticojejunostomy has been reported.9,10 In brief, the stump of the jejunum was everted for a length of 3 cm by applying 2 sutures to the jejunal cut edge to a point in the jejunum 6 cm distal to the cut edge. The jejunal mucosa was destroyed by applying 10% carbolic acid, then rinsed with 75% alcohol and normal saline.
The remnant of the pancreas was dissected for a distance of more than 3 cm from its cut edge. Usually, 2 to 3 small veins, which run between the pancreas and the splenic vein, had to be ligated and divided.
The cut edge of the pancreatic stump and the everted jejunum were anastomosed with 3-0 silk in a circular fashion, and care was taken to suture the jejunal mucosa only to avoid penetrating into the muscular and serosal layers (Fig. 2a). The posterior lip of the pancreatic duct should be involved in the posterior row of suture whenever technically possible.
FIGURE 2. A, Pancreatic stump isolated for >3 cm. End of jejunum everted for 3 cm. Everted jejunal mucosa destroyed with carbolic acid. B, The 2 sutures for everting the jejunum were cut and the everted jejunum was restored to its normal position to wrap over the pancreatic stump. The gap between the jejunum and the pancreatic stump is sealed by compressing from the outside with a binding ligature. C, The binding ligature is the real anastomotic site. Tip of a clamp can pass underneath the binding ligature. D, Injection of saline dyed with methylene blue into jejunal lumen to confirm watertight closure of the anastomosis.
The 2 sutures for everting the jejunum were cut and the everted jejunum was restored to its normal position to wrap over the pancreatic stump (Fig. 2b). An absorbable ligature was then looped around the jejunum, with the invaginated pancreas inside, 1.5 to 2 cm from the cut edge of the jejunum. The ligature was tied just tight enough to allow the tip of a hemostatic clamp to pass underneath the ligature (Fig. 2c). The blood supply to the jejunum distal to the binding ligature was ensured by preserving a bundle of vessels to that portion of the jejunum by placing the binding ligature through a hole in the jejunal mesentery between the last 2 branches of the vessels near the jejunal cut edge (Fig. 2d).
After completion of the pancreaticojejunostomy, a 12-Fr catheter was inserted temporarily through the site where the hepaticojejunostomy was intended to be constructed, for instillation of saline dyed with methylene blue from a height of 40 cm to test for watertight closure at the pancreaticojejunostomy (Fig. 2d).
Conventional Pancreaticojejunostomy
An end-to-end anastomosis was performed between the jejunum and the pancreatic stump. The anastomosis was done in 2 layers. The inner layer consisted of the cut edges of the jejunum and the pancreas. The outer layer was comprised of an inverting seromuscular layer of the jejunum sutured onto the body of the pancreas. The outer layer was about 3 to 4 cm from the inner layer of anastomosis, thus directing the end of the pancreas to invaginate into the jejunum.
Intraoperative and Postoperative Management
Hepaticojejunostomy and gastro/duodeno-jejunostomy were done to the same jejunal loop distal to the pancreaticojejunostomy. A Jackson-Pratt drainage tube was placed near to the pancreaticojejunostomy in both groups of patients and removed when the drain output was ≤10 mL. The volume and the amylase level of the drained fluid were measured every day.
Octreotide was forbidden to be given. All patients received histamine H2-receptor antagonists. Intravenous nutrition, consisting of a solution enriched with 35% branched-chain amino acid at a dosage of 1.5 g of amino acid/kg body weight per day, dextrose and lipid emulsion (50% medium-chain triglycerides) providing 30 kcal/kg body weight per day, 50 g of albumin, and vitamins and trace minerals, was given immediately after the operation until enteral nutrition was started once the bowel function returned, when the patients were encouraged to resume oral feeding.
Outcome Measurements
The primary outcome measurement was pancreaticojejunostomy leakage. The secondary outcome measurements were in-hospital mortality, overall postoperative morbidity, and the duration of postoperative hospital stay.
Definitions of Postoperative Morbidities
Pancreaticojejunostomy leakage was defined as the drain fluid amylase level being ≥3 times the upper limit of the normal amylase level from the third postoperative day onward when the drain output was ≥10 mL. This definition of pancreatic fistula has been commonly used internationally.1
Biliary leakage was defined as bile in the drain fluid from the subhepatic drain with the level of total bilirubin exceeding the upper limit of normal.
Gastro/duodenal-enteric anastomosis leakage was defined as a persistent discharge of digestive juice in the drain for more than 5 days postoperation, and leakage confirmed by the methylene blue test or by radiology.
Delayed gastric emptying was defined to be present when the nasogastric tube was maintained for 10 or more days, combined with at least one of the following: vomiting after removal of nasogastric tube, reinsertion of nasogastric tube, or failure to progress with oral feeding.
Acute pancreatitis was defined as a more than 3-fold increase in serum or lipase from postoperative day 4 onward with a compatible clinical course or findings on computed tomography.
Intraperitoneal hemorrhage was defined to be present when more than 3 units of blood were required in any 24 hours after the operation.
Intraperitoneal fluid collection was defined as a collection of fluid that measured more than 5 cm in diameter on ultrasound or computed tomography.
Intraperitoneal abscess was defined as a collection of pus, with or without necrotic tissue, but with a positive bacterial culture.
Ascites was considered as a complication when the accumulation of fluid was massive, which led to dyspnea, leakage through the abdominal wound, or a diuretic was needed to control the ascites. The diuretic agents, when prescribed, were given by independent assessors who were medical staff members not involved in the study.
Hepatic insufficiency was defined as persistent jaundice, coagulopathy, hypoproteinemia, thrombocytopenia, gross ascites, and encephalopathy.
Renal insufficiency was defined as an elevation of the postoperative serum creatinine to more than 2 times the upper limit of normal.
Wound infection was defined as erythema and induration of a wound with purulent discharge and with a positive bacterial culture.
Wound dehiscence was defined as partial or total disruption of the fascial or all the layers of the incision.
Pulmonary infection was defined as the presence of pneumonia, or atelectatic changes on radiograph, and was associated with a positive sputum bacterial culture.
Pleural effusion was a complication when the collection of fluid caused dyspnea, and chest tapping was required to relieve the symptoms.
Sample Size
We used the pancreaticojejunostomy leakage rate to estimate the sample size for this study. For the conventional pancreaticojejunostomy, the leakage rate was estimated to be 10%, based on previously published data1,5 and our past experience. For the binding pancreaticojejunostomy, the leakage rate was estimated to be 1%, based on our past experience.9,10 Using a 2-tailed test with 90% power at a significant level of 5%, the sample size needed to detect a significant difference was 99 subjects in each treatment group. We randomized 217 patients into this study.
Statistical Analyses
The statistical analyses of the data were performed using SPSS 11.0 statistical software. Comparisons between the 2 groups were done using the Mann-Whitney U test for continuous data, and the χ2 test (with continuity corrected χ2 if the expected count was <5) or the Fisher exact test where appropriate for categorical data. Continuous variables were reported as mean ± SD. All statistical tests were two-sided, and a significant difference was considered when P < 0.05. All analyses were done on the intent-to-treat basis.
RESULTS
The demographic data and the pathologic findings in the 2 groups of patients were comparable (Table 1). The final pathologies of the resected specimens were reviewed by 2 independent pathologists. The commonest pathology was adenocarcinoma of the head of pancreas (41%), followed by carcinoma of the distal bile duct (28%).
TABLE 1. Demographic Data and Pathologic Findings
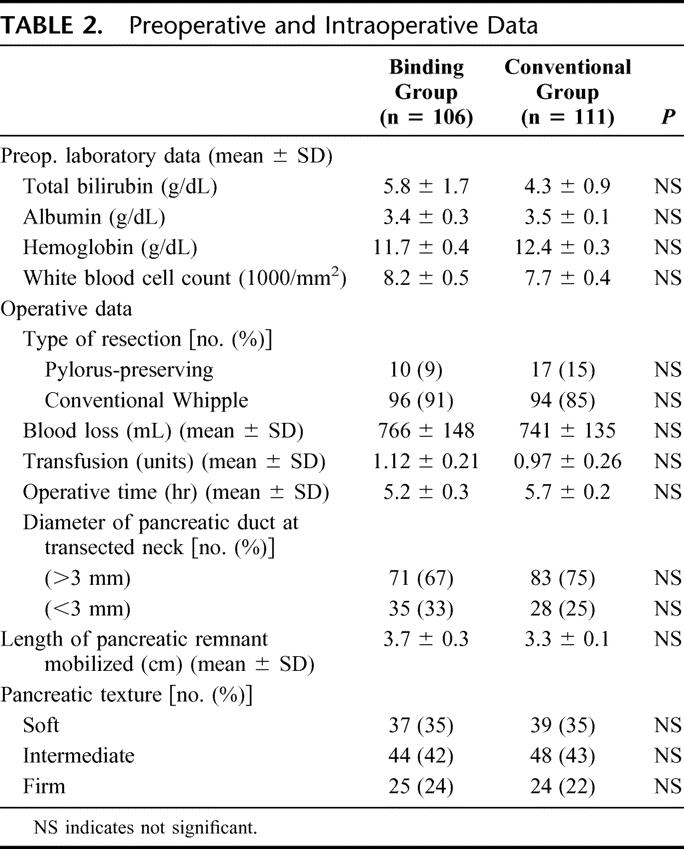
The preoperative and intraoperative data of the 2 groups of patients are presented in Table 2. There were no significant differences in the data between these 2 groups of patients. While classic pancreaticoduodenectomy was done in 190 patients (88%), pylorus-preserving pancreaticoduodenectomy was done in 27 patients (12%). The texture of the pancreas was judged by the surgeons to be soft in 35% of patients, intermediate in 42%, and firm in 23%.
TABLE 2. Preoperative and Intraoperative Data
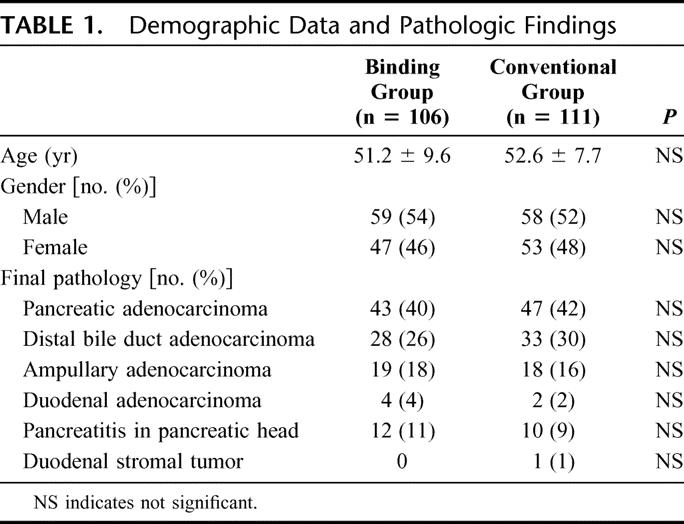
According to the protocol, if there was a discrepancy in the outcome measurement between the 2 assessors, a discussion was made between them to come up with an agreed measurement. But in our real situation, there was no discrepancy between the 2 assessors. The postoperative courses and complications are listed in Table 3. In the binding group, there was a patient who had a transiently high amylase level of 762.5 U/L, which on immediate rechecking on another sample of drain fluid showed the amylase level to return to 25 U/L. The drainage output was less than 50 mL/d. This patient had an uneventful recovery without any complications. Accordingly, the assessors judged that this patient did not have pancreaticojejunostomy leakage. While no pancreatic anastomosis leakage was observed in the binding group, 8 patients developed leakage in the conventional group with the pathology comprising of distal bile duct adenocarcinoma (n = 4), ampullary adenocarcinoma (n = 2), and pancreatic adenocarcinoma (n = 2).
TABLE 3. Postoperative Course and Complications
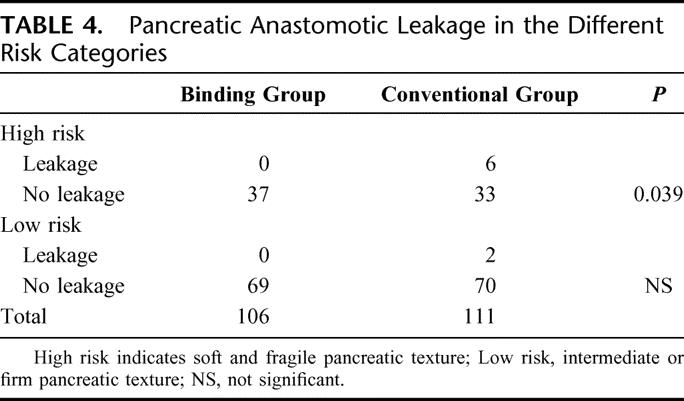
The difference in the pancreatic anastomotic leakage rates between the 2 groups was significant (χ2 test, P = 0.014; relative risk, odds ratio, 1.078; 95% confidence interval, 1.023–1.135). Statistical comparison of the subsets showed that, for the high-risk category (soft and fragile pancreatic texture), there was a significant difference between the 2 groups (χ2 test, P = 0.039; relative risk, odds ratio, 1.182; 95% confidence interval, 1.034–1.351); but for the low-risk category (intermediate or firm pancreatic texture), there was no significant difference between the 2 groups (Table 4).
TABLE 4. Pancreatic Anastomotic Leakage in the Different Risk Categories
There was no significant difference in the drain outputs between the 2 groups, but the difference in the drain fluid amylase levels between the 2 groups was significant (Mann-Whitney U test, P < 0.001). The overall in-hospital mortality was 4.6% (10 of 217). There were 3 deaths in the binding group. These 3 patients died of intraperitoneal hemorrhage, hepatorenal failure, and aspiration pneumonia, respectively. There were 7 deaths in the conventional group. The causes of death were multiorgan failure (n = 3), hepatorenal failure (n = 2), intraperitoneal abscess (n = 1), and chest infection (n = 1). There was no significant difference in the in-hospital mortality between the 2 groups of patients (2.8% vs. 6.3%, χ2 test, P = 0.37; relative risk, odds ratio, 0.433; 95% confidence interval, 0.109–1.720).
For patients who survived the operation, 7 patients required reoperation for incisional wound dehiscence (n = 4), pancreatic anastomosis leakage (n = 2), and intraperitoneal hemorrhage (n = 1). The reoperation rate was 1.9% (2 of 106) in the binding group and 4.5% (5 of 111) in the conventional group.
Overall, 67 of 217 patients (30.8%) developed one or more postoperative complications. Twenty-six of 106 patients (24.5%) in the binding group developed postoperative complications, compared with 41 of 111 patients (36.9%) in the conventional group. The difference between these 2 groups of patients was significant (χ2 test, P = 0.048; relative risk, odds ratio, 0.555; 95% confidence interval, 0.309–0.998).
The duration of the postoperative hospital stay for the binding group was 18.4 ± 4.7 days, compared with 22.4 ± 10.9 days for the conventional group. The difference between the 2 groups was significant (Mann-Whitney U test, P < 0.001).
DISCUSSION
Pancreatic anastomosis leakage remains a major cause of postoperative morbidity after pancreaticoduodenectomy, and it contributes significantly to operative mortality. In an attempt to prevent pancreatic anastomosis leakage, some factors have been identified to be related to the leakage. These factors include the pancreas factor (pancreatic texture, blood supply to the cut end, original pathology, pancreatic duct size, pancreatic juice output), the patient factor (age, gender, level of jaundice, comorbid illness), and the operation factor (total operation time, blood loss, type of anastomosis, stenting of anastomosis).1,5,11–14 Among these factors, pancreatic texture,1,13,15,16 pancreatic duct size,1,16,17 pancreatic stump blood supply,7 and pancreatic juice output1,13,17 have been considered the most significant factors. Some factors such as soft pancreatic texture, nondilated pancreatic duct, and high pancreatic juice output are closely related. It is generally accepted that a fibrotic pancreatic remnant facilitates the pancreaticoenteric anastomosis, whereas a soft and fragile pancreatic remnant frequently results in a high pancreatic anastomosis leakage rate.
A variety of techniques have been used and evaluated over the years in the management of the pancreatic remnant after pancreaticoduodenectomy to reduce the incidence of postoperative pancreatic anastomosis leakage. The high incidence of pancreatic anastomosis leakage following pancreatic duct ligation and the associated pancreatic insufficiency and diabetes have restricted the use of duct occlusion as a reliable method in dealing with the pancreatic remnant.1 Pancreaticogastrostomy has been gaining favor in recent years. Nonrandomized studies have shown low pancreatic anastomosis leakage after pancreaticogastrostomy. Unfortunately, a prospective randomized trial comparing pancreaticogastrostomy and pancreaticojejunostomy showed no difference in the pancreatic anastomosis leakage rate, postoperative complication, and the length of hospital stay following the 2 procedures, thereby indicating no outcome advantage for either group.18
Pancreaticojejunostomy has been the most commonly used method of restoring pancreaticoenteric continuity after pancreaticoduodenectomy. The anastomosis is carried out either as an end-to-end anastomosis with invagination of the pancreatic stump in the jejunum or as an end-to-side anastomosis with or without duct-to-mucosa suturing.19,20 A main pancreatic duct stent may or may not be placed across this anastomosis.21 There is no firm evidence to show which technique of pancreaticojejunostomy is superior. In our study, we decided to use an end-to-end pancreaticojejunostomy with invagination of the pancreatic stump, without duct-to-mucosa suturing and without a stent in the pancreatic duct across the anastomosis in the conventional pancreaticojejunostomy group as this anastomosis is similar to that used in the binding pancraticojejunostomy group.
In recent years, the surgeon has been shown to be one of the most, if not the most, important factor in the prevention of a pancreatic anastomosis leakage.6,7 There is no doubt that the pancreatic anastomosis leakage rate can be reduced in experienced hands using meticulous techniques,6,7,22 and it is logical to relate low operative morbidity and mortality to the surgical volume handled.8 Unfortunately, postoperative morbidity remains high and pancreatic anastomosis leakage remains the single most important cause of morbidity after pancreaticoduodenectomy even in big centers with high volumes of pancreatic resection.1
It occurred to us that leakage from a pancreatic anastomosis can start at a point where a needle inadvertently penetrates a pancreatic ductule, or a suture lacerates the fragile pancreatic parenchyma on suturing or on tying a knot. The resultant minor leak in pancreatic juice gradually leads to a gross anastomoses leakage as a consequence of auto-digestion around the anastomoses. Such a hypothesis forms the basis of the binding pancreaticojejunostomy. In this technique, the cut edge of the pancreas is sutured only to the mucosa of the jejunum. Thus, if there is a leakage, the pancreatic juice goes into the gut lumen. The jejunum, with its mucosa destroyed, is wrapped over the pancreatic remnant. The gap between these 2 structures is sealed by compressing from the outside with a binding ligature. Healing between the jejunum and the pancreas is promoted by destroying the jejunal mucosa with carbolic acid. The real anastomotic site is at the binding ligature where no sutures are applied (Fig. 2b). Such a comprehensive procedure of pancreaticojejunostomy with this binding technique instead of the traditional suturing technique theoretically protects the pancreatic anastomosis from leakage. The blood supply to the jejunal cut end is kept by preserving a branch of the vessels supplying that part of the jejunum beyond the binding ligature. The mobilization of the pancreatic stump for more than 3 cm from the cut edge might possibly compromise the blood supply to the cut end to a certain extent.
The main concern of the binding pancreaticojejunostomy is how tightly the binding ligature should be tied. Should the tie be too loose, watertight closure cannot be achieved and leakage can result. Should the tie be too tight, the blood supply to the distal part of the pancreas can be compromised and the pancreatic duct occluded. The binding ligature should be tied until the jejunum and the pancreas are in close contact with each other, and a dent of 1 to 2 mm is seen in the jejunum under the ligature. The tip of a vascular clamp should be able to pass underneath the ligature. We had carried out animal experiments, and the patency of the pancreatic duct was one of the items that we observed. The results showed no compression of the pancreatic duct.23 We routinely test the anastomosis for a watertight seal by instilling saline dyed with methylene blue at a pressure head of 40 cm.
CONCLUSION
This prospective randomized study showed that pancreatic anastomosis leakage rate was significantly lower with the binding pancreaticojejunostomy technique than the conventional pancreaticojejunostomy technique. The incidence of postoperative complications and the postoperative length of hospital stay were also significantly better using the binding technique.
Footnotes
Reprints: Shu You Peng, MD, FACS(Hon), FRCSG, General Surgery Department, Second Affiliated Hospital, School of Medicine Zhejiang University, #88 Jie Fang Road, Hangzhou, 310009, China. E-mail: zrwkpsy@zjuem.zju.edu.cn.
REFERENCES
- 1.Shrikehande SV, Qureshi SS, Rajneesh N, et al. Pancreatic anastomoses after pancreaticoduodenectomy: do we need further studies. World J Surg. 2005;29:1642–1649. [DOI] [PubMed] [Google Scholar]
- 2.Talamini MA, Moesinger RC, Pitt HA, et al. Adenocarcinoma of the ampulla of Vater, a 28 year experience. Ann Surg. 1997;225:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miedema BW, Sarr MG, van Heerdeen JA, et al. Complications following pancreaticoduodenectomy: current management. Arch Surg. 1992;127:945–950. [DOI] [PubMed] [Google Scholar]
- 4.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. [DOI] [PubMed] [Google Scholar]
- 6.Buechler MW, Friess H, Wagner M, et al. Pancreatic fistula after pancreatic head resection. Br J Surg. 2000;87:833–839. [DOI] [PubMed] [Google Scholar]
- 7.Strasberg SM, Drebin JA, Mokadam NA, et al. Prospective trial of a blood supply-based technique of pancreaticojejunostomy: effect on anastomotic failure in the Whipple procedure. J Am Coll Surg. 2002;197:746–758. [DOI] [PubMed] [Google Scholar]
- 8.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 9.Peng SY, Mou YP, Cai XJ, et al. Binding pancreaticojejunostomy is a new technique to minimize leakage. Am J Surg. 2002;183:283–285. [DOI] [PubMed] [Google Scholar]
- 10.Peng SY, Mou YP, Liu YB, et al. Binding pancreaticojejunostomy: 150 consecutive cases without leakage. J Gastrointest Surg. 2003;7:898–901. [DOI] [PubMed] [Google Scholar]
- 11.Shyr YM, Su CH, Wu CW, et al. Does drainage fluid amylase reflect pancreatic leakage after pancreaticoduodenectomy. World J Surg. 2003;27:607–610. [DOI] [PubMed] [Google Scholar]
- 12.van Berge Henegourven MI, de Wit LT, van Gulik TM, et al. Incidence, risk factors, and treatment of pancreatic leakage after pancreaticoduodenectomy; drainage versus resection of the pancreatic remnant. J Am Coll Surg. 1997;185:18–25. [DOI] [PubMed] [Google Scholar]
- 13.Hamanaka Y, Nishihara K, Hamasaki T, et al. Pancreatic juice output after pancreaticoduodenectomy in relation to pancreatic consistency, duct size, and leakage. Surgery. 1996;119:281–287. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa O, Ohigashi H, Imaoka S, et al. Concomitant benefit of preoperative irradiation in preventing pancreas fistula formation after pancreaticoduodenectomy. Arch Surg. 1991;126:885–889. [DOI] [PubMed] [Google Scholar]
- 15.Al-Sharaf C, Ishes I, Dawiskiba S, et al. Characteristics of the gland remnant predict complications after subtotal pancreaticoduodenectomy. Dig Surg. 1997;14:101–106. [Google Scholar]
- 16.Sato N, Yamaguchi K, Chijiiwa K, et al. Risk analysis of pancreatic fistula after pancreatic head resection. Arch Surg. 1998;133:1094–1098. [DOI] [PubMed] [Google Scholar]
- 17.Marcus SG, Cohen H, Ranson JHC. Optimal management of the pancreatic remnant after pancreaticoduodenectomy. Ann Surg. 1995;221:635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeo CJ, Cameron JL, Maher MM, et al. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580–588; discussion 588–592. [DOI] [PMC free article] [PubMed]
- 19.Sung JP, Steward RD, O'Hara VS, et al. A study of forty-nine consecutive Whipple resections for periampullary adenocarcinoma. Am J Surg. 1997;174:6–10. [DOI] [PubMed] [Google Scholar]
- 20.Aston SJ, Longmire WP Jr. Management of the pancreas after pancreaticoduodenectomy. Ann Surg. 1974;179:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roder JD, Stein HJ, Bottcher KA, et al. Stented versus nonstented pancreaticojejunostomy after pancreaticoduodenectomy: a prospective study. Ann Surg. 1999;1:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grobmyer SR, Rivadenera DE, Goodman CA, et al. Pancreatic anastomotic failure after pancreaticoduodenectomy. Am J Surg. 2000;180:117–120. [DOI] [PubMed] [Google Scholar]
- 23.Bai MD, Peng SY, Liu YB, et al. Wound healing after pancreaticojejunostomy in piglets: a comparison between two anastomotic methods. Zhonghua Wai Ke Za Zhi. 2003;41:458–461. [PubMed] [Google Scholar]