Abstract
Objective:
Determine rates of local excision (LE) over time, and test the hypothesis that LE carries increased oncologic risks but reduced perioperative morbidity when compared with standard resection (SR).
Summary Background Data:
Despite the lack of level I/level II evidence supporting its oncologic adequacy, LE is performed for stage I rectal cancer.
Methods:
Surgical therapy for 35,179 patients with stage I rectal cancer diagnosed in 1989 to 2003 was examined over time, utilizing the National Cancer Database. A special study then analyzed perioperative outcomes, local recurrence and survival in 2124 patients diagnosed between 1994 and 1996, including 765 (T1, 601; T2, 164) treated by LE and 1359 (T1, 493; T2, 866) treated by SR.
Results:
From 1989 to 2003, the use of LE has increased (T1, 26.6–43.7%; T2, 5.8–16.8%; P < 0.001 both). The special study demonstrated significantly lower 30-day morbidity after LE versus SR (5.6% vs. 14.6%; P < 0.001). After adjusting for patient and tumor characteristics, the 5-year local recurrence after LE versus SR was 12.5 versus 6.9% (P = 0.003; hazard ratio = 0.38; 95% CI, 0.23–0.62) for T1 tumors, and 22.1 versus 15.1% (P = 0.01; hazard ratio = 0.69; 95% CI, 0.44–1.07) for T2 tumors. The 5-year overall survival (T1, 77.4% vs. 81.7%, P = 0.09; T2, 67.6% vs. 76.5%, P = 0.01) was influenced by age and comorbidities but not the type of surgery.
Conclusions:
This study provides the best evidence for both the increasing use and the associated risks of LE versus SR. For each individual patient, the benefits of LE must be balanced against the heightened risk of local failure.
Local excision (LE) preserves the sphincter and is performed for stage I rectal cancer despite the lack of level I/level II evidence supporting its oncologic adequacy. This nationwide study investigated time trends in the use of LE and established rates of local recurrence, survival, and perioperative complications for LE versus standard resection.
In recent years, local excision (LE) has gained appeal as a treatment strategy for distal rectal cancer because it eliminates the need for colostomy, spares patients major perioperative risks, and maintains favorable functional results.1,2 However, accepting LE as curative therapy for stage I rectal cancer demands a careful analysis of its oncologic risks because standard resection (SR) procedures that remove the rectum with its surrounding lymph nodes have achieved excellent oncologic outcomes and are regarded as the gold standard.1,3 The oncologic adequacy of LE has remained controversial for several reasons. First, selection criteria for LE have historically been based on tumor location and size,4,5 rather than standard TNM staging.6 Proper TNM staging remains difficult because of the variable accuracy and limitations of preoperative imaging. Second, LE provides inadequate nodal staging and treatment in some patients since LE does not remove regional lymph nodes and up to 20% of stage I rectal cancers harbor occult nodal metastases.7–9 Third, reported oncologic outcomes of LE have been concerning, with local failure rates as high as 18% for T1 and 47% for T2 tumors.9–11 Recurrence rate persists at 11%,12 even with adjuvant therapy, and survival is only 43% to 58% at best after salvage surgery for local failure.9,11,13,14 Finally, prior reports have been limited to single institution studies with small and heterogeneous populations of patients and tumors15,16 treated with or without adjuvant therapy.9–11,17–19
Therefore, while perioperative safety and functional advantages may favor LE versus SR, the oncologic outcomes of survival and local tumor control remain insufficiently defined to accept LE as a curative surgical strategy for stage I rectal cancer. Accordingly, the current study draws on a quality-controlled nationwide oncology database: 1) to determine trends in the use of LE for stage I rectal cancer; and 2) to test the hypothesis that LE is associated with compromised oncologic outcomes but reduced perioperative morbidity, when compared with SR.
METHODS
Sources of Data
The National Cancer Database (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons (ACS) and the American Cancer Society. It currently captures approximately 75% of all new cancer patients diagnosed and treated in the United States annually. Data are collected from tumor registries at over 1400 ACS CoC-approved institutions.20 Additional methodologies of the NCDB have been described previously.21
Between January 1, 1989 and December 31, 2003, 47,241 American Joint Commission on Cancer (AJCC) stage I (T1 and T2) malignant adenocarcinomas of the rectum were reported to the NCDB. For the time-trend analysis, we selected adult (≥18-year-old) patients reported from 997 institutions that submitted to the NCDB every year from 1989 to 2003. Patients who underwent local tumor destruction without pathologic specimen (ie, electrocoagulation, laser or cryosurgery; n = 1590), who did not undergo surgery or whose surgical therapy could not be determined (n = 10,472) were excluded, leaving 35,179 patients meeting selection criteria.
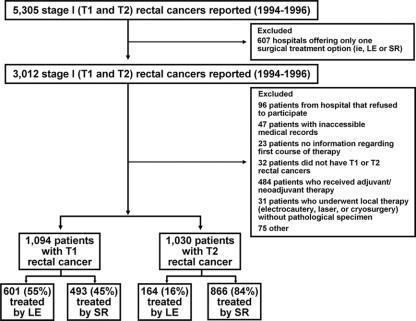
A special study was subsequently performed in a sample of the 5305 patients treated from 1994 through 1996, to allow long-term (5–8 years) follow-up (Fig. 1). To establish perioperative and oncologic outcomes after LE versus SR, detailed clinical data not routinely submitted to the NCDB were specifically requested from 479 hospitals offering both LE and SR as surgical options. A maximum of 11 patients were requested from each hospital since the mean number of eligible patients at each was 6.7, with a standard deviation of 4.1. Hospitals with 11 or fewer eligible patients submitted all patients and those with 12 or more submitted 11 cases identified by a random sample stratified by surgery (LE vs. SR). Patients who received chemotherapy or radiation in neoadjuvant or adjuvant settings in conjunction with surgical intervention, 176 patients with T1 tumors and 308 patients with T2 tumors, were excluded from the study cohort (Fig. 1). After further exclusions (Fig. 1), the final analytic cohort for the special study included a total of 2124 patients: 1094 with T1 and 1030 with T2 rectal tumors.
FIGURE 1. Study cohorts as selected from cases reported to the National Cancer Database.
Submitted data were coded according to the CoC Registry Operations and Data Standards Manual, the AJCC Manual for Staging of Cancer, and the International Classification of Disease for Oncology, 2nd edition. To reduce data errors, underreporting and bias in reporting, all data were extracted from medical records by trained and certified tumor registrars, subsequently verified by a physician member of the hospital cancer committee, and then further reviewed and validated at the institution before submission to the NCDB. The ACS has executed a business agreement that includes a data-use agreement with each of its CoC-approved hospitals. Data were de-identified and submitted to the NCDB in compliance with the Health Insurance Portability and Accountability Act of 1996. The Duke University Institutional Review Board granted this study an exemption waiver.
Data Definitions
Age at diagnosis, sex, and race/ethnicity were assessed because they may affect both the surgical procedure and the outcomes. Race/ethnicity was coded by institutional tumor registrars based on patient-supplied information, according to the NCDB data element descriptions standard among U.S. cancer registries. Household income was estimated from patient’s zip code at diagnosis as linked to U.S. census data. Key comorbid conditions and postoperative complications were defined a priori by the investigators. Comorbidities included: myocardial infarction, angina, arrhythmia, hypertension, congestive heart failure, peripheral vascular disease, chronic obstructive pulmonary disease, coagulopathy, deep venous thrombosis, pulmonary embolism, obesity, diabetes mellitus, cirrhosis, chronic renal failure, cerebrovascular disease, chronic neurologic disease, dementia, paralysis, HIV/AIDS, and other cancer excluding nonmelanoma skin cancer. Postoperative morbidities included: intraabdominal infection, abscess, anastomotic dehiscence, bleeding/hematoma, deep venous thrombosis, dehydration, diarrhea, myocardial infarction, perineal infection, pneumonia, prolonged ileus, pulmonary embolism, and urinary tract infection. These diagnoses were specifically extracted from medical records by tumor registrars accordingly. Treating hospitals were either community cancer centers (treating 300 or fewer cancer cases annually), comprehensive community cancer centers (treating more than 300 cancer cases annually), or teaching-research centers (associated with university medical schools or designated as National Cancer Institute Comprehensive Cancer Care Programs). “Low”-lying tumors were located within 5 cms of the anal verge on preoperative proctoscopy, while “mid to high” tumors were located within 5 to 10 cm. Tumors were measured by their greatest diameter. Histologic grade was either low (moderately well-/well-differentiated) or high (poorly/undifferentiated). The presence of lymphatic, vascular or perineural invasion was noted.
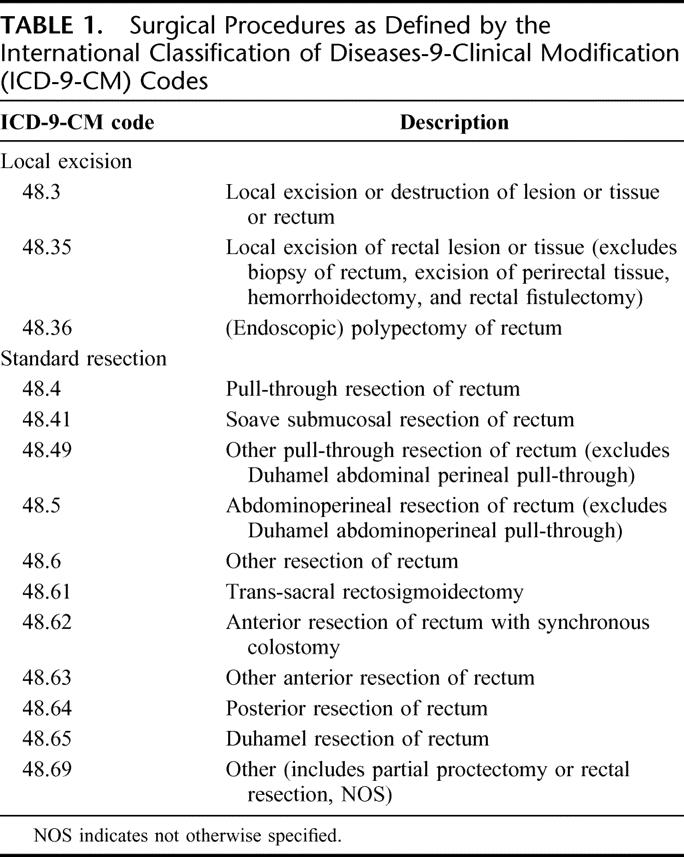
Local excision and SR procedures were defined according to International Classification of Dieases-9-Clinical Modification (ICD-9-CM) codes (Table 1). Postoperative mortality (death within 30 days of surgery or prior to hospital discharge), morbidity (complications within 30 days of surgery leading to rehospitalization), and length of stay were analyzed. The frequency of follow-up was according to the practices of treating physicians. Time to local recurrence (LR) was defined as time from surgical resection to the detection of pelvic recurrence by clinical or radiologic examinations. All patients were followed for a minimum of 5 years or until death (if occurring prior to 5 years). The median lengths of follow-up after LE and SR were 6.3 and 6.4 years (T1 tumors), and 5.7 and 6.3 years (T2 tumors).
TABLE 1. Surgical Procedures as Defined by the International Classification of Diseases-9-Clinical Modification (ICD-9-CM) Codes
Statistical Analysis
After determining the annual rates of LE from 1989 to 2003, the Cochran-Armitage trend test on 1 degree of freedom was used to detect changes in rates over time. In the special study, patient and tumor characteristics, and perioperative outcomes were compared between LE and SR by one-way ANOVA for continuous variables or χ2 test for categorical values. All statistical tests were two-sided with a significance level of 0.05. Overall survival (OS), disease-specific survival (DSS), and LR rates were analyzed using the Kaplan-Meier method. Estimates of statistical power used the two-sided log-rank test for equity of survival curves with a significance level of 0.05 and a power level of 0.9. Prognostic factors for OS and LR were identified using Cox proportional hazard models. All data analyses were performed by standard statistical software (SPSS for Windows, Advanced Statistics, release 12; SPSS Inc., Chicago, IL).
RESULTS
Time Trends
From 35,179 patients with stage I rectal cancer, T1 lesions were identified in 43.8% and T2 in 56.2%. Local excision was performed in a significantly higher proportion of patients with T1 tumors (37.9%) than T2 tumors (12%) for the overall study period (P < 0.001). From 1989 to 2003, rates of LE significantly increased, both for T1 lesions (26.6% in 1989 vs. 43.7% in 2003, P < 0.001) and T2 lesions (5.8% in 1989 vs. 16.8% in 2003, P < 0.001; Fig. 2).
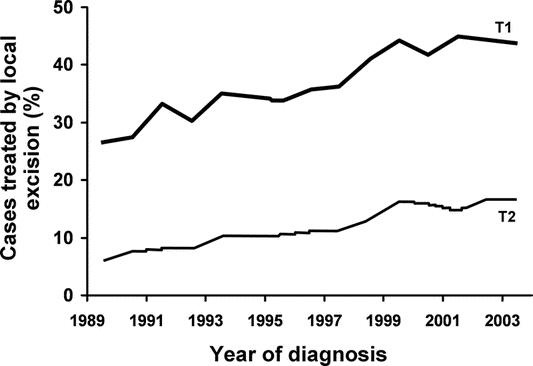
FIGURE 2. Proportion of patients reported to the National Cancer Database with T1 (n = 15,313) and T2 (n = 19,766) AJCC stage I rectal cancer treated by local excision between 1989 and 2003.
Further results, described below, pertain to the 1094 patients with T1 and 1030 patients with T2 tumors investigated in the special study.
Patient and Tumor Characteristics
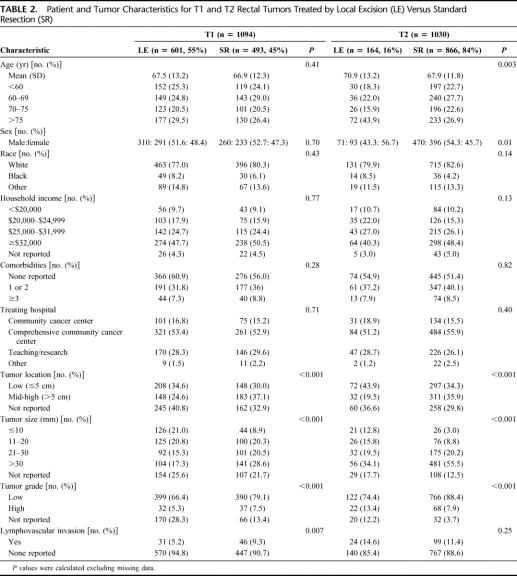
In the special study, 55% of T1 tumors and 16% of T2 tumors were treated by LE. In patients with T1 tumors, the choice of LE versus SR was not influenced by patient age, sex, race/ethnicity, household income, the number of comorbid conditions, or hospital type (Table 2). However, T2 patients treated with LE were more likely to be elderly (P = 0.003) and female (P = 0.01). For both T1 and T2 tumors, selection for LE favored low-lying and small (≤2 cm in size) lesions; however, more low-grade T1 tumors but high-grade T2 tumors were treated by LE.
TABLE 2. Patient and Tumor Characteristics for T1 and T2 Rectal Tumors Treated by Local Excision (LE) Versus Standard Resection (SR)
Preoperatively, 21.3% of T1 and 26.2% of T2 tumors were staged by endorectal ultrasound or magnetic resonance imaging. Surgical resection failed to achieve R0 status (negative gross and microscopic margins) in 35 (4.5%, 29 of T1 and 16 of T2) patients after LE and only 11 (0.81%, 3 of T1 and 8 of T2) patients after SR (P < 0.001). Among LE procedures, endoscopic polypectomy was performed in 178 (17%) T1 tumors and 25 (2.4%) T2 tumors.
Perioperative Outcomes
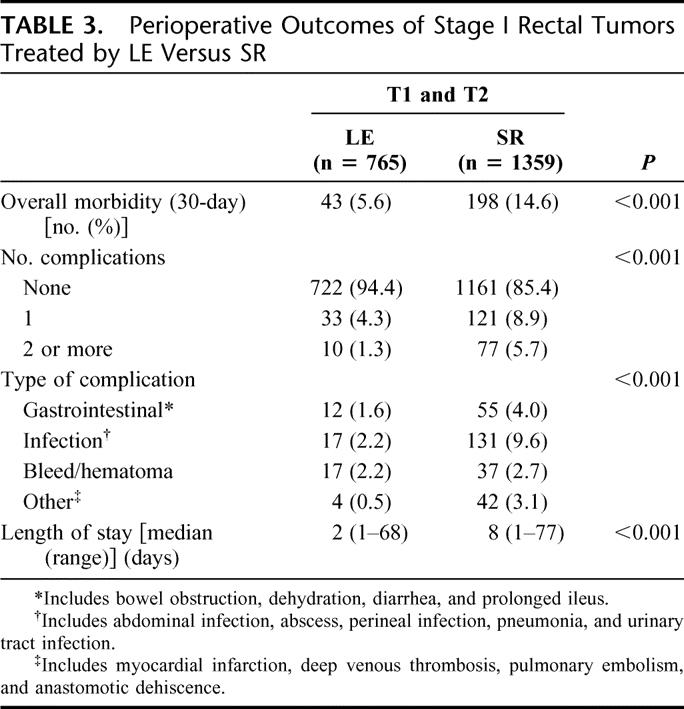
Postoperative mortality was low after both LE (4 patients, 0.5%) and SR (25 patients, 1.8%). However, the overall morbidity rate was significantly lower after LE than SR (Table 2). Gastrointestinal and infectious complications were more common after SR than LE, although the frequency of bleeding/hematoma was similar (Table 3). Significantly longer hospital stay occurred after SR (Table 3).
TABLE 3. Perioperative Outcomes of Stage I Rectal Tumors Treated by LE Versus SR
Oncologic Outcomes
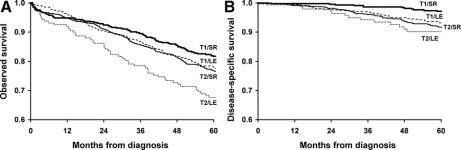
For T1 tumors, the OS after LE versus SR were: 77.4 versus 81.7% at 5 years and 61.7 versus 66.3% at 8 years, and the survival curves did not statistically differ (P = 0.09; Fig. 3A). However, the 5-year DSS was significantly lower after LE (93.2 vs. 97.2%, P = 0.004; Fig. 3B). For T2 tumors, the OS was lower after LE than SR at 5 years (67.6 vs. 76.5%; P = 0.01; Fig. 3A), while the DSS did not differ (90.2 vs. 91.7% at 5 years; P = 0.95; Fig. 3B).
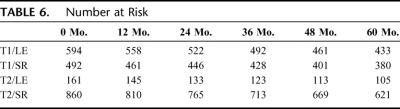
FIGURE 3. Overall survival (OS, A) and disease-specific survival (DSS, B) for T1 and T2 rectal cancer patients treated by local excision (LE) versus standard resection (SR). The number of patients at risk in each group is at time points for every 12 months. (Table 6)
For T1 tumors, disease recurrence was reported as local/regional in 46 (8.2%) and 20 (4.3%), and as distant in 20 (3.6%) and 12 (2.6%) patients after LE and SR respectively. Among T2 tumors, tumor recurrence was local/regional in 20 (12.6%) and 60 (7.2%), and distant in 8 (5.0%) and 64 (7.7%) patients treated by LE and SR respectively. Patients who underwent R0 resection were analyzed for local-regional recurrence over time (Fig. 4). For T1 tumors, the 5-year LR was 12.5% after LE, significantly higher than that after SR (6.9%; P = 0.003), with the difference persisting through 8 years of follow-up (14.3 vs. 8.5%; P = 0.007). For T2 tumors, 5-year LR rates were also higher after LE (22.1 vs. 15.1% in SR; P = 0.01).
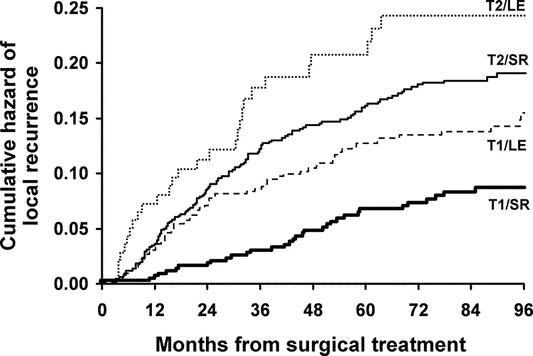
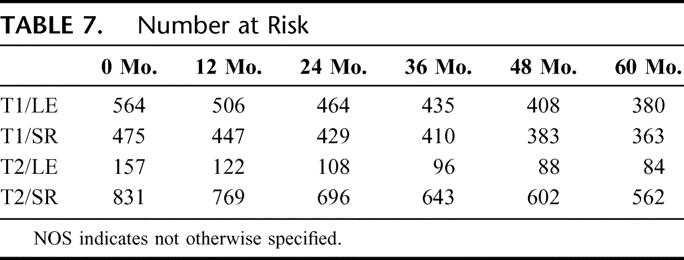
FIGURE 4. Cumulative hazard of local disease recurrence among T1 and T2 rectal cancer patients following R0 surgical resection. The number of patients at risk in each group is at time points for every 12 months. (Table 7)
Factors Influencing Oncologic Outcomes
For both T1 and T2 tumors, significant predictors of poor 5-year OS included: age ≥75-year-old and the presence of 2 or more comorbid conditions (Table 4), but not the type of surgery (T1: hazard ratio, 0.88; 95% CI, 0.68–1.14; T2: hazard ratio, 0.83; 95% CI, 0.61–1.11). Other covariates, including sex, race/ethnicity, income, tumor size, tumor location, histologic grade, or evidence of invasion, did not affect OS (all 95% CIs included 1.0).
TABLE 4. Significant Predictors of Overall Survival and Local Tumor Recurrence for T1 and T2 Rectal Tumors
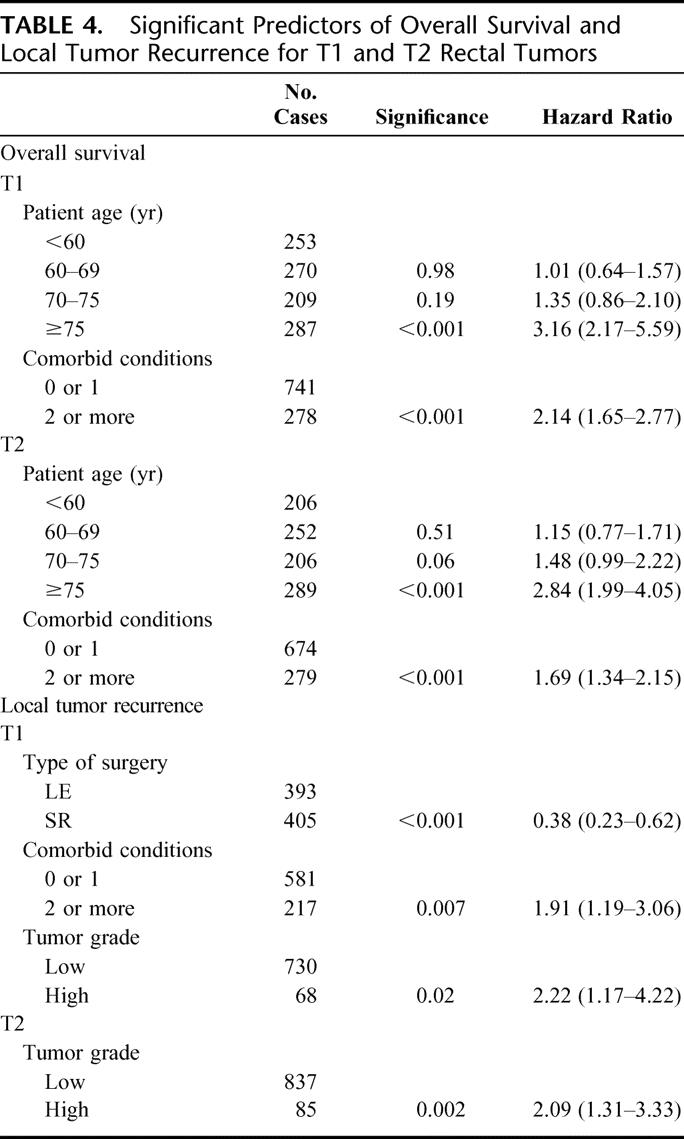
Local failure of T1 tumors was significantly predicted by the type of surgery (Table 4), with LE conferring a nearly 3-fold increase in the risk of LR. Other significant predictors included high tumor grade and the presence of 2 or more comorbidities (Table 4). In T2 tumors, factors predictive of LR included only tumor grade (Table 4). The effect of surgery did not reach statistical significance (hazard ratio, 0.69; 95% CI, 0.44–1.07).
DISCUSSION
Although the rate of LE for stage I rectal cancer has nearly doubled over a 15-year period (almost half of the patients with T1 tumors are currently being treated with LE) until now, there has been no level I or II evidence to support this practice. This large nationwide cohort study represents the best available information defining the perioperative morbidity and oncologic outcomes associated with LE. The NCDB data were collected from nearly 500 hospitals representing diverse practices utilizing both LE and SR for stage I rectal cancer. Furthermore, the quality of the data has met the approval standards of the CoC, including standardized tumor staging, oncologic outcome assessments, and long-term follow-up. The special study (1994–1996) offered a unique opportunity to examine patient and tumor factors influencing the selection of surgical therapy on a national level.
This study quantified the increasing utilization of LE from 1989 to 2003. Furthermore, it confirmed the significant perioperative benefits of LE: less postoperative morbidity and shorter length of hospital stay. Focused examination of the surgical practice during 1994 to 1996 revealed that selection of patients for LE favored tumor characteristics (low-lying, small, low-grade, without evidence of invasion) for T1 rectal cancer, but was more discriminating based on both patient and tumor factors (advanced age, female, low-lying, small, but high-grade tumors) for T2 rectal cancer.
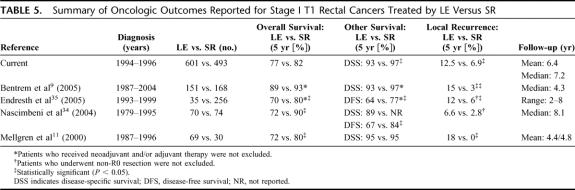
Patients with T1 tumors treated with SR have historically enjoyed excellent oncologic outcomes. In contrast, this study demonstrates that despite favorable tumor characteristics, local failure rates were significantly higher, and DSS lower after LE, as compared with SR. A nearly 3-fold risk for local failure was associated with LE compared with SR, even after adjusting for covariates. These results are not dissimilar to those reported in the 1980s22,23 and recently reestablished (Table 5). Indeed, the LR rates after LE in our study (12.5% in T1 and 22.2% in T2) are less alarming than the 15% to 40% described by others.10,11,17,18,22–24 Taken together, these findings establish that LE is associated with inferior tumor control than SR.
TABLE 5. Summary of Oncologic Outcomes Reported for Stage I T1 Rectal Cancers Treated by LE Versus SR
Despite worse tumor control, a paradoxical, yet unequivocal, increase in the practice of LE for T1 tumors has been observed. Since tumor control is considered an important endpoint for rectal cancer, it is difficult to reconcile the practice trends with inferior oncologic outcomes; several explanations are plausible. It is possible, but not probable, that patients and physicians have had insufficient information or misinformation regarding the oncologic inadequacy of LE. Alternatively, OS rather than disease control may be considered the critical endpoint by patients. The endpoint of OS for T1 tumors was not compromised after LE versus SR with extended follow-up (up to 8 years) in our study, suggesting that in the time period 1994 to 1996, the criteria applied by surgeons in selecting patients for LE have been largely appropriate with respect to OS. Finally, and most likely, complex decision-making processes are occurring and the answer to the increased trend of LE resides in nononcologic factors, including the significant perioperative benefits found in this study, as well as functional and quality of life gains associated with stoma avoidance and sphincter preservation.25
In summary, while the paradoxical increase in the practice of LE for T1 tumors may not be oncologically sound, it is evident that physicians and patients are accepting the trade-offs among local recurrence, overall survival, and perioperative and long-term functional benefits. With proper selection of T1 tumors with favorable biology, acceptable long-term OS can be achieved with LE. However, in exchange for significantly reduced morbidities in the perioperative period, LE is associated with significantly worse local control in the long-term. In current practice, for each individual patient, the benefits of LE must be balanced against the heightened risks of local failure. For the future, research efforts should be placed on enhancing the accuracy of clinical decision-making. This can be accomplished using multiple strategies, including the construction of decision analyses with information from large, standardized databases, such as provided in this report. Additionally, the staging of rectal cancer can be enhanced through the wider availability of endorectal ultrasound and magnetic resonance imaging and/or through the use of genomic and proteomic profiling. Patient interest in LE should be matched by clinical research efforts aimed at optimizing its oncologic outcomes.
Drawing generalized conclusions regarding the use of LE for T2 tumors is more difficult. The significantly smaller sample size of T2 tumors treated by LE in our study limited the ability to assess the true oncologic impact of LE. Although the 5-year OS was inferior after LE versus SR, OS was strongly impacted by nononcologic factors related to patient selection (ie, elderly age and multiple comorbidities) rather than the type of surgery. The presence of strong selection bias is further evidenced by the higher proportion of high-grade T2 tumors treated by LE. While reported survival rates are consistent with those previously reported,11 all studies are limited by selection biases inherent in their observational nature and thus highlight the need for prospective investigations. Furthermore, our study reported substantial rates of local failure after either procedure: 22.1% after LE and 15.1% after SR. Recently, adjuvant or neoadjuvant therapy, commonly used to treat advanced-stage rectal cancers, has been increasingly considered for T2 tumors treated by LE.12,18,26 However, substantial pelvic organ complications (eg, diarrhea, proctitis, fistula formation, and stricture, pelvic fractures)27–31 and dysfunctions32 have been attributed to chemoradiation. The balance between ameliorating adverse oncologic risks and compromising functional outcomes should be adjudicated in the setting of clinical trials involving stage I rectal cancers. Such a trial of neoadjuvant chemoradiation and LE for T2N0 rectal tumors (ACOSOG Z6041) is currently underway at the American College of Surgeons Oncology Group.33 Thus, for T2 tumors, the incomplete understanding of the oncologic outcomes of LE alone based on available retrospective data, and the still ongoing investigation of LE plus additional therapy, caution against a wider application of LE in this patient population.
CONCLUSION
On a national level, the proportion of patients with stage I rectal cancer treated by LE has dramatically increased over time. For patients with T1 rectal tumors, the selection for LE favored small, low-grade, distal tumors without evidence of invasion. Appropriately selected patients may expect acceptable OS after LE but experience a nearly 3-fold increased risk of local failure in the long-term, in exchange for reduced morbidities in the short-term. Thus, the decision regarding LE versus SR in this patient population requires an individualized analysis of the benefits and risks. For patients with T2 tumors, the selection for LE was highly restrictive based on both patient and tumor factors. The observed increase in local failure and compromise in OS may reflect selection bias rather than the true oncologic impact of LE versus SR but raise caution against an expanded practice of LE in T2 tumors.
TABLE 6. Number at Risk
TABLE 7. Number at Risk
Footnotes
Presented at the American Society of Clinical Oncology Gastrointestinal Cancer Symposium, San Francisco, California. January 26–28, 2006.
The National Cancer Database is jointly funded and supported by the American College of Surgeons and the American Cancer Society. The design and conduct of the study as well as the data analyses were the sole responsibilities of the study investigators. Supported by the Ruth L. Kirschstein National Research Service Award Training Grant from the National Institutes of Health (T32CA101695, to Y.N.Y.); the American Society of Clinical Oncology Career Development Award (to N.N.B.); and the American College of Surgeons Oncology Group Grant from the National Cancer Institute (U10CA76001; to H.N.).
Reprints: Heidi Nelson, MD, Division of Colon and Rectal Surgery, Department of Surgery, Mayo Clinic, 200 First Street SW, Rochester, MN 55905. E-mail: nelson.heidi@mayo.edu.
REFERENCES
- 1.Ota DM, Jacobs L, Kuvshinoff B. Rectal cancer: the sphincter-sparing approach. Surg Clin North Am. 2002;82:983–993. [DOI] [PubMed] [Google Scholar]
- 2.Bleday R. Local excision of rectal cancer. World J Surg. 1997;21:706–714. [DOI] [PubMed] [Google Scholar]
- 3.Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–596. [DOI] [PubMed] [Google Scholar]
- 4.Heriot AG, Grundy A, Kumar D. Preoperative staging of rectal carcinoma. Br J Surg. 1999;86:17–28. [DOI] [PubMed] [Google Scholar]
- 5.Rectal Cancer. Clinical practice guidelines in oncology: National Comprehensive Cancer Network.; Version 2, 2006. Available at www.nccn.org. Accessed June 15, 2006.
- 6.Greene FL, Page DL., eds. Colon and rectum. In: American Joint Commission on Cancer Staging Manual. New York: Springer, 2002:113–119. [Google Scholar]
- 7.Nascimbeni R, Burgart LJ, Nivatvongs S, et al. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002;45:200–206. [DOI] [PubMed] [Google Scholar]
- 8.Blumberg D, Paty PB, Guillem JG, et al. All patients with small intramural rectal cancers are at risk for lymph node metastasis. Dis Colon Rectum. 1999;42:881–885. [DOI] [PubMed] [Google Scholar]
- 9.Bentrem DJ, Okabe S, Wong WD, et al. T1 adenocarcinoma of the rectum: transanal excision or radical surgery? Ann Surg. 2005;242:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Aguilar J, Mellgren A, Sirivongs P, et al. Local excision of rectal cancer without adjuvant therapy: a word of caution. Ann Surg. 2000;231:345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellgren A, Sirivongs P, Rothenberger DA, et al. Is local excision adequate therapy for early rectal cancer? Dis Colon Rectum. 2000;43:1064–1071; discussion 1071–1074. [DOI] [PubMed]
- 12.Sengupta S, Tjandra JJ. Local excision of rectal cancer: what is the evidence? Dis Colon Rectum. 2001;44:1345–1361. [DOI] [PubMed] [Google Scholar]
- 13.Hahnloser D, Wolff BG, Larson DW, et al. Immediate radical resection after local excision of rectal cancer: an oncologic compromise? Dis Colon Rectum. 2005;48:429–437. [DOI] [PubMed] [Google Scholar]
- 14.Friel CM, Cromwell JW, Marra C, et al. Salvage radical surgery after failed local excision for early rectal cancer. Dis Colon Rectum. 2002;45:875–879. [DOI] [PubMed] [Google Scholar]
- 15.Kim DG, Madoff RD. Transanal treatment of rectal cancer: ablative methods and open resection. Semin Surg Oncol. 1998;15:101–113. [DOI] [PubMed] [Google Scholar]
- 16.Varma MG, Rogers SJ, Schrock TR, et al. Local excision of rectal carcinoma. Arch Surg. 1999;134:863–867; discussion 867–868. [DOI] [PubMed]
- 17.Taylor RH, Hay JH, Larsson SN. Transanal local excision of selected low rectal cancers. Am J Surg. 1998;175:360–363. [DOI] [PubMed] [Google Scholar]
- 18.Paty PB, Nash GM, Baron P, et al. Long-term results of local excision for rectal cancer. Ann Surg. 2002;236:522–529; discussion 529–530. [DOI] [PMC free article] [PubMed]
- 19.Chakravarti A, Compton CC, Shellito PC, et al. Long-term follow-up of patients with rectal cancer managed by local excision with and without adjuvant irradiation. Ann Surg. 1999;230:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jessup JM, Stewart AK, Menck HR. The National Cancer Data Base report on patterns of care for adenocarcinoma of the rectum, 1985–95. Cancer. 1998;83:2408–2418. [DOI] [PubMed] [Google Scholar]
- 21.Jessup JM, Steele GD Jr, Winchester DP. Introduction. In: Steele G Jr, Jessup JM, Menck HR, et al, eds. National Cancer Data Base Annual Review of Patient Care, 1995. Atlanta: American Cancer Society, 1995:1–11. [Google Scholar]
- 22.Killingback MJ. Indications for local excision of rectal cancer. Br J Surg. 1985;72(suppl):54–56. [DOI] [PubMed] [Google Scholar]
- 23.Biggers OR, Beart RW Jr, Ilstrup DM. Local excision of rectal cancer. Dis Colon Rectum. 1986;29:374–377. [DOI] [PubMed] [Google Scholar]
- 24.Coco C, Magistrelli P, Granone P, et al. Conservative surgery for early cancer of the distal rectum. Dis Colon Rectum. 1992;35:131–136. [DOI] [PubMed] [Google Scholar]
- 25.Temple LK, Naimark D, McLeod RS. Decision analysis as an aid to determining the management of early low rectal cancer for the individual patient. J Clin Oncol. 1999;17:312–318. [DOI] [PubMed] [Google Scholar]
- 26.Rengan R, Paty P, Wong WD, et al. Distal cT2N0 rectal cancer: is there an alternative to abdominoperineal resection? J Clin Oncol. 2005;23:4905–4912. [DOI] [PubMed] [Google Scholar]
- 27.Holm T, Rutqvist LE, Johansson H, et al. Postoperative mortality in rectal cancer treated with or without preoperative radiotherapy: causes and risk factors. Br J Surg. 1996;83:964–968. [DOI] [PubMed] [Google Scholar]
- 28.Sauer R. Adjuvant and neoadjuvant radiotherapy and concurrent radiochemotherapy for rectal cancer. Pathol Oncol Res. 2002;8:7–17. [DOI] [PubMed] [Google Scholar]
- 29.Martling A, Holm T, Johansson H, et al. The Stockholm II trial on preoperative radiotherapy in rectal carcinoma: long-term follow-up of a population-based study. Cancer. 2001;92:896–902. [DOI] [PubMed] [Google Scholar]
- 30.Peeters KC, van de Velde CJ, Leer JW, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients. A Dutch colorectal cancer group study. J Clin Oncol. 2005;23:6199–6206. [DOI] [PubMed] [Google Scholar]
- 31.Baxter NN, Habermann EB, Tepper JE, et al. Risk of pelvic fractures in older women following pelvic irradiation. JAMA. 2005;294:2587–2593. [DOI] [PubMed] [Google Scholar]
- 32.Kollmorgen CF, Meagher AP, Wolff BG, et al. The long-term effect of adjuvant postoperative chemoradiotherapy for rectal carcinoma on bowel function. Ann Surg. 1994;220:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ACOSOG Z6041: A phase II trial of neoadjuvant chemoradiation and local excision for uT2uN0 rectal cancer. Available at www.acosog.org/studies/organ_site/gastrointestinal/index.jsp. Accessed June 15, 2006.
- 34.Nascimbeni R, Nivatvongs S, Larson DR, et al. Long-term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum. 2004;47:1773–1779. [DOI] [PubMed] [Google Scholar]
- 35.Endreseth BH, Myrvold HE, Romundstad P, et al. Transanal excision vs. major surgery for T1 rectal cancer. Dis Colon Rectum. 2005;48:1380–1388. [DOI] [PubMed] [Google Scholar]