Abstract
Ablation of nonmuscle myosin (NM) II-B in mice during embryonic development leads to marked enlargement of the cerebral ventricles and destruction of brain tissue, due to hydrocephalus. We have identified a transient mesh-like structure present at the apical border of cells lining the spinal canal of mice during development. This structure, which only contains the II-B isoform of NM, also contains β-catenin and N-cadherin, consistent with a role in cell adhesion. Ablation of NM II-B or replacement of NM II-B with decreased amounts of a mutant (R709C), motor-impaired NM II-B in mice results in collapse of the mesh-like structure and loss of cell adhesion. This permits the underlying neuroepithelial cells to invade the spinal canal and obstruct cerebral spinal fluid flow. These defects in the CNS of NM II-B–ablated mice seem to be the cause of hydrocephalus. Interestingly, the mesh-like structure and patency of the spinal canal can be restored by increasing expression of the motor-impaired NM II-B, which also rescues hydrocephalus. However, the mutant isoform cannot completely rescue neuronal cell migration. These studies show that the scaffolding properties of NM II-B play an important role in cell adhesion, thereby preventing hydrocephalus during mouse brain development.
INTRODUCTION
Congenital hydrocephalus affects one to three humans per 1000 live births. The results of this abnormality can be devastating in that severe, untreated hydrocephalus can destroy brain tissue and thereby lead to mental retardation. Hydrocephalus occurs when there is an increase in pressure in the ventricular chambers due to a blockage in the circulation of cerebral spinal fluid (CSF) or when there is an increase in production or decrease in the absorption of the CSF. The defect may be accompanied by an obstructed aqueduct of Sylvius, the narrow channel connecting the third and fourth brain ventricles (noncommunicating hydrocephalus), or by a normal aqueduct (communicating hydrocephalus). The pathogenesis of most cases of communicating hydrocephalus is largely unknown (for reviews, see Perez-Figares et al., 2001; Crews et al., 2004).
Nonmuscle myosin (NM) II, one of the major cytoskeletal motor proteins, plays an important role in cell migration (Svitkina et al., 1997; Ma et al., 2004; Even-Ram et al., 2007; Vicente-Manzanares et al., 2007), cell–cell adhesion (Conti et al., 2004; Shewan et al., 2005; Giannone et al., 2007), and cell division (De Lozanne and Spudich, 1987; Takeda et al., 2003; Bao et al., 2005). The molecular structure of NM II is a hexamer consisting of a pair of myosin heavy chains (200 kDa) and two pairs of light chains (20 and 17 kDa). In mammals, three isoforms of the nonmuscle myosin heavy chain (NMHC) have been identified, NMHC II-A, II-B, and II-C, encoded by three different genes, Myh 9, Myh 10, and Myh 14 in humans. The NMHCs share a 60–80% identity in amino acids, but they show significant differences in their motor activities (Golomb et al., 2004; Kim et al., 2005). In general, all three isoforms are ubiquitously expressed in vertebrates with overlapping as well as different expression patterns at the cellular level. Compared with NMHC II-A and II-C, NMHC II-B is enriched in the brain, especially in neural cells. Mice ablated for NMHC II-B (B−/B−) die during the later stages of embryonic development, between embryonic day (E)14.5 and postnatal day (P)0, with abnormalities in the heart and brain (Tullio et al., 1997; Tullio et al., 2001). All B−/B− mice develop a severe, progressive hydrocephalus accompanied by disruption of the cerebral ventricular surface and distortion of the brain architecture. The causes of congenital hydrocephalus remain unknown.
In an effort to produce a mouse model of a human disease, we have generated knockin mice that carry a point mutation in the motor domain of NMHC II-B (Ma et al., 2004). We did this by mutating Arg709 to Cys, thereby duplicating a mutation that had previously been shown to occur in humans for NMHC II-A (Heath et al., 2001). Interestingly, the homologous residue has also been shown to be mutated in NMHC II-C, although, in this case, to a serine residue (Donaudy et al., 2004). In a previous report, we characterized the motor activity of a heavy meromyosin (HMM) derived from the R709C mutant myosin II-B (Kim et al., 2005). We found that, compared with wild-type HMM II-B, the mutant had only 30% of the actin-activated Mg-ATPase activity. The R709C HMM II-B also showed a very high affinity for actin and failed to propel actin filaments in the in vitro motility assay.
In generating the II-B mutant mice, we found that the presence of the cassette encoding Neomycin resistance in the mutant allele resulted in a 75% reduction in the expected mutant myosin II-B protein expression in the homozygous (BCN/BCN) mice (where C represents the R-to-C mutation, and N represents the Neomycin resistance cassette). These mice survived up to P20, and they were characterized with respect to the abnormal migration of three different groups of neurons (Ma et al., 2004). Similar to B−/B− mice, they also developed a severe hydrocephalus, but more gradually than the B−/B− mice. To understand the underlying cause of hydrocephalus in NMHC II-B–ablated and mutated mice, we made a comparative analysis of the B−/B− mice and R709C mutant mice. The purpose of the present study was to identify which properties of NM II-B are important in mouse brain development. These studies should also help to elucidate the cause of human congenital hydrocephalus.
MATERIALS AND METHODS
NMHC II-B R709C Mutant Mice
Generation of BCN/BCN and BC/BC mice was described in a previous article (Ma et al., 2004). The BCN/BCN mice are maintained in a mixed background of 129/Sv and C57BL/6. To generate BC/BC mice, the floxed Neomycin resistance cassette was removed by crossing the B+/BCN mice with BALB/C CMV-Cre mice (The Jackson Laboratory, Bar Harbor, ME). All procedures were conducted using an approved animal protocol in accordance with the National Heart, Lung, and Blood Institute Animal Care and Use Committee.
Histology and Immunofluorescence Staining
The embryos were collected in phosphate-buffered saline (PBS) and directly immersed in 4% paraformaldehyde in PBS, pH 7.4, overnight. Paraffin sections at a thickness of 5 μm were prepared by Histoserv (Germantown, MD) for hematoxylin and eosin (H&E) or immunofluorescence staining. The following primary antibodies were used in this study: polyclonal antibodies against NMHC II-A (1:1000), II-B (1:3000), and II-C (1:1000; Phillips et al., 1995; Buxton et al., 2003); monoclonal antibodies against N-cadherin (1:200; Zymed Laboratories, South San Francisco, CA), β-catenin (1:200; Zymed Laboratories), serine-19 phospho-regulatory myosin light chain (pMLC20) (1:100; Cell Signaling Technology, Danvers, MA), atypical protein kinase C (aPKC)λ (1:100; BD Biosciences, San Diego, CA), and myosin light chain kinase (1:1000; Sigma-Aldrich, St. Louis, MO). Fluorescence secondary antibodies used were Alexa 488 goat anti-rabbit immunoglobulin G (IgG) or Alexa 594 goat anti-mouse IgG (1:250; Invitrogen, Carlsbad, CA). The images were collected using an SP Confocal microscope (Leica, Wetzlar, Germany). In all cases, when possible, comparison was made among littermates. For each genotype in each experiment, we analyzed at least five mice.
RESULTS
Hydrocephalus in BCN/BCN and B−/B− Mice
To study the role of NMHC II-B and its relationship to the etiology of hydrocephalus, we chose two mouse models. Previous work analyzing both NMHC II-B–ablated mice (B−/B− mice) and hypomorphic mice with a point mutation at the motor domain of II-B (BCN/BCN mice) has shown that both lines develop hydrocephalus during embryogenesis (Tullio et al., 2001; Ma et al., 2004). However, the BCN/BCN mice develop hydrocephalus more gradually than the B−/B− mice, and with less destruction and distortion of the ventricles and the surrounding brain tissue. Figure 1 shows sagittal and coronal sections of BCN/BCN mouse brains showing evidence of mild dilatation of the cerebral ventricles at E14.5 (Figure 1b) and increased dilatation of the cerebral ventricles as well as the aqueduct of Sylvius at P0 (Figure 1, d and f). As reported previously, B−/B− mice show marked enlargement of the cerebral ventricles as early as E12.5 and a severe disruption and distortion of the ventricles by P0 (see figure 9 in Tullio et al., 2001).
Figure 1.
Evidence for hydrocephalus in BCN/BCN mice. H&E staining of embryonic mouse brain sections. (a and b) Sagittal sections of B+/B+ (a) and BCN/BCN (b) brains at E14.5 show mild dilatation of the cerebral ventricles (b) and abnormal protrusion of facial neurons (b, arrow) into the fourth ventricle of BCN/BCN mice. (c–f) Coronal sections of B+/B+ (c and e) and BCN/BCN (d and f) brains at P0 show significant dilatation of the cerebral ventricles as well as the aqueduct of Sylvius (AQ) in a BCN/BCN mouse compared with a B+/B+ mouse. LV, lateral ventricle; 3V, third ventricle.
Figure 9.
Abnormal migration of pontine neurons in BC/BC mice. H&E-stained images of sagittal brain sections from B+/B+ and BC/BC mice at E16.5. (a and b) In B+/B+ mice, although some of the pontine neurons were still located in their migrating trajectory (a, arrows), many of them have arrived at their final destination (b, arrow). (c and d) In BC/BC mice by E16.5, many of the pontine neurons have accumulated along their migrating path near to where they were generated (c, arrows), and none of the pontine neurons had reached their destination (d, arrow).
To locate the earliest abnormal changes in the CNS of both B−/B− and BCN/BCN mice, we analyzed serial sections of the brain and spinal cord starting at E11.5. Figure 2 shows cross sections of the spinal canal for B+/B+, BCN/BCN, and B−/B− mice at E11.5 and 12.5. Note that, in contrast to B+/B+ and BCN/BCN mice at E11.5, the spinal canal of the B−/B− mouse is narrow and partially obstructed (Figure 2c). Moreover, by E12.5, the canal is almost entirely obliterated in B−/B− mice (Figure 2f), and it has become partially obstructed at this stage in BCN/BCN mice, where there are neuroepithelial cells protruding into the canal (Figure 2e). Figure 3 shows a cross section of the spinal canal of a BCN/BCN mouse at E14.5 compared with a B+/B+ littermate. At this time, the spinal canal of BCN/BCN mice is completely obstructed by neuroepithelial cells, as shown in both the low-power (Figure 3b) and magnified cross sections of the spinal canal (Figure 3d). As shown in Figure 1, unlike the findings in the spinal canal, there is no evidence for obstruction of the cerebral ventricles at this time. Thus, the earliest change in the CNS that seems to be directly related to the development of hydrocephalus is the obstruction of the spinal canal beginning at E11.5 for B−/B− mice and E12.5 for BCN/BCN mice.
Figure 2.
Blockage of the spinal canal in B−/B− and BCN/BCN mice. H&E-stained images of embryonic mouse spinal cords in cross section. (a–c) At E11.5, the spinal canals of B+/B+ and BCN/BCN mice are patent with intact ventricular surfaces (a and b), whereas the ventricular surface of the spinal canal in B−/B− mice is disrupted with underlying neuroepithelial cells invading the canal (c). (d–f) At E12.5, the B+/B+ canal remains patent (d), the BCN/BCN canal is partially blocked by protruding neuroepithelial cells (e), and the B−/B− spinal canal is almost completely obstructed (f).
Figure 3.
Complete obstruction of the spinal canal in BCN/BCN mice. H&E-stained images of E14.5 mouse spinal cords in cross section. The spinal canal from BCN/BCN mice is completely obstructed (b); in comparison, the spinal canal of a B+/B+ littermate is patent (a). c and d show magnified images for B+/B+ and BCN/BCN mice, respectively.
A Mesh-like Structure at the Apical Border of Neuroepithelial Cells Lining the Spinal Canal
To understand the underlying cause of hydrocephalus in these mice, we examined the spinal cord by using antibodies to NMHC II-A, II-B, and II-C. Figure 4 uses confocal immunofluorescence microscopy to study the localization of all three NM II isoforms as well as N-cadherin in the spinal cords of B+/B+ and BCN/BCN mice at E12.5. Figure 4b shows that NMHC II-B is enriched in the neuroepithelial layers (bracket) surrounding the spinal canal compared with other regions of the spinal cord. Alternatively, as seen in Figure 4c, NMHC II-C is less abundant in the neuroepithelial cells surrounding the canal (bracket) compared with more peripheral regions. NMHC II-A is more enriched in the vasculature than in neural cells (Figure 4a, arrows). Examination of the neuroepithelial cells lining the spinal canal by higher magnification shows that NMHC II-B is the only NMHC II isoform present in the apical border of these cells, because they show up yellow (Figure 4e, arrow) when probed with antibodies to NMHC II-B (green) and N-cadherin (red), indicating the presence of both proteins. Of note is that both N-cadherin and NMHC II-B are more enriched at the apical border than at the cell–cell boundaries in the neuroepithelial layer (Figure 4e; also see Figure 5, f–o). In contrast, when the same cells are stained with antibodies to NMHC II-A (Figure 4, a and d) or II-C (Figure 4, c and f) along with N-cadherin, the cells bordering the canal are red, indicating the presence of cadherin alone. Figure 4, g–i, shows sections of the spinal canal of BCN/BCN mice at E12.5. These panels confirm that NMHC II-B is the sole NMHC II isoform present in the cells lining the spinal canal. They also show that, in BCN/BCN mice, which contain reduced amounts of the mutant form of NMHC II-B, the apical adhesion structure has become discontinuous and that the underlying neuroepithelial cells have protruded into the canal (* in Figure 4, g–i).
Figure 4.
Immunofluorescence confocal images of mouse spinal cords in cross section at E12.5. (a–c, enlarged in d–f) Costaining of the antibodies for NMHC II-A (a and d, green), II-B (b and e, green), and II-C (c and f, green) with antibodies for N-cadherin (a–f, red) in B+/B+ mice shows that only NMHC II-B stains significantly at the ventricular surface (b and e, arrow) of the developing mouse spinal canal together with N-cadherin (b and e, yellow). NMHC II-A predominantly stains the vasculature (a and d, green, arrows), and NMHC II-C stains neural cells but not the apical border (c and f, green). The white brackets delineate the neuroepithelial cells (see text). (g–i) Distribution of NMHC II-A, II-B and II-C in BCN/BCN spinal cords further confirms the predominant expression of NMHC II-B (h) rather than II-A (g) and II-C (i) at the apical border of the ventricular surface. The white asterisks indicate neuroepithelial cells abnormally protruding into the spinal canal in a BCN/BCN mouse.
Figure 5.
Transitory presence of a mesh-like structure at the border of cells lining the spinal canal between E8.5 to E14.5. (a–e) H&E-stained cross sections of representative spinal cords between E8.5 and E14.5 show the changes in the neuroepithelial cell layer (brackets). By E14.5, the spinal canal is lined by a single layer of differentiated ependymal cells. (f–j) Immunofluorescence confocal images of the neuroepithelial cells lining the spinal canal (indicated by dash-lined box in c), and stained for N-cadherin, show a mesh-like adhesion structure that is most obvious at the apical border between E9.5 and E12.5 (g–i). This structure is not obvious at E8.5, and it is markedly reduced at E14.5 (f and j). (k–t) Immunofluorescence confocal images of the neuroepithelial cells lining the spinal canal stained for NMHC II-B (k–o) and β-catenin (p–t) show staining patterns at the apical border of the spinal canal similar to N-cadherin.
We next directed our attention to a mesh-like structure located at the apical border of the neuroepithelial cells present in the developing spinal canal. To examine the time course for the formation of this structure, we analyzed wild-type spinal cords between E8.5 and E14.5. Figure 5 shows sections examined by both light and immunofluorescence microscopy. The former shows the changes in the neuroepithelial cell layers surrounding the spinal canal during this period (Figure 5, a–d, brackets). The latter sections show the presence of a mesh-like structure at the apical border of the neuroepithelial cells in the spinal cord, which is transient, being generated between E8.5 and E9.5 and disappearing by E14.5. Note that this structure is enriched for both NM II-B and the adhesion proteins N-cadherin and β-catenin. It is important to note that, during this period of brain and spinal cord development, many of the neuroepithelial cells extend processes that reach the ventricular border and that the mesh-like structure we describe is present at the apical border of all these cells.
Figure 6, a–c, shows a magnified view of the mesh-like structure bordering the spinal canal immunostained for NM II-B (green), β-catenin (red) at E11.5 in a wild-type mouse. The importance of NM II-B to the structure of this complex is shown by the collapse of the mesh-like configuration in B−/B− mice at E11.5 (Figure 6, compare h and i with g). In BCN/BCN mice, the structure begins to fall apart at E11.5 (Figure 6f), and it is almost gone by E12.5 (Figure 6e). Figure 6f (arrow) shows an early example of the protrusion of a neuroepithelial cell into the spinal canal of a BCN/BCN mouse. This suggests that cell–cell adhesion has already become weakened in this area, although the general configuration still remains. Figure 6h shows that loss of NMHC II-B not only results in the collapse of the mesh-like structure at the apical border but also results in discontinuity of the apical structure lining the spinal canal. The disruption of the structure permits the underlying neuroepithelial cells to invade the spinal canal and obstruct the flow of CSF.
Figure 6.
Immunofluorescence confocal images of the neuroepithelial cells lining the spinal canal stained for NMHC II-B (green) and β-catenin (red) in B+/B+, B−/B−, and BCN/BCN mice. (a–c) The mesh-like cell adhesion structure in the B+/B+ spinal canal at E11.5. NMHC II-B (a, green) and β-catenin (b, red) are present in the structure and colocalize (c, yellow) at the apical border. (d–f) Disrupted adhesion structure in BCN/BCN mice (e) compared with the normal structure found in a B+/B+ littermate at E12.5 (d). At E11.5, the adhesion structure remains in BCN/BCN mice (f), but individual neuroepithelial cells are beginning to protrude through the adhesion complex (f, arrow). (g–i) Disrupted adhesion structures lining the B−/B− spinal canal at E11.5 (h and i) compared with that in B+/B+ canal (g). The adhesion structure is completely discontinuous in some regions (h) or collapsed in other regions (i) in B−/B− mice at E11.5.
Rescue of Hydrocephalus by Increasing Motor-impaired Mutant Nonmuscle Myosin II-B
We then asked what would be the effect of increasing the expression of the endogenous mutant NM II-B (R709C) to wild-type levels in II-B mutant (BC/BC) mice. As demonstrated previously, we increased the quantity of R709C II-B myosin to wild-type levels in BC/BC mice after removal of the Neomycin resistance cassette from the targeted alleles by crossing B+/BCN mice with CMV-Cre transgenic mice (Ma et al., 2004).
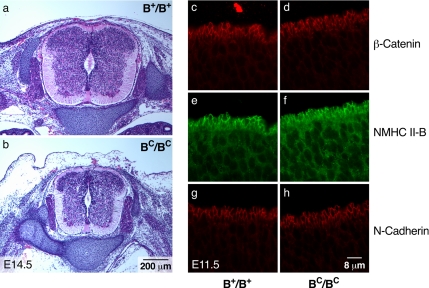
Interestingly, increasing the expression of R709C mutant NMHC II-B in BC/BC mice restored an intact spinal cord ventricular surface. Figure 7b shows that the spinal canal of BC/BC mice is free of obstruction, and Figure 7, d, f, and h, shows that the mesh-like structure present at the apical border of the cells lining the spinal canal has been restored. This finding suggests that the loss of the mesh-like structure and decrease in cell adhesion were not solely due to the mutation in NMHC II-B, but due to the 75% decrease in NMHC II-B. Importantly, no evidence for hydrocephalus was observed in BC/BC mice as late as E16.5, the time when they succumbed to cardiac defects. These results further support the idea that obstruction of the spinal canal seen in B−/B− and BCN/BCN mice during embryonic development causes the development of the hydrocephalus.
Figure 7.
Restoration of an intact ventricular surface in the spinal canal in BC/BC mice. (a and b) Cross sections of the spinal cord from B+/B+ (a) and BC/BC (b) mice stained with H&E show an unobstructed, patent spinal canal in both the B+/B+ and BC/BC mice at E14.5. (c–h) Immunofluorescence confocal images of E11.5 spinal cords from B+/B+ and BC/BC mice stained with β-catenin (c and d, red), NMHC II-B (e and f, green), and N-cadherin (g and h, red). The mutant NMHC II-B as well as adhesion molecules β-catenin and N-cadherin are normally localized and the mesh-like structure is restored to the apical border of the cells lining the spinal canal in BC/BC mice (d, f, and h).
Previous work has demonstrated that baculovirus-expressed HMM II-B with the R709C mutation has only 30% of the actin-activated Mg-ATPase activity compared with wild-type NM II-B HMM and that it is bound to actin in a stable complex. Unlike wild-type HMM II-B, this myosin is unable to propel actin filaments in an in vitro motility assay (Kim et al., 2005). This suggests that the restoration of the integrity of the neuroepithelial cells lining the spinal canal by increasing the amount of mutant NM II-B relies more on the structural properties of NM II-B than on its motor activity. Because the motor activity of NM II is regulated by phosphorylation of the 20-kDa regulatory myosin light chain (MLC20), it was of interest to examine the state of light chain phosphorylation in B+/B+ and BC/BC mice in the cells bordering the spinal canal. No evidence for MLC20 serine-19 phosphorylation could be detected in these cells by using MLC20 serine-19 phospho-specific antibodies (Figure 8a), although there is evidence for serine-19 phosphorylation in the nearby vasculature (arrows). These findings support the idea that, although NM II-B is required to maintain cell–cell adhesion at the apical border of the spinal canal, activation of its motor is not required.
Figure 8.
Localization of aPKCλ and NMHC II-B to the ventricular surface of the spinal cord. Immunofluorescence confocal images of the spinal cord stained for aPKCλ (c) and NMHC II-B (d) show that both are enriched at the ventricular surface lining the spinal canal in B+/B+ mice at E11.5. No significant pMLC20 (a, top) or myosin light chain kinase (b, MLCK) is detected in B+/B+ mice at the ventricular surface. No pMLC20 is detected in the BC/BC ventricular surface (a, bottom). pMLC20 and MLCK are detected in the vasculature of the spinal cord (a and b, arrows).
A recent report by Imai et al. (2006) showed that conditional ablation of aPKCλ in developing mouse brains resulted in the loss of adhesion junctions in the neuroepithelial cells lining the cerebral ventricles. We, therefore, used antibodies to aPKCλ to analyze for the presence of this kinase in the spinal cord at E12.5. As shown in Figure 8c, aPKCλ is enriched at the apical border of the neuroepithelial cells lining the spinal cord similar to NMHC II-B (Figure 8d). A similar result was obtained at E11.5. Conversely, myosin light chain kinase is not present in these cells, although it is expressed in the nearby vasculature (Figure 8b, arrows).
Mutant Myosin II Fails to Fully Rescue Impaired Neuronal Migration
Expression of wild-type quantities of R709C mutant NM II-B in BC/BC mice rescued the cell–cell adhesion defect and hydrocephalus seen in B−/B− and BCN/BCN mice. We next examined whether other brain defects found in the BCN/BCN mice, such as the impaired migration of facial and pontine neurons, were also rescued by increased expression of the mutant NM II-B in BC/BC mice. Because BC/BC mice die by E16.5 from cardiac defects, we are unable to look at cerebellar granule cell migration, which is also impaired in the hypomorphic BCN/BCN mice (Ma et al., 2004). Figure 9 shows H&E-stained sagittal sections of the developing hindbrains from wild-type and BC/BC mice at E16.5. The figure shows pontine neurons in BC/BC mice that were arrested in their migration. The direction of migration is from left to right (arrows in Figure 9, a and c), and many of the BC/BC neurons are delayed in their migration as indicated by their location in the lower left of the lateral sagittal section (Figure 9c). Figure 9, b and d, shows medial sagittal sections in which many of the pontine neurons of B+/B+ mice have reached their final destination by E16.5, whereas none of the pontine neurons have reached their final destination in BC/BC mice (compare arrows in Figure 9, b and d; n = 10 for each). In the case of facial neuron migration, 20% of the BC/BC mice showed an impaired migration similar to that shown for BCN/BCN mice (Figure 1b, arrow).
DISCUSSION
Blockage of the Spinal Canal in NMHC II-B–ablated and Mutated Mice Is the Cause of Hydrocephalus
All of the B−/B− and BCN/BCN mice develop hydrocephalus during embryonic development, and, except for the very late stage of hydrocephalus, no obstruction of the aqueduct of Sylvius is seen in these mice. Thus, based on the current classification, these mice develop a communicating hydrocephalus. Although there was no blockage to the flow of CSF in the cerebral cavities, the spinal canal of these mice was obstructed by the invasion of neuroepithelial cells beginning as early as E11.5 in B−/B− mice and E12.5 in BCN/BCN mice, which is before the development of hydrocephalus. We, therefore, propose that this blockage is the cause for the development of hydrocephalus in B−/B− and BCN/BCN mice. This hypothesis is supported by the following: first, obstruction of the spinal canal occurs before any obvious defects seen in the CNS in B−/B− and BCN/BCN mice. Second, rescuing the blockage of the spinal canal prevents hydrocephalus in BC/BC mice. Increased expression of mutant R709C NM II-B in BC/BC mice restores the mesh-like apical structure and normal cell–cell adhesion to the cells lining the canal. This ability to rescue hydrocephalus occurs despite the fact that increasing the expression of mutant NM II-B causes a number of other abnormalities in BC/BC mice, including defects in ventral body wall closure and heart development (Ma and Adelstein, unpublished data).
Cell–Cell Adhesion of the Neuroepithelial Cells Requires Nonmuscle Myosin II, but Not Activation of Its Motor Domain
Our finding of a transitory mesh-like structure containing a myosin complex is supportive of a structural role for NM II, and it is consistent with our ability to rescue hydrocephalus by using a motor impaired myosin. Because NMHC II-B is the only isoform present in the neuroepithelial cells lining the spinal canal, ablation of this isoform renders these cells more vulnerable to abnormalities than other cells, such as the epithelial cells lining the airways and intestines where all three isoforms of nonmuscle myosin II are expressed (Golomb et al., 2004). The finding of a unique mesh-like adhesion structure that includes NM II-B along with N-cadherin and β-catenin prompted us to postulate that NM II-B is involved in maintaining cell adhesion at the apical border of the neuroepithelial cells lining the spinal canal. As expected, we also detected actin in the mesh-like structure and the actin pattern was similar in both B+/B+ and BC/BC mice (unpublished data). Involvement of NMHC II-B in cell adhesion among these cells was also reported by Hildebrand (2005).
To define which properties of the NMHC II play a role in vivo in maintaining cell adhesion in these cells, we made use of a mutated form of NM II-B (R709C), which has been shown to bind to actin with high affinity, but cannot propel actin filaments in the in vitro motility assay (Kim et al., 2005). Replacement of NM II-B with wild-type amounts of mutant myosin restored normal cell adhesion and prevented hydrocephalus. The observation that increasing the quantity of a mutant form of myosin II can rescue cell adhesion is consistent with the idea that the scaffolding properties of myosin are of greater importance than its motor activity in these circumstances. Previous work by others supports the concept of the importance of the structural properties of myosin II in Dictyostelium (Xu et al., 2001). Further evidence of a structural role for myosin in the apical complex is supported by our inability to detect significant activation of NM II-B, as reflected by the lack of serine-19 phosphorylation of MLC20 at the apical border of the neuroepithelial cells. Furthermore, activation of myosin is not required for maintaining cell adhesion, and it has been reported to disrupt the adhesion junctions in cancer cell lines (Sahai and Marshall, 2002).
Of note are our recent findings that NM II-A can also rescue hydrocephalus and the defects in cell adhesion in the spinal cord of B−/B− mice when it replaces endogenous NM II-B in the neuroepithelial cells of the spinal cord (unpublished data). Rescuing these defects in B−/B− mice with NM II-A further supports the idea that maintaining cell–cell adhesion in the spinal neuroepithelial cells is not dependent on an isoform-specific function of NMHC II-B, but on a function shared by both II-A and II-B. Although both NM II-A and II-B show a high affinity for actin, there are significant differences in their kinetic motor properties (Kovacs et al., 2003). For example, NM II-B has a duty ratio (portion of the kinetic cycle spent in a state strongly bound to actin) that is more than an order of magnitude higher than that of NM II-A. This is consistent with our proposal that a structural property of NM II and not its motor activity is required to maintain cell–cell adhesion in the neuroepithelial cells.
In addition, we show here that aPKCλ is also enriched in this adhesion structure together with NMHC II-B. Although it is possible that NMHC II-B serves as a downstream target of aPKCλ in regulating cell adhesion in the spinal canal, there are other putative targets in the neuronal adherens junction as well, such as PAR3 and Lgl1 (Yamanaka et al., 2003; Klezovitch et al., 2004; Suzuki and Ohno, 2006; Vasioukhin, 2006). In contrast, Even-Faitelson and Ravid (2006) have reported that phosphorylation of the NMHC II-B in the nonhelical tail by an aPKC results in cortical localization of both NM II-B and aPKC in a prostate cancer cell line. The requirement of aPKC in cell–cell adhesion has been demonstrated both in cultured cells (Suzuki et al., 2001, Nunbhakdi-Craig et al., 2002) and neuroepithelial cells in the developing mouse brain (Manabe et al., 2002). As noted above, the importance of aPKCλ in maintaining adherens junctions in the neocortex was demonstrated by conditional loss of this enzyme, which also resulted in hydrocephalus, although at a much later time (P3) than we report here (Imai et al., 2006). This report on the role of the scaffolding properties of NM II raises important and intriguing questions. How does a nonmotoring NM II contribute to the generation of an adhesion structure? What is the exact relationship between NM II and the cell adhesion complex? These questions will be the subject of future experiments both in vitro and in vivo.
Migration of Pontine Neurons Requires NMHC II-B–specific Motor Activity
Although expression of wild-type quantities of the motor-impaired R709C mutant NM II-B in BC/BC mice rescued cell adhesion in the spinal cord, abnormalities in migration of the pontine neurons and facial neurons persisted. Similarly, replacement of NM II-B by II-A also failed to rescue the defects in neuronal migration. Unlike the properties of myosin that are responsible for cell adhesion, migration of these neuronal cells is more likely dependent on the motor function of myosin II-B. This is also consistent with our finding that MLC20 of NM II-B is phosphorylated in the migrating pontine neurons during brain development (Ma et al., 2004).
In summary, our studies show that NM II-B plays an important role in cell adhesion at the apical border of the cells lining the spinal canal during mouse brain development. The cell adhesive properties of the neuroepithelial cells lining the canal depend more on the structural properties of NM II-B than on its motor activity. These findings also shed light on the role of NM II-B in the pathogenesis of congenital hydrocephalus.
ACKNOWLEDGMENTS
We thank Sachiyo Kawamoto, Mary Anne Conti, and members of the Laboratory of Molecular Cardiology for valuable comments on the manuscript. We thank Dr. Stanley Rapoport (National Institute on Aging) for useful discussions and the reviewers of the manuscript for cogent comments. Drs. Chengyu Liu and Yubin Du (National Heart, Lung, and Blood Institute [NHLBI] Transgenic Core) and Dr. Christian A. Combs (NHLBI Light Microscopy Core) provided outstanding service and advice. Antoine Smith provided excellent technical assistance, and Catherine Magruder's editorial assistance is also gratefully acknowledged. This research was supported by the Division of Intramural Research, NHLBI.
Abbreviations used:
- aPKC
atypical protein kinase C
- CSF
cerebral spinal fluid
- E
embryonic day
- HMM
heavy meromyosin
- MLC20
regulatory myosin light chain
- NM
nonmuscle myosin
- NMHC
nonmuscle myosin heavy chain
- P
postnatal day.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-01-0073) on April 11, 2007.
REFERENCES
- Bao J., Jana S. S., Adelstein R. S. Vertebrate nonmuscle myosin II isoforms rescue small interfering RNA-induced defects in COS-7 cell cytokinesis. J. Biol. Chem. 2005;280:19594–19599. doi: 10.1074/jbc.M501573200. [DOI] [PubMed] [Google Scholar]
- Buxton D. B., Golomb E., Adelstein R. S. Induction of nonmuscle myosin heavy chain II-C by butyrate in RAW 264.7 mouse macrophages. J. Biol. Chem. 2003;278:15449–15455. doi: 10.1074/jbc.M210145200. [DOI] [PubMed] [Google Scholar]
- Conti M. A., Even-Ram S., Liu C., Yamada K. M., Adelstein R. S. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J. Biol. Chem. 2004;279:41263–41266. doi: 10.1074/jbc.C400352200. [DOI] [PubMed] [Google Scholar]
- Crews L., Wyss-Coray T., Masliah E. Insights into the pathogenesis of hydrocephalus from transgenic and experimental animal models. Brain Pathol. 2004;14:312–316. doi: 10.1111/j.1750-3639.2004.tb00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987;236:1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Donaudy F., et al. Nonmuscle myosin heavy-chain gene MYH14 is expressed in cochlea and mutated in patients affected by autosomal dominant hearing impairment (DFNA4) Am. J. Hum. Genet. 2004;74:770–776. doi: 10.1086/383285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even-Faitelson L., Ravid S. PAK1 and aPKC. regulate myosin II-B phosphorylation: a novel signaling pathway regulating filament assembly. Mol. Biol. Cell. 2006;17:2869–2881. doi: 10.1091/mbc.E05-11-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even-Ram S., Doyle A. D., Conti M. A., Matsumoto K., Adelstein R. S., Yamada K. M. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat. Cell Biol. 2007;9:299–309. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- Giannone G., et al. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell. 2007;128:561–575. doi: 10.1016/j.cell.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb E., Ma X., Jana S. S., Preston Y. A., Kawamoto S., Shoham N. G., Goldin E., Conti M. A., Sellers J. R., Adelstein R. S. Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family. J. Biol. Chem. 2004;279:2800–2808. doi: 10.1074/jbc.M309981200. [DOI] [PubMed] [Google Scholar]
- Heath K. E., et al. Nonmuscle myosin heavy chain IIA mutations define a spectrum of autosomal dominant macrothrombocytopenias: May-Hegglin Anomaly and Fechtner, Sebastian, Epstein, and Alport-like syndromes. Am. J. Hum. Genet. 2001;69:1033–1045. doi: 10.1086/324267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand J. D. Shroom regulates epithelial cell shape via the apical positioning of an actomyosin network. J. Cell Sci. 2005;118:5191–5203. doi: 10.1242/jcs.02626. [DOI] [PubMed] [Google Scholar]
- Imai F., Hirai S., Akimoto K., Koyama H., Miyata T., Ogawa M., Noguchi S., Sasaoka T., Noda T., Ohno S. Inactivation of aPKCλ results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development. 2006;133:1735–1744. doi: 10.1242/dev.02330. [DOI] [PubMed] [Google Scholar]
- Kim K.-Y., Kovacs M., Kawamoto S., Sellers J. R., Adelstein R. S. Disease-associated mutations and alternative splicing alter the enzymatic and motile activity of nonmuscle myosins II-B and II-C. J. Biol. Chem. 2005;280:22769–22775. doi: 10.1074/jbc.M503488200. [DOI] [PubMed] [Google Scholar]
- Klezovitch O., Fernandez T. E., Tapscott S. J., Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18:559–571. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M., Wang F., Hu A., Zhang Y., Sellers J. R. Functional divergence of human cytoplasmic myosin II. J. Biol. Chem. 2003;278:38132–38140. doi: 10.1074/jbc.M305453200. [DOI] [PubMed] [Google Scholar]
- Ma X., Kawamoto S., Hara Y., Adelstein R. S. A point mutation in the motor domain of nonmuscle myosin II-B impairs migration of distinct groups of neurons. Mol. Biol. Cell. 2004;15:2568–2579. doi: 10.1091/mbc.E03-11-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe N., Hirai S.-I., Imai F., Nakanishi H., Takai Y., Ohno S. Association of ASIP/mPAR-3 with adherens junctions of mouse neuroepithelial cells. Dev. Dyn. 2002;225:61–69. doi: 10.1002/dvdy.10139. [DOI] [PubMed] [Google Scholar]
- Nunbhakdi-Craig V., Machleidt T., Ogris E., Bellotto D., White C. L., III, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J. Cell Biol. 2002;158:967–978. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Figares J. M., Jimenez A. J., Rodriguez E. M. Subcommissural organ, cerebrospinal fluid circulation, and hydrocephalus. Micros. Res. Tech. 2001;52:591–607. doi: 10.1002/1097-0029(20010301)52:5<591::AID-JEMT1043>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Phillips C. L., Yamakawa K., Adelstein R. S. Cloning of the cDNA encoding human nonmuscle myosin heavy chain-B and analysis of human tissues with isoform-specific antibodies. J. Muscle Res. Cell Motil. 1995;16:379–389. doi: 10.1007/BF00114503. [DOI] [PubMed] [Google Scholar]
- Sahai E., Marshall C. J. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat. Cell Biol. 2002;4:408–416. doi: 10.1038/ncb796. [DOI] [PubMed] [Google Scholar]
- Shewan A. M., Maddugoda M., Kraemer A., Stehbens S. J., Verma S., Kovacs E. M., Yap A. S. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol. Biol. Cell. 2005;16:4531–4542. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Ohno S. The PAR-aPKC system: lessons in polarity. J. Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Yamanaka T., Hirose T., Manabe N., Mizuno K., Shimizu M., Akimoto K., Izumi Y., Ohnishi T., Ohno S. Atypical protein kinase C is involved in the evolutionarily conserved PAR protein complex and plays a critical role in establishing epithelia-specific junctional structures. J. Cell Biol. 2001;152:1183–1196. doi: 10.1083/jcb.152.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina T. M., Verkhovsky A. B., McQuade K. M., Borisy G. G. Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J. Cell Biol. 1997;139:397–415. doi: 10.1083/jcb.139.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Kishi H., Ma X., Yu Z.-X., Adelstein R. S. Ablation and mutation of nonmuscle myosin heavy chain II-B results in a defect in cardiac myocyte cytokinesis. Circ. Res. 2003;93:330–337. doi: 10.1161/01.RES.0000089256.00309.CB. [DOI] [PubMed] [Google Scholar]
- Tullio A. N., Accili D., Ferrans V. J., Yu Z.-X., Takeda K., Grinberg A., Westphal H., Preston Y. A., Adelstein R. S. Nonmuscle myosin II-B is required for normal development of the mouse heart. Proc. Natl. Acad. Sci. USA. 1997;94:12407–12412. doi: 10.1073/pnas.94.23.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullio A. N., Bridgman P. C., Tresser N. J., Chan C.-C., Conti M. A., Adelstein R. S., Hara Y. Structural abnormalities develop in the brain after ablation of the gene encoding nonmuscle myosin II-B heavy chain. J. Comp. Neurol. 2001;433:62–74. doi: 10.1002/cne.1125. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V. Lethal giant puzzle of Lgl. Dev. Neurosci. 2006;28:13–24. doi: 10.1159/000090749. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M., Zareno J., Whitmore L., Choi C. K., Horwitz A. F. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J. Cell Biol. 2007;176:573–580. doi: 10.1083/jcb.200612043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. S., Lee E., Chen T.-L., Kuczmarski E., Chisholm R. L., Knecht D. A. During multicellular migration, myosin II serves a structural role independent of its motor function. Dev. Biol. 2001;232:255–264. doi: 10.1006/dbio.2000.0132. [DOI] [PubMed] [Google Scholar]
- Yamanaka T., Horikoshi Y., Sugiyama Y., Ishiyama C., Suzuki A., Hirose T., Iwamatsu A., Shinohara A., Ohno S. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr. Biol. 2003;13:734–743. doi: 10.1016/s0960-9822(03)00244-6. [DOI] [PubMed] [Google Scholar]