Abstract
The 5-hydroxytryptamine4 (5-HT4) receptors have recently emerged as key modulators of learning, memory, and cognitive processes. In neurons, 5-hydroxytryptamine4 receptors (5-HT4Rs) activate cAMP production and protein kinase A (PKA); however, nothing is known about their ability to activate another key signaling pathway involved in learning and memory: the extracellular signal-regulated kinase (ERK) pathway. Here, we show that 5-HT4R stimulation, in primary neurons, produced a potent but transient activation of the ERK pathway. Surprisingly, this activation was mostly PKA independent. Similarly, using pharmacological, genetic, and molecular tools, we observed that 5-HT4Rs in human embryonic kidney 293 cells, activated the ERK pathway in a Gs/cAMP/PKA-independent manner. We also demonstrated that other classical G proteins (Gq/Gi/Go) and associated downstream messengers were not implicated in the 5-HT4R–activated ERK pathway. The 5-HT4R–mediated ERK activation seemed to be dependent on Src tyrosine kinase and yet totally independent of β-arrestin. Immunocytofluorescence revealed that ERK activation by 5-HT4R was restrained to the plasma membrane, whereas p-Src colocalized with the receptor and carried on even after endocytosis. This phenomenon may result from a tight interaction between 5-HT4R and p-Src detected by coimmunoprecipitation. Finally, we confirmed that the main route by which 5-HT4Rs activate ERKs in neurons was Src dependent. Thus, in addition to classical cAMP/PKA signaling pathways, 5-HT4Rs may use ERK pathways to control memory process.
INTRODUCTION
Pharmacological, biochemical, and genetic manipulations both in invertebrates (such as Aplysia) and mammals, have provided convincing evidence that 5-hydroxytryptamine (5-HT, serotonin), one of the oldest signaling substances to appear during evolution (Mattson et al., 1988; Michael et al., 1998), is involved in learning and memory (Kandel and Schwartz, 1982). 5-HT also regulates neurogenesis, neurite outgrowth, dendritic spine densities, and long-term synapse reinforcement (Kater et al., 1988; Gaspar et al., 2003; Banasr et al., 2004). Among the 5-HT receptors (5-HTRs) involved in learning and memory, those coupled to Gs, are particularly important, because cAMP emerges as a key player in memory events and associated synaptic plasticity events, such as long-term potentiation (LTP) (Siegelbaum and Kandel, 1991). In mammals, the 5-HT4Rs-Gs coupled receptors are important modulators of learning and memory (Bockaert et al., 2004; Kemp and Manahan-Vaughan, 2004, 2005). Nothing is known about their action, in vivo, on another key signaling pathway involved in learning and memory: the extracellular signal-regulated kinase (ERK) pathway (Sweatt, 2004). However, 5-HT4Rs have been demonstrated to activate the ERK pathway in human embryonic kidney 293 (HEK293) cells (Norum et al., 2003). Our aim was to examine whether this activation also occurs in neurons. Indeed, we observed a 5-HT4R–induced activation of ERKs in neurons in primary culture, but the unexpected finding was that this activation was mostly a protein kinase A (PKA)-independent event.
There are many possible signaling pathways by which a G protein-coupled receptor (GPCR) can activate ERKs. They can be classified in two main pathways. The first pathway uses a classical G protein activation followed by transduction, via βγ and/or kinases that depend on second messengers (such as PKA, PKC, and phosphatidylinositol 3-kinase [PI3K]), Ras or Rap exchange factors, and finally activation of receptor (epidermal growth factor-receptor [EGF-R]) or nonreceptor (Src) tyrosine kinases (Luttrell, 2005). The second pathway does not require G proteins. Indeed, this pathway occurs after G protein uncoupling. β-Arrestins that participate in this uncoupling also mediate endocytosis of the receptor, via clathrin-coated pits. This change of compartment allows the β-arrestin scaffolding of a new signaling complex, where early signaling proteins, such as Src, are close to members of the ERK pathway, such as Raf and mitogen-activated protein kinase kinase (MEK) (Lefkowitz and Shenoy, 2005; Wang et al., 2006). Interestingly, the time courses of the G-dependent and the β-arrestin–dependent activation of the ERK pathways are very different. The first activation is transient (a few minutes); the second activation is more persistent (up to 1 h) and depends on the duration of the receptor endocytosis, which is much longer for class B than for class A receptors (Oakley et al., 2001).
The aim of this study was to explore the signaling events involved in ERK activation by 5-HT4R, because the PKA pathway, the major signaling cascade of 5-HT4R, is not implicated in neurons.
We report that 5-HT4R activates the ERK pathway independently of G protein cascades and β-arrestin but that it requires Src tyrosine kinase activation. This pattern of signaling events occurs as well in HEK cells as in neurons.
MATERIALS AND METHODS
Plasmids and Construction of Mutated 5-HT4R cDNAs
Plasmid pcDNA-3.1-β-arrestin 2-YFP was generously provided by M. Bouvier (University of Montreal, Montreal, Quebec, Canada). Truncated receptor constructs are already described by Claeysen et al. (1999). Briefly, constructs Δ346 were obtained by inserting a stop codon after residues 346 in the 5-HT4R cDNA sequence, with the QuikChange site-directed mutagenesis kit (Stratagene, Amsterdam, The Netherlands). 5-HT4D66N and W272A were generated using the same protocol.
Antibodies
Anti-phospho PKA substrates, anti-p44/p42 mitogen-activated protein kinase (MAPK) (ERK1/2), anti-phospho-p44/p42 MAPK (Thr202/Tyr204), anti-phospho-Src (Tyr 416), and anti-Src Pan are all polyclonal antibodies purchased from Cell Signaling Technology (Ozyme, France).
The mouse anti-Rho tag antibody was provided by Dr. S. Costagliola (Institut de Recherche en Biologie Humaine et Nucléaire, Brussels, Belgium) (Adamus et al., 1991). The anti-β-arrestin 1 A1CT was a gift from Dr. R. J. Lefkowitz (Duke University Medical Center, Durham, NC). The anti-β-arrestin 2 was purchased from Sigma-Aldrich (St. Louis, MO). Alexa Fluor 488- and Alexa 594-labeled secondary antibodies were purchased from Invitrogen (Cergy-Pontoise, France). The horseradish peroxidase-conjugated anti-rabbit and anti-mouse antibodies were from GE Healthcare (Orsay, France).
Small Interfering RNA (siRNA) Transfection
The double-stranded siRNA sequence 5′-ACCUGCGCCUUCCGCUAUG-3′ was used to simultaneously target human β-arrestin 1 (positions 172–190) and β-arrestin 2 (positions 175–193). Indicated position numbers are relative to the start codon. One small RNA duplex that has no silencing effect was used as a control (5′-AAGUGGACCCUGUAGAUGGCG-3′). All the siRNAs were chemically synthesized (Eurogentec, Seraing, Belgium), and they were described and validated previously (Gesty-Palmer et al., 2006; Kara et al., 2006). Early passage HEK293 cells at 40% confluence were transfected into six-well plates with 300 ng of plasmid encoding the wt-5-HT4R. After cell adhesion, siRNAs were transfected at 200 nM by using the Gene Silencer transfection reagent according to the manufacturer's recommendations (Gene Therapy Systems, San Diego, CA). Briefly, 50 μl of the Gene Silencer transfection reagent was added to 300 μl of minimal essential medium (MEM), whereas RNA mixtures containing 36 μl at 75 μM ≈20 μg of RNA, 240 μl of siRNA diluent, and 180 μl of MEM were prepared. Both solutions were allowed to stand 5–10 min at room temperature, and they were mixed by inversion. After a 10- to 20-min incubation at room temperature, the transfection mixture was divided into six equivalent fractions and added to cells into six-well plates containing 750 μl of fresh, serum-free DMEM. After cells were incubated for 4 h at 37°C, an additional 1 ml of DMEM with 20% fetal calf serum (FCS) and 2% penicillin/streptomycin were added to the wells. All assays were performed 3 d after siRNA transfection.
Cell Cultures and Transfection
Primary cultures of colliculi neurons were prepared as described previously (Dumuis et al., 1988). Briefly, cells dissociated from colliculi of 14- to 15-d-old Swiss mouse embryos were plated in serum-free medium in 12-well culture dishes (0.8 × 106 cells/ml; 1 ml/dish). Cultures were maintained for 6–8 d at 37°C in a humidified atmosphere in 5% CO2/95% H2O/air and in DMEM/F-12 supplemented with 10% of mix hormone (100 μg/ml transferrin, 25 μg/ml insulin, 60 μM putrescine, 20 nM progesterone, and 30 nM sodium selenite) and antibiotics. HEK293 cells were grown in DMEM supplemented with 10% of dialyzed FCS and antibiotics. Once at confluence, cells were transfected by electroporation, as previously described (Claeysen et al., 1999). Colliculi neurons, as well as transfected HEK293 cells, were processed for subsequent experiments, such as immunofluorescence, immunoblotting, or measurement of second messengers (cAMP or inositol phosphates).
Phosphorylation Assays
Cells transfected with the indicated amount of plasmid were plated on six-well dishes and grown for 24 h in DMEM in 10% dialyzed fetal calf serum. Before stimulation, the cells were starved for at least 6 h in serum-free medium. After stimulation, cells were lysed in SDS buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% SDS, proteases inhibitors mixture, and phosphatases inhibitors [1 mM sodium orthovanadate, 10 mM sodium fluoride, and 10 mM pyrophosphate]). Cell lysates were incubated for 20 min at 4°C and subsequently centrifuged at 12,000 rpm for 10 min at 4°C. For each sample, the amount of proteins was determined by bicinchoninic acid method (Sigma-Aldrich). Four times concentrated Laemmli buffer was added to cell lysates, and proteins were separated by electrophoresis on a 10% SDS-polyacrylamide gel. The proteins were transferred to nitrocellulose membranes (Hybond-C; Amersham Biosciences) at 18 V until intensity of current stopped decreasing. Membrane were blocked for 1 h at room temperature using Tris-buffered saline blocking solution, containing 5% (wt/vol) milk powder, 0.25% (vol/vol) Tween 20. Membranes were immunoblotted successively with the primary antibodies overnight at 4°C (either 1:1000 anti-p-PKA substrates, anti-p44/p42 (pERK1/2), total anti-p44/p42 (ERK1/2), anti-p-Src (Tyr416), or Src Pan rabbit polyclonal antibody), washed extensively, and incubated with secondary antibodies. After the detection of phosphorylated bands using a Chemiluminescence Reagent Plus kit (PerkinElmer-Cetus, Courtaboeuf, France) and autoradiography, the membranes were stripped with 100 mM glycine, pH 2.2, 0.2% SDS, and 0.1% NP-40 for 30 min at room temperature, rinsed with wash buffer, and immunoblotted as described above with another primary antibody. Autoradiographs were digitized and subsequent analyses were performed with ImageJ (National Institutes of Health, Bethesda, MD) and GraphPad Prism (GraphPad Software, San Diego, CA).
Determination of cAMP Production in Transfected Cells
HEK293 cells were transfected with the appropriate cDNA and seeded into 24-well plates (500,000 cells/well). Twenty-four hours after transfection, a 5-min-stimulation with the appropriate concentrations of 5-HT, 0.1 mM l-ascorbic acid, and 0.1 mM phosphodiesterase inhibitor Ro-20-1724 was performed at 37°C in 250 μl of HBS (20 mM HEPES, 150 mM NaCl, 4.2 mM KCl, 0.9 mM CaCl2, 0.5 mM MgCl2, 0.1% glucose, and 0.1% bovine serum albumin [BSA]). The same volume of Triton X-100 (0.1%) was added to stop the reaction, and then the cells were incubated 30 min at 37°C. Quantification of cAMP production was performed by homogenous time resolved fluorescence (HTRF) by using the cAMP Dynamic kit (CIS Biointernational, Bagnols-sur-Cèze, France) according to the manufacturer's instructions.
Determination of Inositol Production in Transfected Cells
Cells are plated into 24-well dishes (700,000 cells/well). Twenty-four hours after transfection, 50 mM LiCl was added 10 min before a 30-min stimulation with the appropriate concentration of 5-HT and 0.1 mM l-ascorbic acid in HEPES buffer saline (HBS). Quantification of inositol phosphates (IP) production was performed by HTRF, using the IP-One assay (CIS Biointernational), according to the manufacturer's instructions.
Immunofluorescence Microscopy
HEK293 cells expressing tagged wild type (wt) or Δ346-5-HT4-R with or without yellow fluorescent protein (YFP)-tagged β-arrestin 2 were grown on poly-l-ornithine–coated glass coverslips and incubated in DMEM with 10% fetal calf serum. Thirty-four hours after transfection, cells were serum-starved overnight. To visualize Rho-tagged receptors, cell surface receptors were labeled with 2 μg/ml antibody for 90 min at 4°C. Cells were then washed with serum-free medium and stimulated with 10 μM 5-HT in the same medium at 37°C. Cells were washed with phosphate-buffered saline, fixed with 4% paraformaldehyde for 20 min at room temperature, and then permeabilized with 0.05% Triton X-100. Cells were then incubated for 1 h at room temperature with goat anti-mouse antibody coupled to Alexa Fluor 488 or 594 at 2 μg/ml with or without goat anti-rabbit antibody coupled to Alexa Fluor 594 at 2 μg/ml. After extensive washes, the coverslips were mounted onto slides by using Mowiol mounting media (Hoechst, Frankfurt, Germany). Immunofluorescence microscopy was performed using a Zeiss Axiophot2 microscope (Carl Zeiss, Jena, Germany) with Zeiss 63× numerical aperture (NA) 1.4 oil immersion lenses. Excitation and emission filters for the different labeled dyes were as follow: YFP and Alexa 488 (green), λex = 450–490 nm, λem = 520 nm; and Alexa Fluor 594 (red), λex = 546 nm, λem = 590 nm.
Coimmunoprecipitation Experiments
HEK293 cells were transfected with wt or Δ346 Rho-tagged 5-HT4R construct as indicated in the figure legends. Cells were seeded at 106/150-mm plate 48 h before the experiment. Briefly, a 5-min stimulation with 10−5 M 5-HT was performed at 37°C in DMEM without serum. Then, the cross-linking reaction was realized during 30 min in Locke's buffer completed with 1.25 mM of dithiobis(succinimidyl propionate) (Pierce Chemical, Perbio-Brebières, France) a membrane-permeable, hydrolysable covalent cross-linker. The cross-linking reaction was stopped with Locke 10% FCS. After two washes with Locke's buffer, cells were incubated in lysis/binding buffer (20 mM HEPES, 150 mM NaCl, 1% NP-40, 10% glycerol, 4 mg/ml dodecylmaltoside, 0.8 mg/ml cholesteryl hemisuccinate, phosphatase, and protease inhibitor). After 30 min of incubation at 4°C, the samples were centrifuged at 15,000 rpm for 15 min. The soluble extract was incubated overnight at 4°C with 20 μl of a mixture 1:1 of protein A/protein G-Sepharose beads (Amersham Pharmacia Biotech) precoupled with 8 μg of anti-Rho-tag antibody. After five washes with homogenization buffer, immunoprecipitated proteins were eluted in Laemmli sample buffer, resolved by SDS-polyacrylamide gel electrophoresis, and detected by Western blotting.
Data Analysis
The dose–response curves were fitted using GraphPad Prism, and the following equation for monophasic dose–response curves: y = (ymax − ymin)/1 + [(x/EC50) nH] + ymin, where EC50 is the concentration of the compound necessary to obtain 50% of the maximal effect, and nH is the Hill coefficient. All data represented correspond to the mean ± SEM of three independent experiments performed in triplicate. Statistical analysis was carried out with the t test using GraphPad Prism 3.0 software. p values <0.05 were considered as statistically significant.
RESULTS
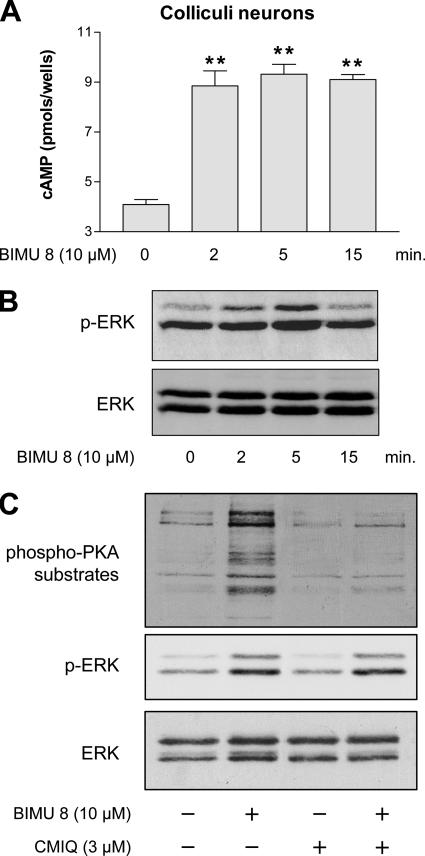
Activation of ERK by the Gs-coupled 5-HT4Rs in Neurons Mostly Takes Place through a cAMP-independent Pathway
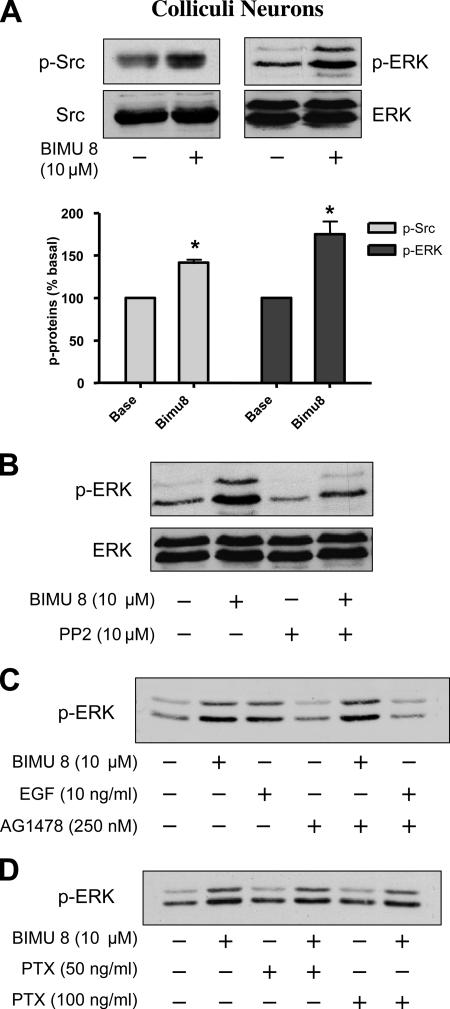
In cultured colliculi neurons, endogenous 5-HT4Rs are coupled to the Gs/cAMP pathway (Dumuis et al., 1988). Indeed, a 2- to 15-min stimulation period with BIMU8 (a selective 5-HT4R agonist) (Bockaert et al., 2004) generated a twofold activation of cAMP production (Figure 1A). BIMU8 also increased the level of p-ERK1/2, and this activation was transient (Figure 1B). Indeed, p-ERK reached a maximal signal after 5-min stimulation and decreased to the basal level after 15 min. This time course of ERK phosphorylation contrasted with those obtained with other GPCRs, such as β2 adrenergic receptors (β2-ARs), vasopressin V2 (V2Rs), or angiotensin II type 1a receptors (AT1AR) (Kim et al., 2005; Ren et al., 2005; Shenoy et al., 2006). In these latter cases, the ERK1/2 activation time courses were divided into two components, a transient G protein-dependent component and a persistent G protein-independent and β-arrestin–dependent component.
Figure 1.
Stimulation of 5-HT4Rs coupled to Gαs in colliculi neurons leads to the activation of ERK, independently of PKA. (A) After 7 d of culture and differentiation, colliculi neurons were incubated in a medium containing 1 mM isobutylmethylxanthine (a phosphodiesterase inhibitor) and 10 μM BIMU8. cAMP accumulation was measured for 2, 5, and 15 min of stimulation at 37°C as indicated in Materials and Methods and expressed as picomoles per well. Results are the mean ± SEM of four independent experiments. **p < 0.01, significantly different from the corresponding cells before BIMU8 treatment. (B) Colliculi neurons were stimulated with 10 μM BIMU8 for the same periods as mentioned in A, and then they were lysed. The phosphorylation state of ERK1/2 of whole cell extract was analyzed by immunoblotting with the antibody to p-ERK1/2. Membranes were then stripped and analyzed with antibody to total ERK. The Western blot shown is representative of five independent experiments. (C) Inhibition of PKA does not lead to the inhibition of p-ERK1/2 in colliculi neurons. Colliculi neurons expressing endogenous 5-HT4R were pretreated with vehicle or 3 μM CMIQ for 30 min before treatment with 10 μM BIMU8 for 5 min. Cell lysates were successively analyzed by immunoblotting with the antibodies to phospho-PKA substrates, to p-ERK1/2, and to total ERK as described in B. The Western blot shown is representative of three independent experiments.
Because 5-HT4R–mediated activation of ERK1/2 was transient, we investigated whether this pathway was G protein signaling dependent. We first attempted to evaluate the contribution of the Gs/cAMP/PKA pathway. To investigate the role of PKA in 5-HT4R–mediated ERK1/2 activation in colliculi neurons, we examined the amount of BIMU8-induced p-ERK1/2 in the presence and absence of 4-cyano-3-methylisoquinoline (CMIQ), a selective PKA inhibitor (He and Yeung, 2003). Before this experiment, we determined the concentration of CMIQ necessary to inhibit PKA in neuronal cultures. Immunoblotting with an anti-phospho-PKA substrate revealed that pretreatment of neurons with a concentration of 3 μM CMIQ was sufficient to completely inhibit the 5-HT4R–induced activation of PKA (Figure 1C). We found that BIMU8 still generated ERK1/2 activation in the presence of 3 μM CMIQ with only a slight decrease in p-ERK1/2 signal (Figure 1C).
Our results suggest that activation of 5-HT4Rs in neurons could induce activation of ERK through a pathway largely independent of PKA. In neurons, a Gq-dependent ERK activation can also be excluded, because we detected no IP accumulation after activation of endogenous 5-HT4Rs (data not shown).
G Protein-independent ERK1/2 Activation by 5-HT4Rs in HEK293 Cells
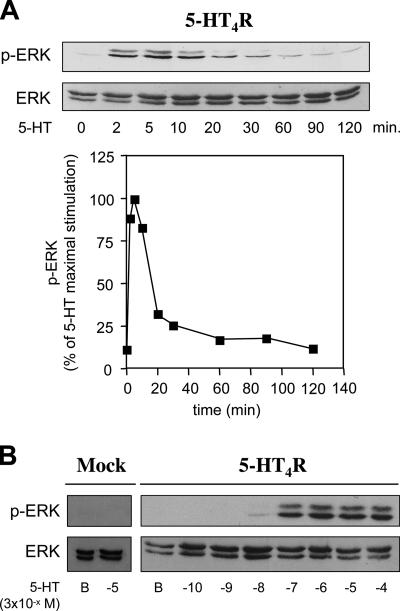
In an effort to better understand the molecular mechanisms involved in 5-HT4R–mediated ERK phosphorylation, we used HEK293 cells transiently transfected with 5-HT4Rs. We chose HEK293 cells to take advantage of the genetic and molecular tools difficult to set up in primary cultured neurons, such as transfection of cDNA, as well as siRNA. As in colliculi neurons, the time course of 5-HT4R–induced p-ERK1/2 accumulation observed in HEK293 cells was very transient with a maximum effect after 5 min of stimulation, and the effect returned to the basal level after 10–20 min of stimulation (Figure 2A). The concentration–response curve of the 5-HT4R ligand-mediated p-ERK1/2 indicated an EC50 of 3 ± 0.2 × 10−7 M (Figures 2B and 3), a value much higher than the EC50 determined for 5-HT4R–mediated cAMP accumulation in the same cells (0.9 ± 0.1 × 10−9 M) (Figure 3).
Figure 2.
Time course of agonist-stimulated ERK1/2 phosphorylation. (A) HEK293 cells were transfected with 100 ng of 5-HT4R. Twenty-four hours later, the cells were starved in serum-free medium overnight and stimulated with 10 μM 5-HT for the indicated periods at 37°C. Phosphorylated (p-ERK1/2) and total ERK1/2 of the same cell lysate were determined by immunoblotting with the antibody to p-ERK1/2 or to total ERK. p-ERK1/2 bands were quantified by densitometry and normalized to ERK level and expressed as a percentage of the maximal p-ERK1/2 (obtained at 5 min). Data from four independent experiments are represented as the mean ± SEM and plotted in the graph below. (B) HEK293 cells expressing the 5-HT4R were starved in serum-free medium overnight and stimulated with increasing concentrations of 5-HT (3 × 10−10 – 3× 10−4 M). Representative blots of p-ERK1/2 and ERK are shown in A and B.
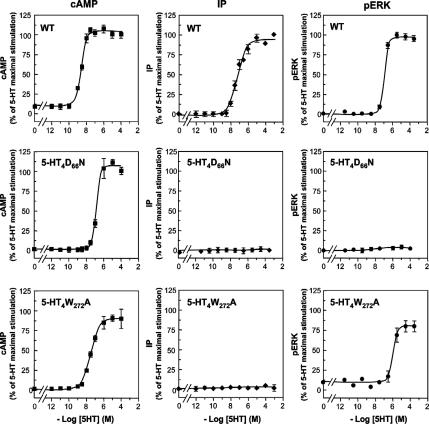
Figure 3.
5-HT-stimulated second messengers (cAMP and IP) and phosphorylation of ERK1/2 in HEK293 cells. Comparison between the effects of 5-HT4R WT and defective mutants. HEK293 cells transiently expressing either 5-HT4R WT or mutants (D66N or W272A) were treated with increasing concentrations of 5-HT for cAMP production (5 min), IP production (30 min), and p-ERK1/2 (5 min). Receptor expression levels were similar for all the receptors as revealed by enzyme-linked immunosorbent assay that allowed quantifying relative cell surface receptor expression before agonist stimulation (data not shown). Basal and 5-HT–stimulated cAMP values were 1 ± 0.5 and 9 ± 2 pmol/well, respectively. Basal and 5-HT-stimulated IP values were 0.15 ± 0.5 and 10 ± 3 pmol/well, respectively. For WT, data are expressed as a percentage of the maximum second messenger (cAMP or IP) or p-ERK produced after stimulation with 5-HT 10 μM for 5 min (cAMP and p-ERK) or for 30 min (IP) (top). For mutants, data are expressed as a percentage of the maximum cAMP, IP, or p-ERK1/2 produced by the WT stimulated with 10 μM 5-HT, as indicated above. Analysis of p-ERK1/2 and total ERK content was performed by immunoblotting and quantified by densitometry. The amounts of p-ERK1/2 were always normalized to the total ERK signal obtained by stripping the same immunoblot and determined with the antibody to ERK42/44. Curve fitting was performed with the GraphPad Prism software. For all the curves, the data are the mean ± SEM of at least four independent experiments.
We then attempted to evaluate how much ERK1/2 activation by 5-HT4R was dependent on Gs, Gq, or Gi signaling cascades. First, note that in HEK293 cells, 5-HT4Rs were also able to stimulate IP accumulation with an EC50 for 5-HT slightly higher than its EC50 to stimulate p-ERK accumulation (Figure 3). We generated several 5-HT4R mutants with different specificities of G protein pathway activation. When the highly conserved aspartate D66 (D2.50) in the second transmembrane domain was replaced by an asparagine (N), the receptor was still able to stimulate cAMP production (Figure 3). However, without losing its coupling to Gs, this mutant was absolutely unable to stimulate p-ERK1/2 accumulation or stimulate IP accumulation (Figure 3). This was the first initial evidence to prove that the Gs/cAMP pathway was not involved in p-ERK1/2 accumulation after 5-HT4R activation. To exclude the involvement of the Gq/inositol-1,4,5-triphosphate pathway, we used the W272A (W6.48) mutant, which was completely unable to induce an increase in IP accumulation, but it was still able to increase ERK1/2 phosphorylation in response to 5-HT stimulation, although with a lower potency (EC50 = 30 ± 4 × 10−7 M) and efficacy (78 ± 6% of the WT) than the native receptor (Figure 3). It was also able to increase cAMP. Results with both mutants suggest that neither cAMP nor IP was involved in 5-HT4R–mediated ERK1/2 activation.
To further validate the absence of a role for the Gs/cAMP pathway in 5-HT4Rs–mediated p-ERK1/2 accumulation in HEK293 cells, we examined the effect of 1 mM SQ 22536, an adenylyl cyclase inhibitor. This drug inhibited 5-HT4R–stimulated cAMP accumulation by >85% (Figure 4B). However, the accumulation of p-ERK1/2 in the presence of 5-HT remained unchanged (Figure 4A). Similarly to the experiment carried out in colliculi neurons, we determined the concentration of CMIQ necessary to inhibit PKA in HEK293 cells lines. We also tested H-89, another PKA inhibitor. Immunoblotting with an anti-phospho-PKA substrate revealed that pretreatment of HEK293 cells with either a concentration of 3 μM CMIQ or 1 μM H-89 was sufficient to completely inhibit the 5-HT4R–stimulated activation of PKA (Figure 4C). In parallel, we found that 5-HT still generated ERK1/2 activation in the presence of 3 μM CMIQ or 1 μM H-89. Furthermore, we observed that forskolin did not activate ERK1/2 in HEK293 cells (data not shown). This has also been reported by Rey et al. (2006) who concluded that in HEK cells, ERK activation is not responsive to direct stimulation of adenylyl cyclase with forskolin.
Figure 4.
cAMP and downstream effector PKA do not participate in 5-HT4R–mediated ERK activation. (A) Inhibition of cAMP formation does not prevent ERK1/2 activation. HEK293 cells expressing 100 ng of 5-HT4R WT plasmid were serum starved overnight and exposed or not to 1 mM SQ 22536 for 30 min (an inhibitor of cAMP accumulation) before the stimulation by 10 μM 5-HT for 5 min. The cell lysates were analyzed by immunoblotting with antibody to p-ERK1/2. A representative immunoblot is shown. (B) In parallel, HEK293 cells coming from the same transfection as described in A for each condition were seeded into 24-well plates, stimulated with 10 μM 5-HT plus Ro-20-1724 (a phosphodiesterase inhibitor), and lysed in HBS plus Triton 0.1% as indicated in Materials and Methods. cAMP levels accumulated during the stimulation period of 5 min are expressed as the percentage of maximum cAMP response to 10 μM 5-HT, and they are reported in the absence or presence of pretreatment with SQ 22536. Values are the mean ± SEM of four independent experiments. **p < 0.01, significantly different from the corresponding cells stimulated with 5-HT before SQ 22536 treatment. (C) ERK phosphorylation does not depend on PKA activation. HEK293 cells expressing 5-HT4R were pretreated with either vehicle or 3 μM CMIQ or 1 μM H-89 for 30 min before treatment with 10 μM 5-HT for 5 min. Cell lysates were analyzed by immunoblotting successively with the antibodies to phospho-PKA substrates and to p-ERK1/2. The Western blot shown is representative of three independent experiments.
To confirm that 5-HT4R–stimulated ERK1/2 activity did not depend on the Gq/IP/phospholipase C (PLC) pathway, we exposed the cells to a PLC inhibitor, U 73122 at 10 μM, and to its inactive analogue U 73434 at 10 μM. Neither of these inhibitors could suppress ERK1/2 activation (Figure 5A); however, U 73122 inhibited ∼75% of IP accumulation produced under 5-HT4R activation (Figure 5B). To independently assess the role of Gi in the activation of ERK1/2, we also determined the effect of pertussis toxin (PTX) preincubation of HEK293 cells expressing the 5-HT4R. β2-ARs can switch from a Gs to a Gi coupling and subsequently activate the ERK pathway via βγ. We observed no such event for 5-HT4Rs. Indeed, a PTX treatment of HEK293 cells had no effect on 5-HT4R–mediated accumulation of p-ERK1/2 (Figure 5C). Altogether, the present data indicate that 5-HT-stimulated p-ERK accumulation in cells expressing 5-HT4R does not require the generation of a classical second messenger that is dependent on G protein (Gs, Gi, and Gq) signaling.
Figure 5.
5-HT4R-mediated activation of p-ERK1/2 is independent of PLC, Gi/Go, and EGF receptors. (A) 5-HT4R–mediated ERK activation does not require PLC activation. Serum-starved HEK293 cells expressing 5-HT4R were pretreated with 10 μM U 73122, a PLC inhibitor, or by its inactive analogue U 73343 for 30 min before stimulation with 10 μM 5-HT for 5 min. Total lysates were analyzed by immunoblotting with antibody to p-ERK1/2. (B) In parallel, HEK293 cells coming from the same transfection as described in A for each condition were seeded into 24-well plates, pretreated by LiCl 10 min before a 30-min stimulation by 10 μM 5-HT. Quantification of IP production was performed by HTRF by using the IP-One assay as described in Materials and Methods. IP levels accumulated during the stimulation time are expressed as the percentage of maximum IP response to 10 μM 5-HT, and they are reported in the absence or presence of pretreatment with U 73122 and U 73343. Values are the mean ± SEM of four independent experiments. **p < 0.01, significantly different from the corresponding cells before U 73122-treatment. (C) 5-HT4R–mediated ERK activation does not require G protein PTX sensitive (Gi/Go). HEK293 cells expressing 5-HT4R were pretreated with 100 ng/ml PTX overnight before treatment with 10 μM 5-HT for 5 min. Total lysates were analyzed by immunoblotting with antibody to p-ERK1/2. (D) 5-HT4R–mediated ERK activation does not require transactivation of EGF-R tyrosine kinase. HEK293 cells expressing 5-HT4R were pretreated with 250 nM tyrphostin/AG1478 for 30 min before stimulation with 10 μM 5-HT. Total lysates were analyzed by immunoblotting with antibody to p-ERK 1/2. A representative blot of each experiment is shown in A, C, and D.
To exclude that ERK activation could perhaps result from the transactivation of EGF-R as described for some GPCRs in HEK293 cells (Turner et al., 2001; Beom et al., 2004), we tested the effect of AG1478, a selective EGF-R inhibitor, on ERK1/2 activation in response to 5-HT. As shown in Figure 5D, 250 nM tyrphostin/AG1478 does not reduce ERK1/2 activation induced by 5-HT4R activation, whereas at this concentration EGF-R–mediated ERK activation was inhibited. Moreover, pretreating HEK293 cells with 100 nM wortmannin 30 min before activation of the 5-HT4R excludes the involvement of PI3 kinase in 5-HT4R–mediated activation of ERK1/2 (data not shown).
5-HT4R-induced ERK Phosphorylation Involves Src Kinase
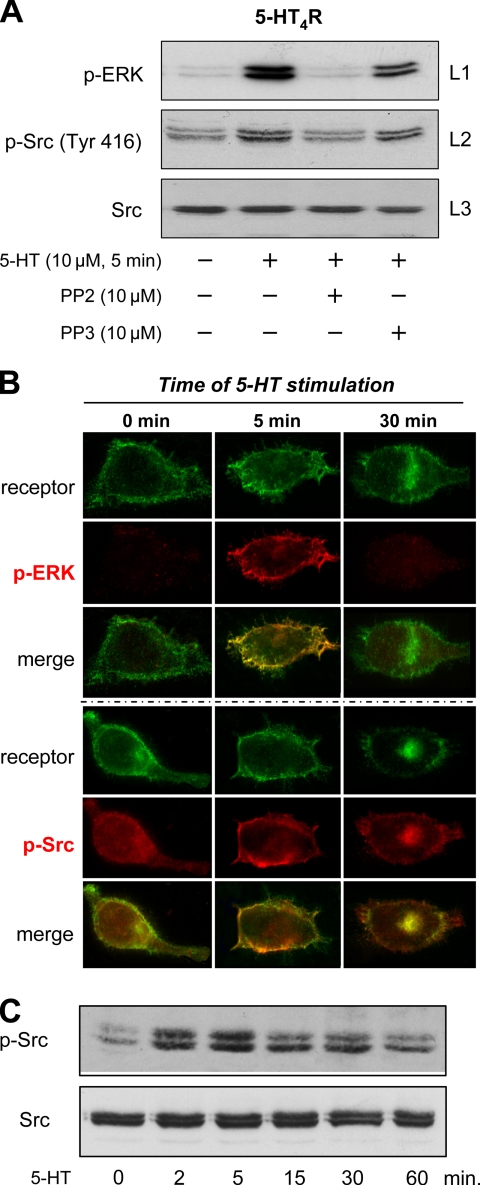
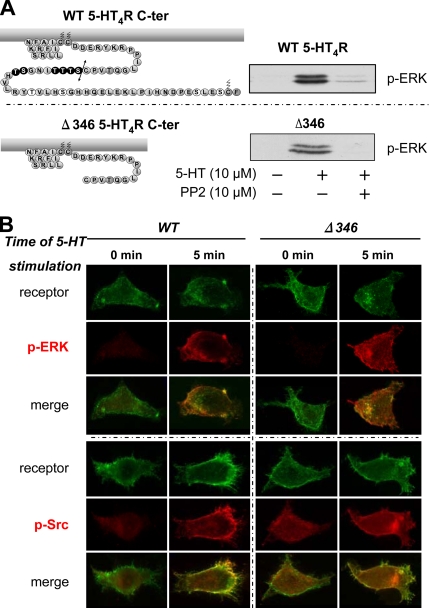
Previous studies have demonstrated that activation of the 5-HT4Rs present in the enterocyte Caco-2 cell line inhibits apical Cl−/OH− exchange activity by activating the nonreceptor tyrosine kinase Src (Saksena et al., 2005). Moreover, Src has been shown to play a prominent role in GPCR-induced ERK activation (Luttrell and Luttrell, 2004). In a few situations, Src may directly be activated by binding to the GPCR, although a G protein was involved (β3-AR and P2Y2 receptors) (Cao et al., 2000; Liu et al., 2004). Src can also be activated after its recruitment by β-arrestin together with members of the ERK cascade such as Raf and MEK during β-arrestin–dependent endocytosis of the receptor (Wang et al., 2006). To determine whether one of these mechanisms was required for 5-HT4R–induced activation of ERK1/2, we first tested the sensitivity of this activation on the Src-specific tyrosine kinase inhibitor PP2 (Bain et al., 2003). As illustrated in Figure 6A, line 1, pretreating the cells with 10 μM PP2 resulted in a dramatic decrease (> 80%) in p-ERK accumulation. In contrast, 5-HT4R–dependent ERK1/2 phosphorylation was only slightly reduced by PP3, a structural analogue of PP2 that does not inhibit Src kinase. To further establish the role of Src, we demonstrated that phosphorylation of Src kinase at Tyr 416 was induced by 5-HT4Rs stimulation, an effect blocked by PP2 but not PP3 (Figure 6A, line 2).
Figure 6.
5-HT4R–mediated ERK1/2 activation depends on Src tyrosine kinase activation. (A) Stimulation of 5-HT4R activates Src. Inhibition of Src prevents ERK activation. Serum-starved HEK293 cells expressing 5-HT4R were pretreated with the Src kinase inhibitor PP2 at 10 μM or with the inactive analogue PP3 at 10 μM 30 min before a 5-min stimulation with 10 μM 5-HT. The cell lysates were analyzed by immunoblotting. The blots were probed sequentially to detect p-ERK1/2 with antibody against p-ERK1/2 (line 1), active Src with antibody to p-Src (Tyr 416) (line 2), and total Src with Pan antibody to Src inactive (line 3). (B) Colocalization of p-ERK1/2 and p-Src (Tyr 416) after stimulation of 5-HT4R WT. HEK293 cells transfected with 500 ng of Rho-tagged 5-HT4R were seeded onto coverslips. Twenty-four hours after transfection, cells were serum starved overnight. Cells were incubated 90 min with antibody against Rho-tagged 5-HT4R at 4°C before a 5- or 30-min stimulation with 10 μM 5-HT. After fixation and permeabilization, cells were sequentially incubated with primary antibody against p-ERK1/2 or p-Src (Tyr 416) and with fluorochrome-labeled secondary antibody. Fluorescence microscopy was then used to visualize the distribution of antibody-labeled receptors (green channel) and the appearance of phosphorylated form of ERK (p-ERK1/2) and Src (p-Src) (red channel). Immunofluorescence microscopy was performed using a Zeiss Axiophot2 microscope with Zeiss 63× NA 1.4 oil immersion lenses. Representative images from several independent experiments are shown. Top, distribution of Rho-tagged 5-HT4R before (basal) or after 5- and 30-min addition of 10 μM 5-HT to the culture medium at 37°C. The left column shows the distribution of the receptor and the absence of p-ERK1/2 before activation. An increase in phosphorylation state of ERK1/2 at the plasma membrane is visualized after 5-min treatment with 5-HT. Merged images were magnified to show colocalization of 5-HT4R with p-ERK1/2 at the plasma membrane after 5 min of stimulation. Bottom, phosphorylation state of p-Src (Tyr 416) before and after 5-HT4R stimulation. Phosphorylation state and colocalization with 5-HT4R is longer for p-Src than for p-ERK1/2. (C) HEK293 cells were transfected with 5-HT4R. Twenty-four hours later, the cells were starved in serum-free medium overnight and stimulated with 10 μM 5-HT for the indicated periods at 37°C. Phosphorylated Src (Tyr 416) of all the cell lysates was determined by immunoblotting with the antibody to active p-Src (Tyr 416) and to total Src.
Data in Figure 6B show immunofluorescence detection of Rho-tagged 5-HT4Rs, p-ERK1/2 as well as p-Src (Tyr 416) in HEK293 cells under basal conditions or after stimulation with 10 μM 5-HT for 5 and 30 min. Under basal conditions, a very low level of p-ERK1/2 was detected, whereas an intense accumulation of p-ERK1/2 was observed after 5-HT stimulation for 5 min. Note that there was a clear colocalization between the receptor and p-ERK1/2 at the submembrane level. p-Src was already detected under basal conditions and stimulation of 5-HT4Rs for 5 min with 5-HT, further increased p-Src levels. As illustrated in Figure 6B, a colocalization between 5-HT4Rs and p-Src at the plasma membrane level can be observed. As already described using immunoblotting experiments (Figure 2), the immunofluorescence experiments in Figure 6 further confirmed that a 30-min stimulation with 5-HT was not associated with a persistent accumulation of p-ERK1/2. At 30 min, the receptors could still be detected at the cell surface and also in a perinuclear area, as described previously (Barthet et al., 2005). One very interesting observation was that the receptor-induced Src phosphorylation was still present even after a 30-min stimulation with 5-HT (Figure 6B). Note that this p-Src (Tyr 416) pool colocalized with cell surface receptors at 5 min as well as with internalized receptors in perinuclear compartments at 30 min (Figure 6B). Immunoblotting experiments confirmed that the activation of Src after stimulation with 5-HT, in contrast to the activation of ERK1/2, was persistent up to 30 min (Figure 6C).
The 5-HT4R–induced Accumulation of p-ERK1/2 Is Not Dependent on β-Arrestin Recruitment
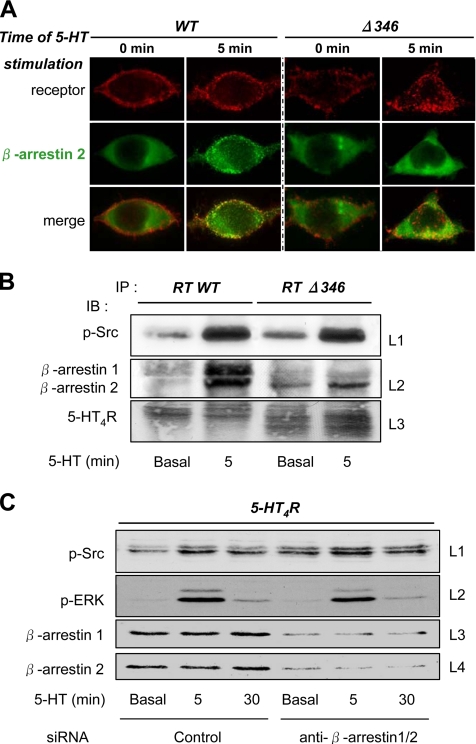
Recently, a very well documented concept proposed that GPCRs can trigger non-G protein-mediated signaling events. These events are generally mediated via the association of GPCRs with GPCR-associated proteins, such as β-arrestin. In particular, the activation of ERK1/2 by scaffolding complexes composed by β-arrestin, Src, and MAPK cascade effectors have already been reported (for review, see Lefkowitz and Shenoy, 2005). The possibility that the G protein-independent, Src-dependent activation of ERK1/2 by 5-HT4Rs involves β-arrestin was analyzed. The first argument against this possibility was the transient time course of ERK1/2 activation by 5-HT4R, compared with the generally persistent β-arrestin–mediated activation of ERKs. The second counter-argument was that the C-terminal cluster of Ser/Thr, which was necessary for the recruitment of β-arrestin by the 5-HT4Rs and for their endocytosis after 15 min of stimulation (Barthet et al., 2005), was not required for 5-HT4Rs–induced accumulation of p-ERKs (Figure 7). We particularly studied the Δ346 mutant that lacks this Ser/Thr cluster (Figure 7A) and showed that a 5-min agonist stimulation period of the truncated Δ346 mutant induced a robust activation of ERK1/2 that was blocked by PP2 (Figure 7A). A larger truncation of the C-terminal domain Δ327 did not modify the receptor's ability to mediate p-ERK1/2 accumulation, an effect blocked by PP2 (data not shown). We also confirmed that phosphorylation of ERKs by the Δ346 mutant was associated with an increase in p-Src (Tyr 416) (Figure 7B), which colocalized with the receptor at the plasma membrane. However, a short 5-min stimulation provided a way for the native 5-HT4R WT to recruit β-arrestin 2-YFP and form complexes localized on the plasma membrane (Figure 8A). On the contrary, no complexes could be detected between Δ346 and β-arrestin 2-YFP (Figure 8A). Thus, the Δ346 mutant was unable to recruit β-arrestin, but at the same time, it was still able to activate p-ERK1/2 and p-Src accumulations (Figure 7A). These data exclude a role for β-arrestin in the 5-HT4R–mediated activation of ERKs.
Figure 7.
5-HT4R–mediated ERK phosphorylation through Src activation does not involve the C-terminal domain of 5-HT4R. (A) The C terminus is not implicated in Src-dependent 5-HT4R–mediated ERK activation. Topology of the C-terminal domain of 5-HT4R WT and Δ346 is represented on the left. Putative Ser/Thr phosphate acceptor sites are represented by black circles. HEK293 cells were transfected with 100 ng of Rho-tagged WT and Δ346 5-HT4R. Serum-starved HEK293 cells expressing the WT and the mutant were pretreated or not with the Src kinase inhibitor PP2 at 10 μM for 30 min before the stimulation with 10 μM 5-HT for 5 min. Whole cell lysates were prepared and analyzed by immunoblotting with antibody to p-ERK1/2. A representative blot of each experiment is shown. Both WT and truncated mutant Δ346 phosphorylate ERK1/2 in the same manner, dependent on Src kinase. (B) Colocalization of p-ERK1/2 and active p-Src after WT and Δ346 stimulation. HEK293 transfected with 500 ng of Rho-tagged 5-HT4R or Rho-tagged Δ346 were seeded onto coverslips. Twenty-four hours after transfection, cells were serum starved overnight. Cells were incubated 90 min with antibody against Rho-tagged 5-HT4R at 4°C before a 5- or 30-min stimulation period with 10 μM 5-HT. After fixation and permeabilization, the cells were sequentially incubated with primary antibody against p-ERK1/2 or p-Src (Tyr 416) and with fluorochrome-labeled secondary antibody. Fluorescence microscopy was then used to visualize distribution of antibody-labeled receptors (green channel) and appearance of the phosphorylated form of active ERK1/2 and active Src (red channel). Immunofluorescence microscopy was performed using a Zeiss Axiophot2 microscope with Zeiss 63× NA 1.4 oil immersion lenses. Representative images from several independent experiments are shown. Top, distribution of Rho-tagged 5-HT4R or Rho-tagged Δ346 before (basal) or after 5-min treatment with 10 μM 5-HT at 37°C. An increase in phosphorylation of p-ERK1/2 is visualized at the plasma membrane after 5-min stimulation with 5-HT of both WT and Δ346. Merged images were magnified to show colocalization of both receptors (WT and Δ346) with p-ERK1/2 at the plasma membrane after stimulation. Bottom, phosphorylation state of active Src before (basal) and after (5 min) 5-HT4R stimulation. Phosphorylation states were enhanced after stimulation with 5-HT and localization of p-ERK and p-Src do not differ between WT and Δ346 after 5-min stimulation.
Figure 8.
Arrestins are not implicated in Src-dependent 5-HT4R–mediated ERK1/2 activation. (A) Δ346 does not promote β-arrestin 2 redistribution to the plasma membrane after stimulation. HEK293 transfected with 500 ng of Rho-tagged 5-HT4R or Rho-tagged Δ346 were seeded onto coverslips. Twenty-four hours after transfection, cells were serum starved overnight. Cells were incubated 90 min with antibody against Rho-tagged 5-HT4R at 4°C before a 5-min stimulation with 10 μM 5-HT. After fixation and permeabilization, cells were incubated with Alexa Fluor 594-labeled secondary antibody. Fluorescence microscopy was then used to visualize the distribution of antibody-labeled receptors (red channel) and the redistribution of YFP-β-arrestin 2 (green channel). Immunofluorescence microscopy was performed using a Zeiss Axiophot2 microscope with Zeiss 63× NA 1.4 oil immersion lenses. Representative images from several independent experiments are shown. Left, distribution of Rho-tagged WT and β-arrestin 2-YFP. WT-5-HT4R is expressed at the plasma membrane under basal conditions, whereas β-arrestin 2-YFP is homogenously present in the cytosol. After 5-min stimulation with 10 μM 5-HT at 37°C, β-arrestin 2-YFP is redistributed to the plasma membrane where the stimulated 5-HT4Rs are localized. Merged images were magnified to show colocalization of wt 5-HT4R with β-arrestin 2-YFP at the plasma membrane. Right, distribution of Rho-tagged Δ346 and β-arrestin 2-YFP. Δ346, initially characterized to lack the ability to traffic in endosomes with β-arrestin 2 after a long-term stimulation, also lack the ability to promote the translocation of β-arrestin to the plasma membrane after a 5-min period of stimulation. (B) Δ346 activates Src despite its inability to interact with β-arrestins. Cells transfected with Rho-tagged 5-HT4R WT or Rho-tagged Δ346 (800 ng) were incubated with 10 μM 5-HT for 5 min. 5-HT4Rs were purified from proteins extracts by immunoprecipitation by using anti-Rho-tag antibody. Coprecipitated proteins (p-Src and β-arrestins 1 and 2) were analyzed by Western blotting by using the antibody to p-Src (Tyr 416) (line 1) and the antibody to β-arrestins 1 and 2 (line 2). The presence of the receptor was revealed by using the antibody anti-Rho-tag (line 3). (C) Down-regulation of β-arrestin expression by siRNA does not inhibit ERK phosphorylation mediated by 5-HT4R. Early passage HEK293 were transfected with 5-HT4R and with control or β-arrestin 1- and 2-specific siRNA. Seventy-two hours after transfection, cells were stimulated 5 or 30 min before lysis. p-Src, p-ERK, and β-arrestin contents were analyzed by Western blotting. The same blot was probed and stripped, sequentially, for p-Src (Tyr 416) and p-ERK1/2 (lines 1 and 2) and β-arrestins (lines 3 and 4). A representative illustration of three experiments is shown in A–C.
We then analyzed by coimmunoprecipitation the physical interactions between p-Src and the receptors (WT and Δ346) and between β-arrestins and the same receptors. Immunoprecipitation of Rho-tagged WT and Δ346–5-HT4Rs resulted in a significant basal coimmunoprecipitation of p-Src that was largely increased after a 5-min stimulation with 5-HT (Figure 8B). The amounts of p-Src coimmunoprecipitated were comparable between the WT and the Δ346 (Figure 8B, line 1). The presence of endogenous β-arrestins coimmunoprecipitated was solely observed after 5 min of stimulation of the WT-5-HT4R. These data confirm that β-arrestins were tightly associated with activated 5-HT4R WT. On the contrary, Δ346 failed to interact with β-arrestin 1 and only weakly with β-arrestin 2 after stimulation (Figure 8B, line 2). These huge differences in β-arrestin recruitment between WT and Δ346 contrast with the equal aptitude of both WT and Δ346 to recruit p-Src (Figure 8B, line 1) and induce p-ERK1/2 activation (Figure 7A). Thus, Src and ERK1/2 activation did not seem to depend on physical interaction between 5-HT4Rs and β-arrestins.
Nonetheless, these results did not completely exclude the possibility that Src and ERK activations by 5-HT4Rs were dependent on β-arrestins. Thus, we examined the effect of β-arrestin depletion, by RNA interference, on 5-HT4R–mediated activation of p-ERK. As illustrated in Figure 8C, the depletion of both β-arrestins 1 and 2 (Figure 8C, lines 3 and 4) does not affect the 5-HT4R–mediated Src and ERK1/2 activation (Figure 8C, lines 1 and 2, respectively). The latter signal remains unaffected, whereas the amounts of β-arrestins 1 and 2 were reduced by >70%. Altogether, these data further confirm that ERK1/2 activation stimulated by 5-HT4R was not mediated by β-arrestin.
Moreover, neither β-arrestin nor receptor endocytosis were required. Overexpression of the dominant-negative β-arrestin (319–418) (which blocked 5-HT4R endocytosis; Barthet et al., 2005) as well as inhibition of endocytosis with hypertonic 0.4 M sucrose or with concanavalin A had any effect on 5-HT4R–mediated ERK stimulation (data not shown). These observations support the immunofluorescence studies indicating that p-ERK1/2 is present only at the plasma membrane level (Figures 6B and 7B).
Stimulation of Endogenous 5-HT4Rs in Neurons Induces pERK1/2 Accumulation in a Src-dependent Manner
It was important to establish whether the activation of ERK1/2 by endogenous 5-HT4Rs also involves Src phosphorylation in a pure neuronal cell population. This was particularly interesting to analyze because a 5- to 200-fold higher level of this protein is expressed in brain, compared with other tissues, as reported by Thomas and Brugge (1997). The data reported in Figure 9A revealed that in spite of a high basal content of p-Src (on Tyr 416), the 5-HT4R agonist BIMU8 increased p-Src (Tyr 416) content by 40 ± 3%, as well as p-ERK1/2 accumulation by 76 ± 5%. Moreover, pretreatment of neurons with 10 μM PP2 markedly decreased phosphorylation of p-ERK1/2 (Figure 9B). These results further support the involvement of Src kinases in 5-HT4R–mediated ERK phosphorylation in neurons. Then, we examined a pathway often implicated in Src activation by a GPCR in neurons, the transactivation of EGF-R (Shah and Catt, 2004). We tested the effect of AG1478, a selective EGF-R inhibitor, on ERK1/2 activation in response to 5-HT. As shown in Figure 9C, 250 nM AG1478 does not reduce ERK1/2 activation induced by 5-HT4R agonist in neurons, whereas it totally inhibits EGF-mediated stimulation. To study a possible role for Gi, we treated neuronal cells with PTX (Figure 9D). This treatment had no effect on 5-HT4R–induced ERK phosphorylation in neurons. These data clearly indicated that 5-HT4R expressed in HEK 293 cells or 5-HT4R endogenously expressed in colliculi neurons used the same pathway dependent on Src kinase to activate ERK phosphorylation.
Figure 9.
Src-dependent 5-HT4R–mediated ERK1/2 activation also occurs in neurons. (A) Colliculi neurons endogenously expressing 5-HT4R were stimulated with vehicle or BIMU8 (a 5-HT4R agonist). Total neuron lysates were analyzed by immunoblotting with the indicated antibodies. The same immunoblot was probed sequentially for p-Src with antibody to active p-Src (Tyr 416), for total inactive Src with antibody against pan Src, for p-ERK1/2 with the antibody to activate ERK1/2, and for total ERK1/2 with the antibody to ERK42/44. Quantification of p-Src and p-ERK1/2 was performed by densitometric analysis using NIH Image software. Data are means ± SEM of results obtained in four independent experiments. *p < 0.05 versus corresponding values measured in untreated neurons. (B) Colliculi neurons were pretreated with the Src kinase inhibitors PP2 at 10 μM for 30 min before the stimulation with 10 μM BIMU8 for 5 min. Neuron lysates were analyzed by immunoblotting with antibodies against p-Src and p-ERK1/2 as indicated in A. (C) 5-HT4R–mediated ERK activation does not require transactivation of EGF-R tyrosine kinase. Colliculi neurons were pretreated with 250 nM tyrphostin/AG1478 for 30 min before stimulation with 10 μM BIMU8 or 10 ng/ml EGF during 5 min. Total lysates were analyzed by immunoblotting with antibody to p-ERK1/2. (D) 5-HT4R–mediated ERK activation does not require G protein PTX sensitive (Gi/Go). Colliculi were pretreated with PTX at 50 or 100 ng/ml overnight before treatment with 10 μM BIMU8 for 5 min. Total lysates were analyzed by immunoblotting with antibody to p-ERK1/2. A representative blot of each experiment is shown in A–D.
DISCUSSION
The present study provides evidence that 5-HT4R stimulation, which mediates ERK activation, involves activation of the nonreceptor tyrosine kinase Src as a signaling event. If a number of GPCRs can function either independently of their G protein partners, or independently of β-arrestin, 5-HT4R mediated ERK activation is the first example that involves neither a classical Gα protein signaling (Gs/Gq/Gi) and associated messengers, nor β-arrestins. β3-AR does not require β-arrestins to induce ERK1/2 activation through a direct interaction with Src kinase, but it does require Gi proteins (Cao et al., 2000). V2 vasopressin receptors act through a mechanism independently of G protein signaling, by involving Src and β-arrestin (Charest et al., 2007).
In colliculi neurons, 5-HT4R–mediated ERK activation seems to be largely PKA independent, because BIMU8 still generated a strong ERK1/2 activation in the presence of 3 μM CMIQ, a selective PKA inhibitor (Figure 1D). We checked that 3 μM CMIQ was a concentration high enough to fully inhibit PKA in neurons (Figures 1C and 4C). Similarly, in HEK293 cells, inhibiting PKA either with 3 μM CMIQ or 1 μM H-89 did not result in inhibition of 5-HT4R–induced stimulation of ERK in these cells (Figure 4C). These results were unexpected, because in a previous report on HEK293 cells transfected with 5-HT4Rs, 20 μM H-89 inhibited 5-HT–induced ERK1/2 activation (Norum et al., 2003). The reasons for this discrepancy are not known. One possible explanation could be that at 20 μM H-89 loses its specificity and inhibits other kinases in addition to PKA. Indeed, a previous report mentions that from 10 μM, H-89 inhibited many kinases even stronger than PKA (Davies et al., 2000).
Based on these observations, we can postulate that the Gs/cAMP/PKA pathway and activation of ERK1/2 could be two independent 5-HT4R–mediated signaling both in neurons and HEK293 cells. It is interesting to note that Dyer et al. (2003) demonstrated that 5-HT–mediated ERK activation in Aplysia sensory neurons was not dependent on cAMP. These data do not indicate that cAMP is not able to stimulate ERK activation in neurons but only that activation of ERK by 5-HT4Rs is mostly cAMP/PKA independent. Indeed, activation of ERKs via a cAMP route is known to occur in several cells, such as primary neurons in culture (Vincent et al., 1998), PC12 cells, melanocytes, and thyroid cells, and it generally occurs via a B-Raf–MEK pathway (Dumaz and Marais, 2005). In contrast, in many other cells, cAMP inhibits ERK via inhibition of C-Raf, which leads to the well-known antiproliferate effects of cAMP (Dumaz and Marais, 2005).
We have used several pharmacological and genetic tools to obtain further arguments to exclude the Gs/cAMP/PKA pathway in HEK293 cells. SQ 22536, a potent inhibitor of the adenylyl cyclase, suppresses 5-HT4R–induced cAMP production without suppressing 5-HT4R–induced ERK activation. Finally, the D66N receptor mutants that stimulate cAMP production are unable to activate the ERK1/2 pathway. We also exclude any implication of the Gq/PLC pathway. For unknown reasons, 5-HT4Rs activate the production of IP in HEK293 cells but not in neurons (our unpublished data). Thus, we looked for a possible role of the Gq/PLC pathway in 5-HT4R–induced ERK activation in HEK293 cells. The role of PLC activation was excluded by showing that U 73122, an inhibitor of PLC, but not its inactive analogue, does not suppress the effect of 5-HT4Rs on p-ERK1/2, whereas it decreases their ability to activate IP production ∼75%. In addition, the W272A (W6.48) receptor mutants do not activate IP production, but they stimulate ERK activation. Finally, we eliminated a contribution of Gi by pretreating cells with PTX. Indeed, a switch from Gs to Gi can occur for Gs-coupled receptors, as demonstrated for β2-AR. In this latter case, the switch to Gi after Gs activation allows a subsequent stimulation of Gi, and the release of βγ subunits that causes ERK activation (Daaka et al., 1997). Our data also eliminate the PI3K pathway and transactivation of EGF receptor tyrosine kinases as contributors to 5-HT4R–mediated ERK activation.
It was surprising to find that classical G protein signaling pathways are not implicated in 5-HT4R–mediated ERK activation. Indeed, the rapid and transient time course of this activation was similar to the G protein-dependent components of ERK activation by other GPCRs, such as AT1AR, V2R and also β2-AR (Shenoy et al., 2006). The classical paradigm that relates the strong but transient increase of the p-ERK1/2 signal to the G protein-dependent pathway (Lefkowitz and Shenoy, 2005) seems to present some exceptions.
Indeed, 5-HT4R stimulation of p-ERK is totally dependent of Src activation. Moreover, the concentration–response curve of the 5-HT4R ligand-mediated p-ERK indicated that only high concentrations of agonist contributed to p-ERK phosphorylation (>10−7 M). No p-ERK signal was observed before administering 10−7 M 5-HT4R agonist, whereas cAMP formation was maximal at 10−8 M 5-HT. Our observations are consistent with those by Sun et al. (2007) who recently reported that β2-AR G protein-coupled signaling needs low concentrations of isoproterenol, whereas β2-AR non-G protein signaling needs high concentrations of this agonist (Sun et al., 2007). Why do Gs protein/cAMP- and Src-dependent signaling pathways require such different agonist concentrations when they involve the same 5-HT4R? One likely explanation is that the receptor can adopt two different activated (R*) conformations. One conformation is stabilized by low 5-HT concentrations (activating the Gs signaling pathway), and the other conformation is stabilized by high 5-HT concentrations (activating the Src signaling pathway). In this regard, we have already provided evidence for the occurrence of multiple and well-defined conformational states of the 5-HT4R, depending on the ligand (Baneres et al., 2005).
Previous observations demonstrate that activation of 5-HT4Rs in the enterocyte cell line Caco2 inhibits apical Cl−/OH− exchange activity, via activation of the nonreceptor tyrosine kinase Src (Saksena et al., 2005). This result does not seem to be cell specific, because we demonstrated that 5-HT4Rs also stimulated Src activation, both in epithelial HEK293 cell lines and in neurons in primary culture. In these cells, activation of Src was required to activate the ERK1/2 pathway by 5-HT4Rs.
By immunoblotting and immunofluorescence staining, we observed that the ERK1/2 activation by 5-HT4Rs was very transient. In contrast, the activation of Src was more persistent and still present at 30 min. Active Src remained associated with the internalized receptor and was accumulated in a perinuclear compartment. Moreover, upon stimulation, 5-HT4Rs coimmunoprecipitated with p-Src. Further work is needed to determine the putative cellular role, if any, of this endocytosed receptor–p-Src complex.
One of the key questions was to know how 5-HT4Rs mediate Src activation. Do 5-HT4Rs recruit Src kinase directly, or do they require β-arrestin recruitment, as shown for some GPCRs (Lefkowitz and Shenoy, 2005)? We used many approaches to demonstrate that β-arrestin recruitment was not necessary to observe a 5-HT4R–mediated phosphorylation of both ERK and Src. First, the time course of 5-HT–induced ERK activation (Figures 1B and 2A) did not support the involvement of β-arrestin in the 5-HT4R–induced p-ERK1/2 accumulation. Indeed, the β-arrestin–dependent activation of ERK1/2 by GPCRs was associated with receptor endocytosis and was generally slow and persistent (Shenoy et al., 2006), whereas the 5-HT4R activation of ERK1/2 was very rapid and transient. Second, the absence of involvement of β-arrestin in this pathway was also supported by our previous and present reports demonstrating that the Δ346 mutant, devoid of the C-terminal tail cluster of serine/threonine, failed to bind to β-arrestin, but it was able to stimulate ERK1/2 in a Src-dependent manner, like native 5-HT4Rs. Third, the depletion by RNA interference of β-arrestins 1 and 2 did not modify the ability of 5-HT4Rs to induce ERK1/2 and Src phosphorylations. All these data clearly confirm that ERK activation stimulated by 5-HT4R was not mediated by β-arrestin. This was an unexpected finding, because 5-HT4Rs belong to class B type, and they have been shown to be stably associated with β-arrestin without recycling or degradation over a long period (Barthet et al., 2005).
Arrestin seems to be an absolute requirement for ERK activation by some GPCRs, including α2-ARs (Wang et al., 2004), gonadotropin-releasing hormone receptors (Benard et al., 2001), and the D2 and D3 dopamine receptors (Beom et al., 2004). However, 5-HT4Rs seem to be the only GPCRs that activate ERK1/2 in a G protein signaling-independent and β-arrestin–independent pathway. Note also that 5-HT4R–mediated ERK1/2 activation was also independent of receptor endocytosis, as already demonstrated for the α2-AR (Wang et al., 2004). To date, we have not been able to determine whether Src and p-Src interact directly or indirectly via other unknown scaffolding proteins to 5-HT4Rs. The absence of a PXXP sequence in 5-HT4R sequences excludes the possibility that the receptors bind to the Src homology 3 (SH3) domain. In contrast, there are several Tyr residues within the cytoplasmic domains of the 5-HT4Rs (loops i2, i3) and carboxy terminal (C-t) that are all putative SH2-binding sites after phosphorylation. The different C-t truncated 5-HT4Rs (Δ327 and Δ346) were still able to activate the ERK cascade through Src. This indicates that the Tyr residues of C-t were not implicated in Src recruitment. This is in contrast with what has been reported for the β2-AR. Indeed, Fan et al. (2001) demonstrated that phospho-Tyr 350 in the C-t of β2-AR is important for Src binding. However, in contrast to our data, Src binds the β2-AR to mediate agonist-induced receptor desensitization but not to mediate ERK activation (Fan et al., 2001). We have generated several mutants in which, Tyr was substituted by Phe (Tyr 119, Tyr 129, Tyr 212, and Tyr 216) present in i2 and i3 loops. Unfortunately, all the mutants could still recruit p-Src and stimulate ERK1/2, in a pathway sensitive to PP2 (data not shown). Therefore, it is possible that either an intermediary protein is necessary for the 5-HT4R–pSrc association or that a conformational epitope of the receptor is needed for p-Src recruitment.
The ERK1/2 signaling pathway is critical for multiple cellular processes that include proliferation, differentiation, survival, and migration. Kinetics and subcellular compartmentalization of p-ERKs are the major factors determining which cellular responses are triggered by active ERK signaling (Caunt et al., 2006). In our study, we clearly observed a plasma membrane-restricted ERK activation by immunofluorescence (Figures 6B and 7B). These data are similar to previous findings by Scott et al. (2006) demonstrating a role of activated ERK1/2 at the plasma membrane. In this compartment, p-ERK seems to be implicated in membrane ruffling and cell migration in Hep2 epithelial cells. In neurons, ERK activated at the plasma membrane level could participate in morphological modifications involved in neuroplasticity: neurite outgrowth, dendritic spine maturation, or synapse formation. The finding that 5-HT4Rs activate ERK in neurons sheds light on the capacity of 5-HT4R agonists to improve recognition memory, spatial learning, and cognition (Marchetti et al., 2000, 2004; Micale et al., 2006). Moreover, this signaling pathway could participate in a 5-HT4R–generated shift from long-term depression to LTP in the hippocampal CA1 region, a process that supports memory formation (Kemp and Manahan-Vaughan, 2004, 2005).
Our data highlight an emerging role for 5-HT4Rs in mediating ERK activation that could be important in learning and memory. We propose that the link between 5-HT4Rs and memory could involve both the cAMP/PKA and the Src/ERK signaling pathways. Nonetheless, a direct relationship between 5-HT4R–mediated ERK activation and memory processing requires further investigation.
ACKNOWLEDGMENTS
We are grateful to Leigh-Anne Swayne for constructive discussion and critical reading of the manuscript and to Angie Turner-Madeuf for help in language revision. We thank the Pharmacological screening platform of the Federal Institute of Research (IFR3) for the use of its facilities. This work was supported by grants from the Centre National de la Recherche Scientifique, the Génopole de Montpellier–Languedoc-Roussillon, La Region–Languedoc-Roussillon, Pfizer Japan, and the European Community's Sixth Framework Program (grant LSHB-CT-2003-503337). Gaël Barthet was supported by grants from the Fondation pour la Recherche Medicale.
Abbreviations used:
- β2AR
β2-adrenergic receptor
- AT1A
angiotensin II type 1A receptor
- BSA
bovine serum albumin
- C-t
carboxy terminal
- ERK
extracellular signal-regulated kinase
- GPCR
G protein-coupled receptor
- HA
hemagglutinin
- HEK
human embryonic kidney
- 5-HT
5-hydroxytryptamine (serotonin)
- 5-HT4R
5-hydroxytryptamine4 receptor
- IP
inositol phosphates
- PI3K
phosphatidylinositol 3-kinase (PI3Ks)
- PKA
protein kinase A
- V2R
vasopressin 2 receptor
- WT
wild type
- YFP
yellow fluorescent protein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-12-1080) on March 21, 2007.
REFERENCES
- Adamus G., Arendt A., Hargrave P. A. Genetic control of antibody response to bovine rhodopsin in mice: epitope mapping of rhodopsin structure. J. Neuroimmunol. 1991;34:89–97. doi: 10.1016/0165-5728(91)90118-q. [DOI] [PubMed] [Google Scholar]
- Bain J., McLauchlan H., Elliott M., Cohen P. The specificities of protein kinase inhibitors: an update. Biochem. J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M., Hery M., Printemps R., Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- Baneres J. L., Mesnier D., Martin A., Joubert L., Dumuis A., Bockaert J. Molecular characterization of a purified 5-HT4 receptor: a structural basis for drug efficacy. J. Biol. Chem. 2005;280:20253–20260. doi: 10.1074/jbc.M412009200. [DOI] [PubMed] [Google Scholar]
- Barthet G., Gaven F., Framery B., Shinjo K., Nakamura T., Claeysen S., Bockaert J., Dumuis A. Uncoupling and endocytosis of 5-hydroxytryptamine 4 receptors. Distinct molecular events with different GRK2 requirements. J. Biol. Chem. 2005;280:27924–27934. doi: 10.1074/jbc.M502272200. [DOI] [PubMed] [Google Scholar]
- Benard O., Naor Z., Seger R. Role of dynamin, Src, and Ras in the protein kinase C-mediated activation of ERK by gonadotropin-releasing hormone. J. Biol. Chem. 2001;276:4554–4563. doi: 10.1074/jbc.M006995200. [DOI] [PubMed] [Google Scholar]
- Beom S., Cheong D., Torres G., Caron M. G., Kim K. M. Comparative studies of molecular mechanisms of dopamine D2 and D3 receptors for the activation of extracellular signal-regulated kinase. J. Biol. Chem. 2004;279:28304–28314. doi: 10.1074/jbc.M403899200. [DOI] [PubMed] [Google Scholar]
- Bockaert J., Claeysen S., Compan V., Dumuis A. 5-HT4 receptors. Curr. Drug Targets CNS Neurol. Disord. 2004;3:39–51. doi: 10.2174/1568007043482615. [DOI] [PubMed] [Google Scholar]
- Cao W., Luttrell L. M., Medvedev A. V., Pierce K. L., Daniel K. W., Dixon T. M., Lefkowitz R. J., Collins S. Direct binding of activated c-Src to the beta 3-adrenergic receptor is required for MAP kinase activation. J. Biol. Chem. 2000;275:38131–38134. doi: 10.1074/jbc.C000592200. [DOI] [PubMed] [Google Scholar]
- Caunt C. J., Finch A. R., Sedgley K. R., McArdle C. A. Seven-transmembrane receptor signalling and ERK compartmentalization. Trends Endocrinol. Metab. 2006;17:276–283. doi: 10.1016/j.tem.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Charest P. G., Oligny-Longpre G., Bonin H., Azzi M., Bouvier M. The V2 vasopressin receptor stimulates ERK1/2 activity independently of heterotrimeric G protein signalling. Cell Signal. 2007;19:32–41. doi: 10.1016/j.cellsig.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Claeysen S., Sebben M., Becamel C., Bockaert J., Dumuis A. Novel brain-specific 5-HT4 receptor splice variants show marked constitutive activity: role of the C-terminal intracellular domain. Mol. Pharmacol. 1999;55:910–920. [PubMed] [Google Scholar]
- Daaka Y., Luttrell L. M., Lefkowitz R. J. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- Davies S. P., Reddy H., Caivano M., Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumaz N., Marais R. Integrating signals between cAMP and the RAS/RAF/MEK/ERK signalling pathways. Based on the anniversary prize of the Gesellschaft fur Biochemie und Molekularbiologie Lecture delivered on 5 July 2003 at the Special FEBS Meeting in Brussels. FEBS J. 2005;272:3491–3504. doi: 10.1111/j.1742-4658.2005.04763.x. [DOI] [PubMed] [Google Scholar]
- Dumuis A., Bouhelal R., Sebben M., Bockaert J. A 5-HT receptor in the central nervous system positively coupled with adenylate cyclase is antagonized by ICS 205 930. Eur. J. Pharmacol. 1988a;146:187–188. doi: 10.1016/0014-2999(88)90503-1. [DOI] [PubMed] [Google Scholar]
- Dyer J. R., Manseau F., Castellucci V. F., Sossin W. S. Serotonin persistently activates the extracellular signal-related kinase in sensory neurons of Aplysia independently of cAMP or protein kinase C. Neuroscience. 2003;116:13–17. doi: 10.1016/s0306-4522(02)00566-3. [DOI] [PubMed] [Google Scholar]
- Fan G., Shumay E., Malbon C. C., Wang H. c-Src tyrosine kinase binds the beta 2-adrenergic receptor via phospho-Tyr-350, phosphorylates G-protein-linked receptor kinase 2, and mediates agonist-induced receptor desensitization. J. Biol. Chem. 2001;276:13240–13247. doi: 10.1074/jbc.M011578200. [DOI] [PubMed] [Google Scholar]
- Gaspar P., Cases O., Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat. Rev. Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gesty-Palmer D., et al. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J. Biol. Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- He Y., Yeung E. S. High-throughput screening of kinase inhibitors by multiplex capillary electrophoresis with UV absorption detection. Electrophoresis. 2003;24:101–108. doi: 10.1002/elps.200390000. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning. Science. 1982;218:433–438. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Kara E., Crepieux P., Gauthier C., Martinat N., Piketty V., Guillou F., Reiter E. A phosphorylation cluster of five serine and threonine residues in the C-terminus of the follicle-stimulating hormone receptor is important for desensitization but not for beta-arrestin-mediated ERK activation. Mol. Endocrinol. 2006;20:3014–3026. doi: 10.1210/me.2006-0098. [DOI] [PubMed] [Google Scholar]
- Kater S. B., Mattson M. P., Cohan C., Connor J. Calcium regulation of the neuronal growth cone. Trends Neurosci. 1988;11:315–321. doi: 10.1016/0166-2236(88)90094-x. [DOI] [PubMed] [Google Scholar]
- Kemp A., Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc. Natl. Acad. Sci. USA. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A., Manahan-Vaughan D. The 5-hydroxytryptamine4 receptor exhibits frequency-dependent properties in synaptic plasticity and behavioural metaplasticity in the hippocampal CA1 region in vivo. Cereb. Cortex. 2005;15:1037–1043. doi: 10.1093/cercor/bhh204. [DOI] [PubMed] [Google Scholar]
- Kim J., Ahn S., Ren X. R., Whalen E. J., Reiter E., Wei H., Lefkowitz R. J. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc. Natl. Acad. Sci. USA. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R. J., Shenoy S. K. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Liu J., Liao Z., Camden J., Griffin K. D., Garrad R. C., Santiago-Perez L. I., Gonzalez F. A., Seye C. I., Weisman G. A., Erb L. Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J. Biol. Chem. 2004;279:8212–8218. doi: 10.1074/jbc.M312230200. [DOI] [PubMed] [Google Scholar]
- Luttrell D. K., Luttrell L. M. Not so strange bedfellows: G-protein-coupled receptors and Src family kinases. Oncogene. 2004;23:7969–7978. doi: 10.1038/sj.onc.1208162. [DOI] [PubMed] [Google Scholar]
- Luttrell L. M. Composition and function of g protein-coupled receptor signalsomes controlling mitogen-activated protein kinase activity. J. Mol. Neurosci. 2005;26:253–264. doi: 10.1385/JMN:26:2-3:253. [DOI] [PubMed] [Google Scholar]
- Marchetti E., Chaillan F. A., Dumuis A., Bockaert J., Soumireu-Mourat B., Roman F. S. Modulation of memory processes and cellular excitability in the dentate gyrus of freely moving rats by a 5-HT4 receptors partial agonist, and an antagonist. Neuropharmacology. 2004;47:1021–1035. doi: 10.1016/j.neuropharm.2004.06.033. [DOI] [PubMed] [Google Scholar]
- Marchetti E., Dumuis A., Bockaert J., Soumireu-Mourat B., Roman F. S. Differential modulation of the 5-HT(4) receptor agonists and antagonist on rat learning and memory. Neuropharmacology. 2000;39:2017–2027. doi: 10.1016/s0028-3908(00)00038-1. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Guthrie P. B., Kater S. B. Intracellular messengers in the generation and degeneration of hippocampal neuroarchitecture. J. Neurosci. Res. 1988;21:447–464. doi: 10.1002/jnr.490210236. [DOI] [PubMed] [Google Scholar]
- Micale V., Marco Leggio G., Mazzola C., Drago F. Cognitive effects of SL65.0155, a serotonin 5-HT(4) receptor partial agonist, in animal models of amnesia. Brain Res. 2006;1121:207–215. doi: 10.1016/j.brainres.2006.08.108. [DOI] [PubMed] [Google Scholar]
- Michael D., Martin K. C., Seger R., Ning M. M., Baston R., Kandel E. R. Repeated pulses of serotonin required for long-term facilitation activate mitogen-activated protein kinase in sensory neurons of Aplysia. Proc. Natl. Acad. Sci. USA. 1998;95:1864–1869. doi: 10.1073/pnas.95.4.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norum J. H., Hart K., Levy F. O. Ras-dependent ERK activation by the human G(s)-coupled serotonin receptors 5-HT4(b) and 5-HT7(a) J. Biol. Chem. 2003;278:3098–3104. doi: 10.1074/jbc.M206237200. [DOI] [PubMed] [Google Scholar]
- Oakley R. H., Laporte S. A., Holt J. A., Barak L. S., Caron M. G. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-beta-arrestin complexes after receptor endocytosis*. J. Biol. Chem. 2001;276:19452–19460. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- Ren X. R., Reiter E., Ahn S., Kim J., Chen W., Lefkowitz R. J. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc. Natl. Acad. Sci. USA. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A., Manen D., Rizzoli R., Caverzasio J., Ferrari S. L. Proline-rich motifs in the PTH/PTHrP-receptor C-terminus mediate scaffolding of c-Src with β-arrestin2 for ERK1/2 activation. J. Biol. Chem. 2006;281:38181–38188. doi: 10.1074/jbc.M606762200. [DOI] [PubMed] [Google Scholar]
- Saksena S., Gill R. K., Tyagi S., Alrefai W. A., Sarwar Z., Ramaswamy K., Dudeja P. K. Involvement of c-Src and protein kinase C δ in the inhibition of Cl(−)/OH− exchange activity in Caco-2 cells by serotonin. J. Biol. Chem. 2005;280:11859–11868. doi: 10.1074/jbc.M411553200. [DOI] [PubMed] [Google Scholar]
- Scott M. G., Pierotti V., Storez H., Lindberg E., Thuret A., Muntaner O., Labbe-Jullie C., Pitcher J. A., Marullo S. Cooperative regulation of extracellular signal-regulated kinase activation and cell shape change by filamin A and beta-arrestins. Mol. Cell. Biol. 2006;26:3432–3445. doi: 10.1128/MCB.26.9.3432-3445.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah B. H., Catt K. J. GPCR-mediated transactivation of RTKs in the CNS: mechanisms and consequences. Trends Neurosci. 2004;27:48–53. doi: 10.1016/j.tins.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Shenoy S. K., Drake M. T., Nelson C. D., Houtz D. A., Xiao K., Madabushi S., Reiter E., Premont R. T., Lichtarge O., Lefkowitz R. J. beta-arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J. Biol. Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Kandel E. R. Learning related synaptic plasticity: LTP and LTD. Curr. Opin. Neurobiol. 1991;1:113–120. doi: 10.1016/0959-4388(91)90018-3. [DOI] [PubMed] [Google Scholar]
- Sun Y., Huang J., Xiang Y., Bastepe M., Juppner H., Kobilka B. K., Zhang J. J., Huang X. Y. Dosage-dependent switch from G protein-coupled to G protein-independent signaling by a GPCR. EMBO J. 2007;26:53–64. doi: 10.1038/sj.emboj.7601502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt J. D. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr. Opin. Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Thomas S. M., Brugge J. S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Turner N. A., Ball S. G., Balmforth A. J. The mechanism of angiotensin II-induced extracellular signal-regulated kinase-1/2 activation is independent of angiotensin AT(1A) receptor internalisation. Cell Signal. 2001;13:269–277. doi: 10.1016/s0898-6568(01)00135-8. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Sebben M., Dumuis A., Bockaert J. Neurotransmitter regulation of MAP kinase signaling in striatal neurons in primary culture. Synapse. 1998;29:29–36. doi: 10.1002/(SICI)1098-2396(199805)29:1<29::AID-SYN3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Wang Q., Lu R., Zhao J., Limbird L. E. Arrestin serves as a molecular switch, linking endogenous {alpha}2-adrenergic receptor to SRC-dependent, but not SRC-independent, ERK activation. J. Biol. Chem. 2006;281:25948–25955. doi: 10.1074/jbc.M605415200. [DOI] [PubMed] [Google Scholar]
- Wang Q., Zhao J., Brady A. E., Feng J., Allen P. B., Lefkowitz R. J., Greengard P., Limbird L. E. Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science. 2004;304:1940–1944. doi: 10.1126/science.1098274. [DOI] [PubMed] [Google Scholar]