Abstract
Serum- and glucocorticoid-induced kinase 1 is a ubiquitous kinase that regulates diverse processes such as ion transport and cell survival. We report that a single SGK1 mRNA produces isoforms with different N-termini owing to alternative translation initiation. The long isoforms, 49 and 47 kDa, are the most abundant, localize to the ER membrane, exhibit rapid turnover, their expression is decreased by ER stress, activate the epithelial sodium channel (ENaC) and translocate FoxO3a transcriptional factors from the nucleus to the cytoplasm. The short isoforms, 45 and 42 kDa, localize to the cytoplasm and nucleus, exhibit long half-life and phosphorylate glycogen synthase kinase-3β. The data indicate that activation of Sgk1 in different cellular compartments is key to providing functional specificity to Sgk1 signaling pathways. We conclude that the distinct properties and functional specialization of Sgk1 given by the N-terminus confer versatility of function while maintaining the same core kinase domain.
INTRODUCTION
Serum- and glucocorticoid-induced kinases are S/T protein kinases structurally related to the large AGC family of protein kinases that include PKA, atypical isoforms of PKC, and PKB/Akt. The mouse and human genomes contain three independent genes that encode closely related proteins known as Sgk1, Sgk2, and Sgk3 (Kobayashi and Cohen, 1999; Kobayashi et al., 1999). These kinases differ in the N-terminus but share a high degree of homology, 74% amino acid identity, in the catalytic domain.
Sgk1 is distinct among other AGC kinases by being regulated at multiple levels. Transcription of Sgk1 is induced rapidly and differentially in many tissues by serum, hormones (cortisol, aldosterone, and follicle-stimulating hormone), transforming growth factor β (Waldergger et al., 1999; Gomis et al., 2006), and stress conditions (UV light, changes in osmolarity and oxidative stress; Firestone et al., 2003, Gomis et al., 2006). Posttranscriptionally, Sgk1 is activated by sequential phosphorylation on residues S422 and T253 by a still unidentified kinase (PDK2) and phosphatidylinositol-3-phosphate dependent kinase 1 (PDK1), respectively (Kobayashi and Cohen, 1999). The abundance of Sgk1 protein is maintained at low basal levels by rapid degradation mediated by the ubiquitin proteasomal machinery associated to the endoplasmic reticulum (ER) (Arteaga et al., 2006; Bogusz et al., 2006). Lastly, under certain stimuli Sgk1 translocates from the cytoplasm to the nucleus where it phosphorylates transcriptional factors (Firestone et al., 2003).
Sgk1 participates in many cellular processes including regulation of activity/traffic of plasma membrane ion channels and transporters (ENaC, ROMK, Na/H-exchanger, Na, K-ATPAse) (Lang et al., 2006). The main phenotype of mice null for Sgk1 (Sgk1−/−) is indeed defective sodium retention when the animals are deprived of salt (Wulff et al., 2002). Sgk1 also increases cell survival after various stress conditions, a process that is in part mediated by phosphorylation of FoxO family of transcriptional factors (FoxO1, FoxO3, and FoxO4), whereby FoxO proteins translocate from the nucleus to the cytoplasm (You et al., 2004). In Caenorhabditis elegans the homolog of Sgk1 directly phosphorylates DAF-16/FoxO3A and is crucial for the control of development, stress response, and longevity (Hertweck et al., 2004). There is also strong evidence in C. elegans that both Sgk1 and Akt1 are required for signaling downstream of insulin receptor activation. Similarly, in mammalian cells Sgk1 directly or indirectly phosphorylates GSK3β, a downstream component of the insulin pathway (Kobayashi and Cohen, 1999). However, the final effects, i.e., either enhancement of sodium or glucose transport activity (Arteaga and Canessa, 2005), DNA synthesis or oncogenic transformation of cells, depend on whether Akt or Sgk1 is the activate kinase (Sakoda et al., 2003), indicating that functional specificity of these kinases goes beyond selectivity of substrate phosphorylation.
To date, it is not known how Sgk1 mediates such a broad spectrum of effects while still maintaining specificity within and among cells. In this work we report that Sgk1 expresses as four different isoforms with distinct properties, differential targeting and activation in subcellular compartments thereby the isoforms confer functional specificity to the many signaling pathways mediated by Sgk1.
MATERIALS AND METHODS
Cell Lines and Culture
Chinese hamster ovary (CHO), COS, M1, and HEK-293 cell lines were maintained in α-MEM or MEM supplemented with 10% fetal bovine serum, penicillin, and streptomycin in an incubator set at 37°C and 5% CO2. A6 cells were maintained in amphibian medium at 29°C and 1.5% CO2 (Alvarez de la Rosa and Canessa, 2003). Stable cell lines were generated by lipofectamine transfection of a 5:1 mixture of two plasmids pcDNA6/TR and pcDNA4/ TO containing the following mouse cDNAs: Sgk1/HA, Sgk1S422D/HA, Δ60Sgk1/HA and Δ60Sgk1S422D/HA. Clones were selected in medium containing 500 μg/ml zeocin and 10 μg/ml blasticidin as described previously (Alvarez de la Rosa and Canessa, 2003). Cells were expanded on plastic dishes and seeded on Transwell permeable supports, 0.33 cm2 (Corning, NY) for experiments. Expression of Sgk1 constructs was examined by Western blotting with anti-HA antibody in cells treated ±1 μg/ml tetracycline.
Generation of SGK1-HA Transgenic Mouse
A mouse BAC containing the whole SGK1 gene (RP24–332J7 from CHORI) was modified by the addition of three repeats of the HA tag at the C-terminus of SGK1 gene by homologous recombination in Escherichia coli as described (Lalioti and Heath, 2001). Linearized BAC was injected into fertilized oocytes. Founders were identified by PCR of genomic tail DNA with specific primers. Expression of Sgk1-HA mRNA in tissues was ascertained by RT-PCR using specific primers (Supplemental Figure 3).
Other cDNA Constructs and Mutagenesis
Human Sgk1 and FoxO3a cDNAs were from TrueClone (OriGene Technologies, Rockville, MD). Mouse and Xenopus Sgk1 and mouse GSK3β were cloned by RT-PCR from kidney RNA. HA, FLAG, or V5 tags were added to the C-terminus of the cDNAs by PCR or ligation of an oligonucleotide, and the products were subcloned into pcDNA3.1/V5-His TOPO vector (Invitrogen, Carlsbad, CA). Site directed mutagenesis of mouse Sgk1 cDNA was conducted with QuickChange (Stratagene, La Jolla, CA). All final constructs were sequenced at Yale University's Keck Facility (New Haven, CT).
Pulse Chase and Rate of Synthesis
Cells were transfected with Lipofectamine 2000 (Invitrogen). After 16 h cells were washed with DMEM serum-free medium without methionine and cysteine. Cells were subsequently labeled with 150 μCi/ml Express Cell Labeling Mix (Perkin Elmer-Cetus Life Sciences, Boston, MA) for 20 min. When appropriate, chase medium containing a 10-fold molar excess of both methionine/cysteine and 0.1 mg/ml cycloheximide was added, and cells were returned to the incubator for different periods of time. For rate of synthesis, cells were labeled with labeling mix for increasing time periods, 0–30 min. Cells were washed with phosphate-buffer saline (PBS), suspended in 0.2 ml of lysis buffer (in mM: 150 NaCl, 5 EDTA, 50 HEPES, pH 7.5, and 1.0% Triton X-100). After centrifugation (15,000 × g for 15 min), supernatants were denatured in 1% SDS and heated at 95°C for 3 min. SDS concentration was brought to 0.1% by addition of lysis buffer. Protein concentration was measured with the bicinchoninic acid procedure (Pierce Chemical Co., Rockford, IL), and equal amounts were immunoprecipitated with 5 μl anti-Sgkct antibody (Alvarez de la Rosa et al., 2003) and protein A beads. Beads were washed with 1 ml lysis buffer and eluted with SDS-PAGE sample buffer followed by electrophoresis. Gels were exposed to x-ray film and analyzed by densitometry with Bio-Rad G800 (Richmond, CA) and QuantityOne software.
Phosphorylation Assay
CHO cells cotransfected with GSK3β-HA and different constructs of Sgk1-FLAG were washed with medium without phosphate, followed by labeling with 0.5 mCi of [32P]orthophosphate for 2 h. Cells were homogenized in lysis buffer supplemented with phosphatase inhibitors (in mM: 2 pyrophosphate, 2 Na3VO4, 1 NaF, and 10 nM microcystin) and subjected to immunoprecipitation with anti-HA antibody as formerly indicated.
Western Blotting
Before electrophoresis samples were treated with Endo-H or PNGase F (New England Biolabs, Beverly, MA), calf intestinal phosphatase (CIP), shrimp alkaline phosphatase (SAP), protein phosphatase 2A (PP2A; Sigma), or protease inhibitors (Complete, Roche, Alameda, CA). SDS-PAGE, 7.5%, was transferred to Immobilon-P membrane. After blocking with 5% dry milk, the membranes were probed with primary antibody, either anti-hyaluronic acid (HA)-horseradish peroxidase (HRP; Santa Cruz Biotechnology, Santa Cruz, CA) or anti-Sgk1 antibodies. Signals were developed with ECL+ (Amersham, Piscataway, NJ), and blots were exposed to BioMax MR film (Eastman Kodak, Rochester, NY).
Immunofluorescence Microscopy
Transfected cells were fixed with 4% formaldehyde, washed with PBS, permeabilized with 1% Triton X-100 in PBS, and blocked with 10% goat serum and 0.5% Triton X-100 for 30 min. Primary antibodies (monoclonal anti-HA, anti-FLAG, and anti-V5 (1:200–1:500 dilutions) were incubated for 1 h. After washes with PBS secondary antibody AlexaFluor-488 or AlexaFluor-594 goat anti-mouse IgG (Molecular Probes, Eugene, OR) were added at a 1:400 dilution and incubated for 1 h. Slides were covered with mounting solution containing DAPI. Cells were examined with a Zeiss Axiophot microscope (Thornwood, NY), and images were acquired with a Zeiss IE-24 LSM 510 Meta confocal microscope.
Short-Circuit Current Measurement
Transepithelial voltage (VT) across A6 cells grown on Transwells was measured with Ag/AgCl2 electrodes (electronic shop, Yale University) and short circuit current (Isc) was measured in cells clamped at 0 mV with a DVC-1000 voltage/current clamp apparatus (WPI Industries, Sarasota, FL). Transepithelial resistance (RT) was calculated from the change in current induced by a 20-mV pulse in the voltage clamp. Amiloride, 10 μM, was added to the apical side at the end of experiments to calculate the amiloride-sensitive component of the Isc. Cells were maintained in serum-free medium for 12 h before experiments. Tetracycline, 1 μg/ml, was added to the basolateral side when indicated.
RESULTS
Mouse Sgk1 mRNA Generates Four Protein Species with Different Molecular Weights
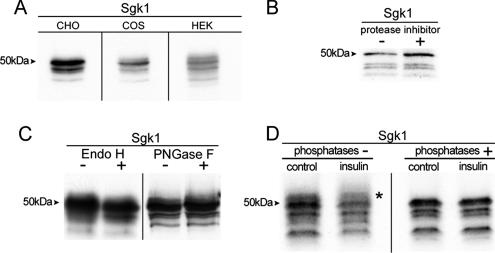
Transfection of mouse Sgk1 cDNA into three different cell lines CHO, COS, and HEK-293 results in expression of four bands of 49, 47, 45, and 42 kDa when resolved on denaturing 7.5% polyacrylamide gels (Figure 1A). This finding suggests that a posttranslational modification gives rise to bands of apparent smaller molecular weight that the one predicted from the amino acid composition. We examined whether partial proteolysis, glycosylation, or phosphorylation is the source of the modification. Cell lysates were treated with or without a mixture of several protease inhibitors (PMSF and Complete from Roche), N-glycosidase F or Endo-H, or phosphatases (CIP, SAP, and PP2A). These treatments did not change the size or intensity of the four bands (Figure 1, B–D); although, after phosphatase treatment, we noticed the disappearance of a fuzzy band above the 49-kDa one (asterisk in Figure 1D), which may represent a phosphorylated form of Sgk1 as previously described (Park et al., 1999). We confirmed activity of the phosphatases by digestion of [32P]-labeled proteins (Supplemental Figure 1). Furthermore, the mutant Sgk1T253A/S422A, which cannot be phosphorylated in the two activating residues, reproduced the four described bands, indicating that they do not arise from phosphorylation (Supplemental Figure 2). Together these results indicate that the usual posttranslational processes do not generate the multiple bands observed in Sgk1.
Figure 1.
Immunoprecipitation of Sgk1 from transfected CHO labeled with [35S]methionine/cysteine resolved on 7.5% SDS-PAGE gels. (A) Mouse Sgk1 cDNA transfected in various mammalian cell lines. (B) Cell lysates ± protease inhibitors. (C) IPs were treated with or without glycanases. (D) Control or cells pretreated with insulin were lysed and IP products were exposed to ± phosphatases. Arrowhead indicates molecular weights. Asterisk indicates a high-molecular-weight band removed by phosphatases.
Alternative Translation Initiation Produces Four Sgk1 Isoforms
Analysis of the sequence of mouse Sgk1 cDNA reveals the presence of seven in-frame methionine codons in the N-terminus, some of which are surrounded by bases that conform to the canonical Kozak sequence recognized by the translation initiation machinery in eukaryotes (Table 1).
Table 1.
cDNA sequences surrounding methionine residues in the N-terminus of human, mouse, frog, and shark Sgk1
| M1 | M17 | M20 | M28 | M33 | M60 | M69 | |
|---|---|---|---|---|---|---|---|
| Human | GTGGTGATGA | TCCAGGATGA | AGGGGCATGG | GCTTTCATGA | AGCAGGATGG | GAGCTTATGA | |
| Mouse | GGGACGATGA | TCCAGAATGA | AGGGGAATGG | GCTTTTATGA | AGAAGGATGG | TTGAAAATGT | GAGCTTATGA |
| Frog | CCAACCATGA | TCTAAGATGA | AGGGGAATGG | GCTTTTATGA | CGAAGAATGG | ||
| Shark | TCAGCCATGA | TCCAAAATGA | GCTTTTATGA | AAACGAATGG | CCAGCCATGT | ACTGAGATGA |
The ATG codons are underlined, and the bases at positions −3 and +4 that conform to the canonical Kozak sequence are in bold.
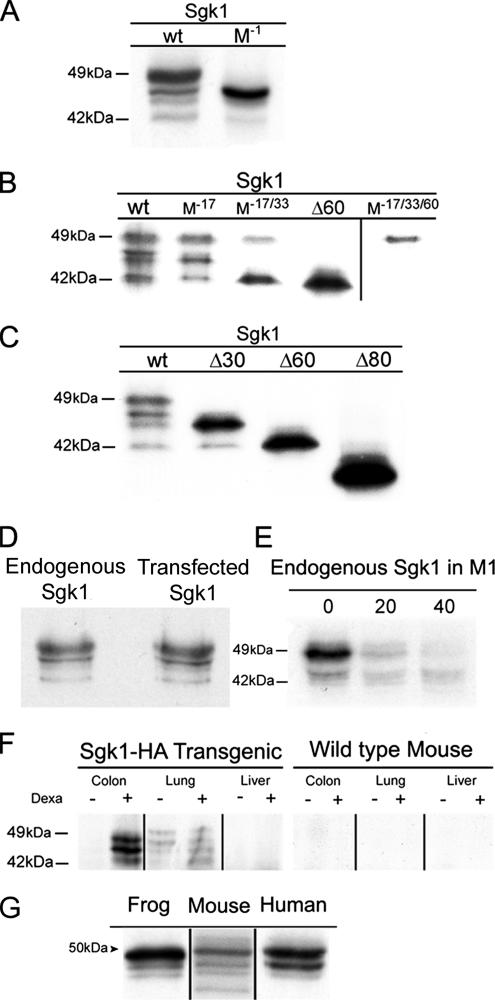
Mutation of the start codon corresponding to the first methionine of mouse Sgk1 cDNA eliminates the Kozak sequence; thereby, it should prevent translation of the protein unless other initiation sites are present downstream of Met1. Transfection of such mutant in CHO cells resulted in robust expression of Sgk1 with a slight decrease in molecular size compared with wild-type Sgk1 (Figure 2A), demonstrating that downstream in frame translational initiation sites in Sgk1 mRNA are functional and are recognized by the translation machinery.
Figure 2.
Alternative initiation of translation produces Sgk1 isoforms in cells and mouse tissues and other vertebrates. (A) Immunoprecipitation (IP) of Sgk1 wild type and with deletion of the first Met (M−1). (B) IP of Sgk1 with mutations on Met17 (M−17), double (M−17/33), and triple mutant (M−17/33/60). (C) Similar experiments in cells transfected with Sgk1-truncated forms Δ33, Δ60, and Δ80. (D) Comparison of transfected and endogenous Sgk1 from M1 cells pretreated with 50 mM dexamethasone for 8 h. (E) Pulse-chase experiment of endogenous Sgk1 in M1 cells illustrating faster degradation of the 49 than the 42-kDa isoform. (F) IP of tissue homogenates from transgenic Sgk1-HA and wild-type mice ± pretreatment with dexamethasone using a polyclonal anti-HA followed by IB with monoclonal HA-HRP. (G) IP of frog, mouse, and human Sgk1 from transfected cell labeled with [35S]methionine/cysteine.
We next analyzed all other methionines that could be used as initiation sites of translation by changing the ATG sequence to CTG of Met17, Met33, and Met60. Figure 2B shows that these mutations eliminate expression of three species of Sgk1, leaving only the 49-kDa Sgk1 protein with the entire N-terminus. For comparison, Figure 2C shows the pattern of bands obtained with N-terminal truncated forms of Sgk1 missing the first 33 (Δ33Sgk1), 60 (Δ60Sgk1), or 80 (Δ80Sgk1) residues. Therefore, alternative initiation of translation of a single Sgk1 mRNA gives rise to four Sgk1 isoforms, 49, 47, 45, and 42 kDa, each with a progressively shorter N-terminus.
Endogenous Sgk1 in Mouse Cells and in Tissues Expresses as Several Isoforms
We investigated the pattern of band expression of endogenous Sgk1 in M1 cells derived from mouse distal renal tubule. In M1 cells the level of expression of endogenous Sgk1 is low requiring treatment of cells with dexamethasone and long exposure of gels, ∼1 wk, to detect the signal from immunoprecipitated Sgk1. Figure 2D shows Sgk1 as four bands with molecular weights identical to the ones seen in cells transfected with Sgk1; hence, the isoforms are intrinsic to Sgk1 and not overexpression artifacts. Moreover, the endogenous Sgk1 also exhibits a short half-life (t1/2 ∼30 min) as the transfected Sgk1 (Figure 2E).
We next examine endogenous Sgk1 in mouse tissues. As detection of Sgk1 in vivo is compounded by close sequence similarity with Sgk2 and Sgk3, insufficient specificity of available antibodies to distinguish them and the fact that many tissues express more than one SGK gene, we generated a transgenic mouse by pronuclear injection of a bacterial artificial chromosome (BAC) containing the whole mouse SGK1 gene modified by insertion of 3xHA epitopes added to the C-terminus (Supplementary Figure 3). The tag allows detection of the Sgk1 transgene with a monoclonal anti-HA antibody avoiding the former uncertainties. The BAC contains the promoter, enhancers, and other regulatory elements of the SGK1 gene, assuring the transgene keeps the same transcriptional control and processing as the endogenous SGK1 alleles.
We found low basal level of expression of Sgk1-HA protein in transgenic tissues. To increase transcription, Sgk1-HA transgenics and wild-type littermates were injected with dexamethasone (1 mg/kg) subcutaneously 8 h before harvesting tissues. For detection of Sgk1-HA protein we first immunoprecipitated Sgk1-HA from tissue homogenates with a rabbit polyclonal anti-HA followed by western blotting with anti-HA monoclonal conjugated with HRP. Figure 2F shows that the HA antibody recognizes the expected molecular weight bands for Sgk1 isoforms. Moreover, the intensity of these bands increased after dexamethasone injection indicating that the Sgk1 transgene is regulated by glucocorticoids equally as the endogenous Sgk1. As expected, no signal was detected in wild-type littermates because the endogenous Sgk1 is not recognized by HA antibody. These results demonstrate in vivo expression of Sgk1 isoforms.
Finally, we show that Sgk1 isoforms are conserved across evolutionary distant species of vertebrates. Table 1 lists the coding region of Sgk1 from several species cloned to date. A common feature in the N-terminus of these proteins is the presence of several methionine residues flanked by Kozak sequences. To examine whether these methionines also give expression to Sgk1 isoforms, we transfected human or frog Sgk1 in CHO cells and examined the pattern of band expression. Figure 2G shows that Sgk1 from these species express at least three different size proteins. The shortest isoform, 42 kDa, is absent as predicted from the cDNA sequences (Table 1).
Sgk1 Isoforms Localize to Different Cellular Compartments
Recently we have shown that the N-terminus of Sgk1 contains an amphipathic α-helix that targets the protein to the ER membrane (Arteaga et al., 2006; Bogusz et al., 2006). This finding posed the question of how ER localization is compatible with the functions hitherto proposed for Sgk1, some of which require translocation of Sgk1 to the nucleus. Because the N-termini of the related kinases Akt and Sgk3 contain sequences that target these proteins to the plasma membrane or to early endosomes, we reason that the differences in the N-terminus could also determine distinct subcellular localization of the Sgk1 isoforms.
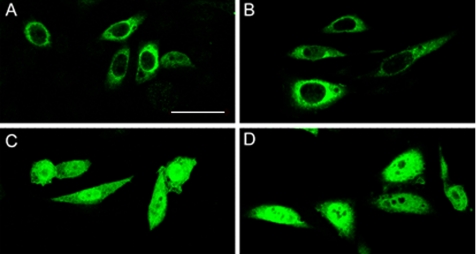
CHO cells transfected with each of the four Sgk1 isoforms were examined by immunofluorescence. Figure 3shows that 49- and 47-kDa isoforms localize primarily around the nucleus in a reticular pattern, whereas 45- and 42-kDa isoforms distribute homogeneously over the cytoplasm and nucleus. Therefore, expression of isoforms provides a means for differential localization of Sgk1 in cellular compartments including the nucleus.
Figure 3.
Subcellular localization of Sgk1 isoforms. Immunofluorescence of CHO cells transfected with the four Sgk1 isoforms: (A) 49, (B) 47, (C) 45 and (D) 42 kDa. Sgk1 proteins were detected with anti-HA monoclonal conjugated with fluorescein isothiocyanate on a confocal macroscope. Bar, 15 μm.
Regulation of Expression Levels of the Sgk1 Isoforms
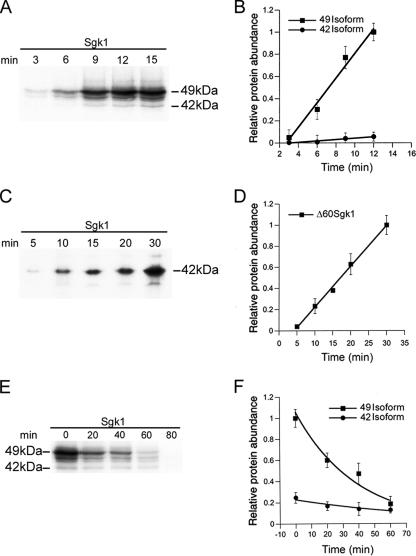
The Sgk1 isoforms differ in levels of expression (Figures 1 and 2), the 49 and 47-kDa isoforms are more abundant than the 45- and 42-kDa isoforms. Because the balance of synthesis and degradation determines the abundance at steady state, we examined these processes for the long and short Sgk1 isoforms. Cells were transfected with Sgk1 and labeled with [35S]methionine for the indicated time periods (Figure 4A). The rate of synthesis was calculated for the 49- and 42-kDa isoforms, revealing a 10-fold faster synthesis rate for the 49- than for the 42-kDa isoform (Figure 4B). However, when the rate of synthesis of the 42-kDa isoform was examined using a Δ60Sgk1 construct, which does not contain the coding region for the first 59 residues, we found a rate of synthesis similar to that of the long isoforms (Figure 4, C and D). These results are consistent with leaky scanning of ribosomes due to the presence of several AUG start codons in the 5′ end of Sgk1 mRNA. Translation of Sgk1 preferentially starts at Met1, and the probability of initiation of translation at a downstream methionine decreases progressively as the position is more distal from the first Kozak sequence. This mechanism explains the relative low rate of synthesis of the short isoforms compared with the long ones when they are translated from the same mRNA.
Figure 4.
Rates of synthesis and degradation of 49- and 42-kDa Sgk1 isoforms. (A and B) Differential rate of synthesis of 49- and 42-kDa isoforms estimated by cumulative incorporation of [35S]methionine using Sgk1 full length. (C and D) Rate of synthesis of 42-kDa isoforms estimated with Δ60Sgk1 construct. (E and F) Differential rates of degradation of 49 and 42 isoforms estimated by pulse-chase experiments with [35S]methionine. Data points in graphs represent average of four independent experiments ± SD.
Analysis of the degradation rate of Sgk1 by pulse-chased experiments revealed a t1/2 of 30 min for the 49-kDa isoform and t1/2 >180 min for the 42-kDa isoform (Figure 4, E and F). The high turnover rate of the long isoforms results from the presence of a localization signal (encompassed by residues 18–30) that is recognized by the ubiquitin/proteasomal machinery associated to the ER (Arteaga et al., 2006; Belova et al., 2006). In contrast, the short isoforms are stable because they do not have this sequence in the N-terminus. Thus, the level of expression of the long isoforms is the highest among all the Sgk1 isoforms spite of a high turnover rate because they are synthesized more efficiently than the short ones.
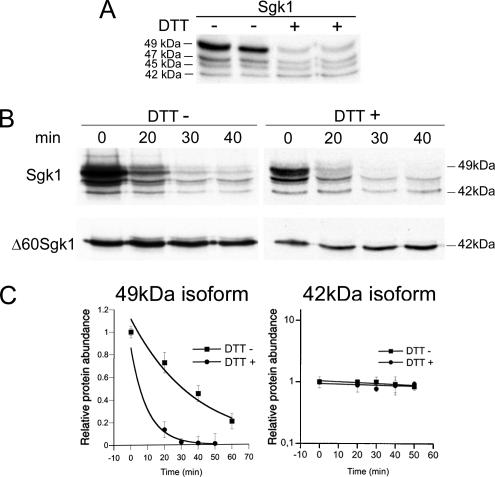
However, the relative abundance of the Sgk1 isoforms is not fixed; it changes under certain stress conditions. CHO cells expressing Sgk1 were exposed to heat shock (42°C for 1 h) or to 2 mM DTT for 1 h to induce cytoplasmic or ER stress. Both treatments decreased incorporation of [35S]methionine into the whole protein pool estimated as radioactive counts/mg protein, 19% reduction by heat shock, and 73% by DTT (Supplementary Figure 4A). This global decrease in translation is part of the response to environmental stresses that allows cells to conserve resources and direct them to the expression of genes that alleviate stress conditions (Wek et al., 2006). The specific effect on Sgk1 was assessed by counts recovered after Sgk1 immunoprecipitation, a 20 and 50% reduction induced by heat shock and DTT, respectively (Supplementary Figure 4B). Only DTT treatment markedly reduced the abundance of the 49-kDa isoform (Figure 5A), due to a selective increase in the rate of degradation of this isoform (Figure 5, B and C), whereas the 42-kDa isoform remained stable (Figure 5, D and E). This effect is consistent with DTT triggering the unfolded protein response (UPR) (Zhang and Kaufman, 2006) and activating the ER-associated degradation machinery (ERAD), which increases expression of the ubiquitin ligase HRD1, the enzyme involved in Sgk1 degradation (Arteaga et al., 2006).
Figure 5.
Effect of ER stress on expression of Sgk1 isoforms. (A) Steady state level of expression of Sgk1 isoforms in cells treated ± DTT. (B) Representative pulse-chase experiment of Sgk1 full-length and Δ60Sgk1 construct ± DTT. (C) Rate of degradation of 49- and 42-kDa isoforms ± DTT; n = 3.
As indicated previously, distinctive of SGK1 gene is a high sensitivity to transcriptional regulation by many external stimuli (Firestone et al., 2003). The results from this section indicate that in addition, posttranslational mechanisms play a role in regulating differentially levels of expression of the various Sgk1 isoforms.
Functional Specificity of Sgk1 Isoforms
Many cellular functions have been attributed to Sgk1; the best characterized are modulation of sodium absorption in epithelia by ENaC, a role in cell survival mediated by translocation of FoxO3a (Tran et al., 2003; Wu et al., 2006) and in insulin signaling by phosphorylation of GSK3β (Kobayashi and Cohen, 1999). We investigated whether the Sgk1 isoforms exhibit functional differences in any of these targets.
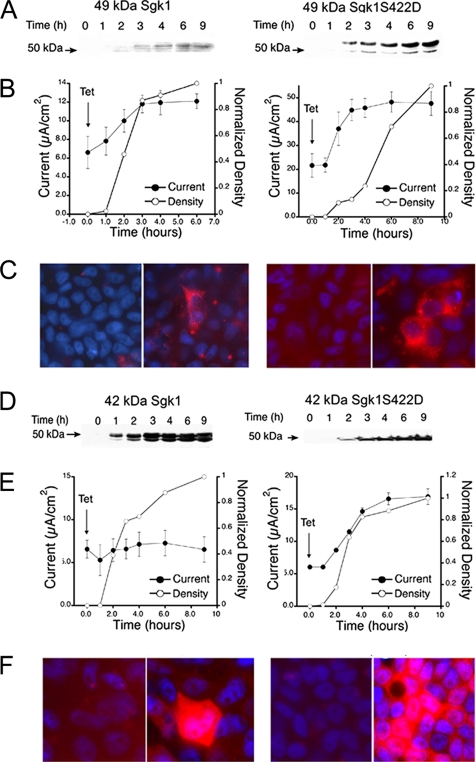
We first evaluated the effect on endogenous ENaC expressed in the epithelial cell line A6, which was stably transfected with the 49- or 42-kDa Sgk1 isoforms with or without the mutation S422D that renders the kinase constitutively active (Park et al., 1999). At least three clones from each transfection were analyzed. Activity of ENaC was measured as the amiloride-sensitive component of Isc in cells grown on Transwells. Tetracycline-induced expression and localization of Sgk1 were examined by Western blotting and immunofluorescence with a monoclonal HA antibody. All experiments were conducted in cells deprived of serum for 16 h. Figure 6 shows representative clones expressing the indicated Sgk1 isoforms. The 49-kDa Sgk1 with and without the activating mutation increased ENaC activity to a similar degree, whereas the 42-kDa isoform affected ENaC only when modified by the S422D mutation. Of notice, we observed little correlation between the level of expression of Sgk1, assessed by Western blotting, and the degree of ENaC activation, Isc (Figure 6, A and B). In some of the clones the maximal increase in sodium transport was achieved when Sgk1 was barely detectable by Western blotting, indicating that the differential functional effects cannot be attributed simply to differences in levels of expression of the Sgk1 isoforms. Figure 6C shows confocal images of the indicated clones ± tetracycline grown on permeable supports and stained with anti-HA and DAPI. A plane was chosen for good visualization of the subcellular distribution of Sgk1. As in transfected CHO cells, 49-kDa Sgk1 in A6 cells exhibits reticular pattern with higher intensity around the nucleus, whereas 42-kDa Sgk1 expresses more abundantly and distributes homogeneously over the cell. Of notice, despite being single clones, heterogeneity was found in the level of expression among cells.
Figure 6.
Functional effects of 49- and 42-kDa Sgk1 isoforms on ENaC current. Clones of A6 cells expressing 49- or 42-kDa Sgk1 isoforms ± activating mutation S422D under control of tetracycline. (A and D) Time course of Sgk1 expression assessed by Western blotting at the indicated times. (B and E) Time course of amiloride-sensitive Isc after induction with tetracycline at time 0 (●) and quantification by densitometry of western blots at the corresponding time points (○). (C and F) Immunofluorescence of each clone grown of Transwells ± tetracycline with anti-HA antibody.
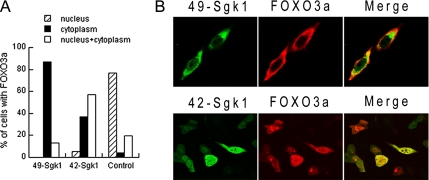
We next examined the ability of the Sgk1 isoforms to translocate FoxO3a from the nucleus to the cytoplasm (Figure 7). FoxO3a localizes to the nucleus in serum-deprived cells where it represses/activates many sets of genes involved in survival and proliferation. On addition of serum or growth factors, kinases such as Akt1 and Sgk1 phosphorylate FoxO3a in specific S/T residues, inducing inactivation and translocation to the cytoplasm (Tran et al., 2003). CHO cells were transfected with FoxO3a alone as control or together with 49-kDa Sgk1 or 42-kDa Sgk1 and examined by immunofluorescence. Cells were deprived of serum for 16 h before experiments in order to minimize FoxO3a phosphorylation by endogenous kinases. Cells transfected only with FoxO3a showed mainly nuclear localization (80% nuclear and 20% in nucleus and cytoplasm; control in Figure 7A). Cotransfection with 49-kDa Sgk1 significantly shifted FoxO3a localization to the cytoplasm (87% in cytoplasm and 13% in nucleus and cytoplasm), whereas in cells expressing the 42-kDa Sgk1 isoform only 37% of cells exhibited FoxO3a in the cytoplasm and 57% in cytoplasm and nucleus (Figure 7, A and B). Therefore, the 49-kDa isoform is much more efficient at inducing FoxO3a cytoplasmic translocation. Transfection of 49- or 42-kDa isoforms with the mutation S422D produced strong and equal cytoplasmic translocation of FoxO3a (data not shown).
Figure 7.
Effects of 49- and 42-kDa Sgk1 isoforms on Foxo3a translocation. (A) Cells transfected with FoxO3a-V5 alone or with Sgk1 49 or 42 isoforms were examined with anti-V5 antibody. Serum was removed 16 h before experiments. Quantification of FoxO3a localization in nucleus (hatched), cytoplasm (black), and nucleus+cytoplasm (white) in 100–150 cells. (B) Identification of Sgk1 isoforms (green) with anti-FLAG and FoxO3a (red) with anti-V5.
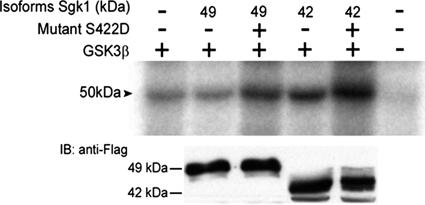
As an additional potential target of Sgk1, we examined phosphorylation of GSK3β by the Sgk1 isoforms (Figure 8). Cells were cotransfected with GSK3β and 49- or 42-kDa Sgk1 isoforms with or without the mutation S422D. Cells were serum deprived for 24 h before experiments to minimize phosphorylation of GSK3β by endogenous kinases. The 49-kDa isoform did not phosphorylate GSK3β, whereas the 42-kDa isoform produced a 60% increase in phosphorylation (Figure 8). This result was not due to different expression levels of the isoforms as demonstrated by Western blotting of Sgk1 in the same experiment (bottom panel in Figure 8). When the Sgk1 isoforms were rendered constitutively active, both phosphorylated GSK3β with similar efficiency, indicating again that subcellular localization determines the state of activation of the Sgk1 isoforms in basal conditions.
Figure 8.
Representative autoradiography of immunoprecipitated GSK3β-HA after phosphorylation in cells cotransfected with empty vector or the Sgk1-FLAG isoforms indicated in the top panel. Immunoblot of expression levels of Sgk1 isoforms in the same experiments.
DISCUSSION
Functional Implications of Sgk1 Isoforms
We report that Sgk1 is expressed as isoforms with different length of the N-terminus. Conservation of the isoforms in evolutionary distant species including human, mouse, and frog underscores their functional significance. In mouse, we identified four Sgk1 isoforms that exhibit distinct properties allowing their classification in two groups: long, 49 and 47 kDa, and short Sgk1 isoforms, 45- and 42-kDa. The long Sgk1 isoforms localize to the ER membrane, are rapidly degraded by the ERAD system, and have the highest level of expression, and their abundance is regulated by conditions that activate the UPR. The short Sgk1 isoforms exhibit low basal level of expression and low protein turnover and distribute in the cytoplasm and nucleus. Most significant, the isoforms are functionally distinct. The 49-kDa isoform stimulates ENaC activity and translocates FoxO3 from the nucleus to the cytoplasm but does not phosphorylate GSK3β. In contrast, the 42-kDa isoform phosphorylates GSK3β but does not activate ENaC and poorly translocates FoxO3a to the cytoplasm.
Differences in functional effects of the Sgk1 isoforms could be explained on the basis of local activation in different subcellular compartments and access to substrates. Results from ENaC and FoxO3a indicate that the 49-kDa Sgk1 isoform is functional under basal conditions because further activation by introduction of the S422D mutation does not significantly increase the functional effects. These results imply phosphorylation of the 49-kDa Sgk1 isoform by a kinase, i.e., a putative PDK2, that resides at the ER membrane or has access to the ER-associated proteins and that is also active in the absence of external stimuli. This is consistent with observations in oocytes where expression of Sgk1 wild-type or mutant S422D does not change significantly the degree of activation of membrane transporters (Boehmer et al., 2006; Shojaiefard and Lang, 2006). In contrast the 42-kDa Sgk1 is more efficient in phosphorylating GSK3β and the S422D mutation increases effect, indicating that a different and stimulus-dependent PDK2 activates the cytosolic 42-kDa Sgk1. This form of regulation by compartmentation is disrupted by expression of constitutively active Sgk1 isoforms, which results in indiscriminate activation of all pathways. Subsequent phosphorylation of the activation loop of Sgk1 by PDK1 (T253) is not restricted by compartmentalization because, as elegantly demonstrated by Alessi's group (Biondi et al., 2001), this phosphorylation event is independent of phosphatidylinositol-3-phosphate and follows a mechanism different from the one used in the phosphorylation of the equivalent residue of Akt.
Although N-terminal truncations of Sgk1 have been used as research tools (Kobayashi et al., 1999; Arteaga et al., 2006), it was not known that such proteins exist in vivo. Our findings demonstrate that they are an intrinsic feature of endogenous Sgk1 expressed in cells, in tissues, and in many vertebrate species. Because most Sgk1 is expressed as the ER-associated 49-kDa isoform, hitherto it was difficult to reconcile previous reports of Sgk1 in the cytoplasm or its translocation into the nucleus by stress or hormonal stimulation (Firestone et al., 2003; Leong et al., 2003). It is now apparent that the 45- and 42-kDa Sgk1 isoforms are the ones that can shuttle between cytoplasm and nucleus. Although these isoforms exhibit low level of expression, sometimes below the limits of conventional detection methods, the results also demonstrate that low levels of Sgk1 protein are sufficient to elicit functional effects.
The products of the Sgk1, Sgk2, and Sgk3 genes and other closely related kinases, such as Akt, share a high degree of homology in the catalytic domain (52–89%) and discriminate poorly among substrates (Murray et al., 2004), raising the question of how functional specificity is achieved in vivo. This problem has been solved in evolution by targeting the kinases to different cellular compartments through N-terminal localization signals. Thus, Akt localizes to the plasma membrane through a pleckstrin homology domain, 49-kDa Sgk1 to the ER through an amphipathic α-helix (Arteaga et al., 2006), and Sgk3 to endosomes through a Phox homology domain (Xu et al., 2001; Xing et al., 2004). The 45- and 42-kDa Sgk1 and Sgk2 do not have signals to direct them to membrane-formed compartments; instead they remain in the cytoplasm but can enter the nucleus using a nuclear localization signal present in the catalytic domain (Maiyar et al., 2003). Our data indicate that in each of these compartments the respective Sgk1 isoforms are locally activated and have access to their substrates, conferring specificity to their distinct signaling pathways.
Mechanism That Produces the Sgk1 Isoforms
The isoforms of Sgk1 are generated by alternative initiation of translation from a single SGK1 mRNA due to leaky scanning of the ribosomes. Translation of the overwhelming majority of eukaryotic mRNAs occurs by a scanning mechanism, in which the 40 S ribosomal subunit binds to the mRNA immediately downstream of the 5′-cap. On recognition of the first AUG triplet scanning ceases, and the 60 S ribosomal subunit joins the complex. However, if the first AUG is in a suboptimal context, it may not be recognized, and translation can then initiate at downstream AUG(s). In this way, a single mRNA can produce several variant products.
The Kozak consensus sequence was originally defined as (A/G)CCAUGG (Kozak, 1984). The A in the underlined AUG start codon is coordinate 1 and at position −3 could be an A or G. A purine, usually A, in position −3 is crucial for efficient initiation of translation, and in its absence, a G at position +4 is essential. Thus, according to Table 1, sequences surrounding Met1, Met17, Met20, Met33, and Met60 in the mouse Sgk1 are all good sites for translation initiation and are indeed used in vivo to synthesize Sgk1 isoforms. The same applies for other species as indicated in Table 1 and Figure 2F.
In light of these new results it is important to reconsider the previous interpretation of the meaning of multiple bands of Sgk1 resolved on SDS-PAGE. The different molecular-weight bands of Sgk1 have been attributed exclusively to aberrant migration, owing to phosphorylation of residues T253 and S422 upon activation of Sgk1 by insulin or other agonists (Park et al., 1999; Rozansky et al., 2002). We demonstrate that additional bands of lower molecular weight than Sgk1 correspond to different translational isoforms. Moreover, treatment with phosphatases does not alter the pattern of the four bands reported here. It is likely that changes in intensity of bands in previous reports represent changes in relative expression of the Sgk1 isoforms, which could have been induced by exposing cells to various stress conditions. Indeed, we demonstrate here that activation of the ERAD changes relative abundance of Sgk1 isoforms.
In conclusion, alternative initiation of translation provides an additional level of regulation that increases the diversity of Sgk1 proteins. In turn this mechanism provides a means to target Sgk1 to different cellular compartments where local activation confers functional specificity to Sgk1-dependent cellular processes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institute of Health Grant DK054062.06A1 and AHA0555777T to C.M.C.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-10-0968) on March 21, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Alvarez de la Rosa D., Canessa C. M. Role of SGK in hormonal regulation of epithelial sodium channel in A6 cells. Am. J. Physiol. 2003;284:C404–C414. doi: 10.1152/ajpcell.00398.2002. [DOI] [PubMed] [Google Scholar]
- Alvarez de la Rosa D., Coric T., Todorovic N., Shao D., Wang T., Canessa C. M. Distribution and regulation of expression of serum- and glucocorticoid-induced kinase-1 in the rat kidney. J. Physiol. 2003;551:455–466. doi: 10.1113/jphysiol.2003.042903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga M. F., Wang L., Ravid T., Hochstrasser M., Canessa C. M. An amphipathic helix targets serum and glucocorticoid-induced kinase 1 to the endoplasmic reticulum-associated ubiquitin-conjugation machinery. Proc. Natl. Acad. Sci. USA. 2006;103:11178–11183. doi: 10.1073/pnas.0604816103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga M. F., Canessa C. M. Functional specificity of Sgk1 and Akt1 on ENaC activity. Am. J. Physiol. 2005;289:F90–F96. doi: 10.1152/ajprenal.00390.2004. [DOI] [PubMed] [Google Scholar]
- Belova L., Sharma S., Brickley D. R., Nicolarsen J. R., Patterson C., Conzen S. D. Ubiquitin/proteasome degradation of serum and glucocorticoid-regulated kinase-1 (SGK-1) is mediated by the chaperone-dependent E3 ligase CHIP. Biochem. J. 2006 doi: 10.1042/BJ20060905. [Epub ahead of print] PMID: 16895519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi R. M., Kieloch A., Currie R. A., Deak M., Alessi D. R. The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. EMBO J. 2001;20:4380–4390. doi: 10.1093/emboj/20.16.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer C., Palmada M., Rajamanickam J., Schniepp R., Amara S., Lang F. Post-translational regulation of EAAT2 function by co-expressed ubiquitin ligase Nedd4–2 is impacted by SGK kinases. J. Neurochem. 2006;97:911–921. doi: 10.1111/j.1471-4159.2006.03629.x. [DOI] [PubMed] [Google Scholar]
- Bogusz A. M., Brickley D. R., Pew T., Conzen S. D. A novel N-terminal hydrophobic motif mediates constitutive degradation of serum- and glucocorticoid-induced kinase-1 by the ubiquitin-proteasome pathway. FEBS J. 2006;273:2913–2928. doi: 10.1111/j.1742-4658.2006.05304.x. [DOI] [PubMed] [Google Scholar]
- Firestone G. L., Giampaolo J. R., O'Keeffe B. A. Stimulus-dependent regulation of serum and glucocorticoid inducible protein kinase (SGK) transcription, subcellular localization and enzymatic activity. Cell Physiol. Biochem. 2003;13:1–12. doi: 10.1159/000070244. [DOI] [PubMed] [Google Scholar]
- Gomis R. R., Alarcon C., He W., Wang Q., Seoane J., Lash A., Massagué J. A FoxO-Smad synexpression group in human keratinocytes. Proc. Natl. Acad. Sci. USA. 2006;103:12747–12752. doi: 10.1073/pnas.0605333103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertweck M., Gobel C., Baumeister R. C. elegansSGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev. Cell. 2004;6:577–588. doi: 10.1016/s1534-5807(04)00095-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Deak M., Morrice N., Cohen P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem. J. 1999;344:189–197. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Cohen P. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem. J. 1999;339:319–328. [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984;12:857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalioti M., Heath J. K. A new method for generating point mutations in bacterial artificial chromosomes by homologous recombination in Escherichia coli. Nucleic Acids Res. 2001:e14. doi: 10.1093/nar/29.3.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F., Bohmer C., Palmada M., Seebohm G., Strutz-Seebohm N., Vallon V. (Patho)physiological significance of serum- and glucocorticoid-inducible kinase isoforms. Physiol. Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- Leong M. L., Maiyar A. C., Kim B., O'Keeffe B. A., Firestone G. L. Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. J. Biol. Chem. 2003;278:5871–5882. doi: 10.1074/jbc.M211649200. [DOI] [PubMed] [Google Scholar]
- Maiyar A. C., Leong M. L., Firestone G. L. Importin-alpha mediates the regulated nuclear targeting of serum- and glucocorticoid-inducible protein kinase (Sgk) by recognition of a nuclear localization signal in the kinase central domain. Mol. Biol. Cell. 2003;14:1221–1239. doi: 10.1091/mbc.E02-03-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. T., et al. Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. Biochem. J. 2004;384:477–488. doi: 10.1042/BJ20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Leong M. L., Buse P., Maiyar A. C., Firestone G. L., Hemmings B. A. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI3-kinase-stimulated signaling pathway. EMBO J. 1999;18:3024–3033. doi: 10.1093/emboj/18.11.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozansky D. J., Wang J., Doan N., Purdy T., Faulk T., Bhargava A., Dawson K., Pearce D. Hypotonic induction of SGK1 and Na+transport in A6 cells. Am. J. Physiol. 2002;283:F105–F113. doi: 10.1152/ajprenal.00176.2001. [DOI] [PubMed] [Google Scholar]
- Sakoda H., et al. Differing roles of Akt and serum- and glucocorticoid-regulated kinase in glucose metabolism, DNA synthesis, and oncogenic activity. J. Biol. Chem. 2003;278:25802–25807. doi: 10.1074/jbc.M301127200. [DOI] [PubMed] [Google Scholar]
- Shojaiefard M., Lang F. Stimulation of the intestinal phosphate transporter SLC34A2 by the protein kinase mTOR. Biochem. Biophys. Res. Commun. 2006;345:1611–1614. doi: 10.1016/j.bbrc.2006.05.067. [DOI] [PubMed] [Google Scholar]
- Tran H., Brunet A., Griffith E. C., Greenberg M. E. The many forks in FOXO's road. Sci. STKE. 2003;172:RE5. doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- Waldegger S., Klingel K., Barth P., Sauter M., Rfer M. L., Kandolf R., Lang F. h-sgk serine-threonine protein kinase gene as traqnscriptional target of transforming growth factor β in human intestine. Gastroenterology. 1999;116:1081–1088. doi: 10.1016/s0016-5085(99)70011-9. [DOI] [PubMed] [Google Scholar]
- Wu W., Zou M., Brickley D. R., Pew T., Conzen S. D. Glucocorticoid receptor activation signals through forkhead transcription factor 3a in breast cancer cells. Mol. Endocrinol. 2006;20:2304–2314. doi: 10.1210/me.2006-0131. [DOI] [PubMed] [Google Scholar]
- Wulff P., et al. Impaired renal Na+retention in the sgk1-knockout mouse. J. Clin. Invest. 2002;110:1263–1268. doi: 10.1172/JCI15696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Liu D., Zhang R., Joachimiak A., Songyang Z., Xu W. Structural basis of membrane targeting by the Phox homology domain of cytokine-independent survival kinase (CISK-PX) J. Biol. Chem. 2004;279:30662–30669. doi: 10.1074/jbc.M404107200. [DOI] [PubMed] [Google Scholar]
- Xu J., Liu D., Gill G., Songyang Z. Regulation of cytokine-independent survival kinase (CISK) by the Phox homology domain and phosphoinositides. J. Cell Biol. 2001;154:699–705. doi: 10.1083/jcb.200105089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H., Jang Y., You-Ten A. I., Okada H., Liepa J., Wakeham A., Zaugg K., Mak T. W. p53-dependent inhibition of FKHRL1 in response to DNA damage through protein kinase SGK1. Proc. Natl. Acad. Sci. USA. 2004;101:14057–14062. doi: 10.1073/pnas.0406286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Kaufman R. J. From acute ER stress to physiological roles of the unfolded protein response. Cell Death. Differ. 2006;13:374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.